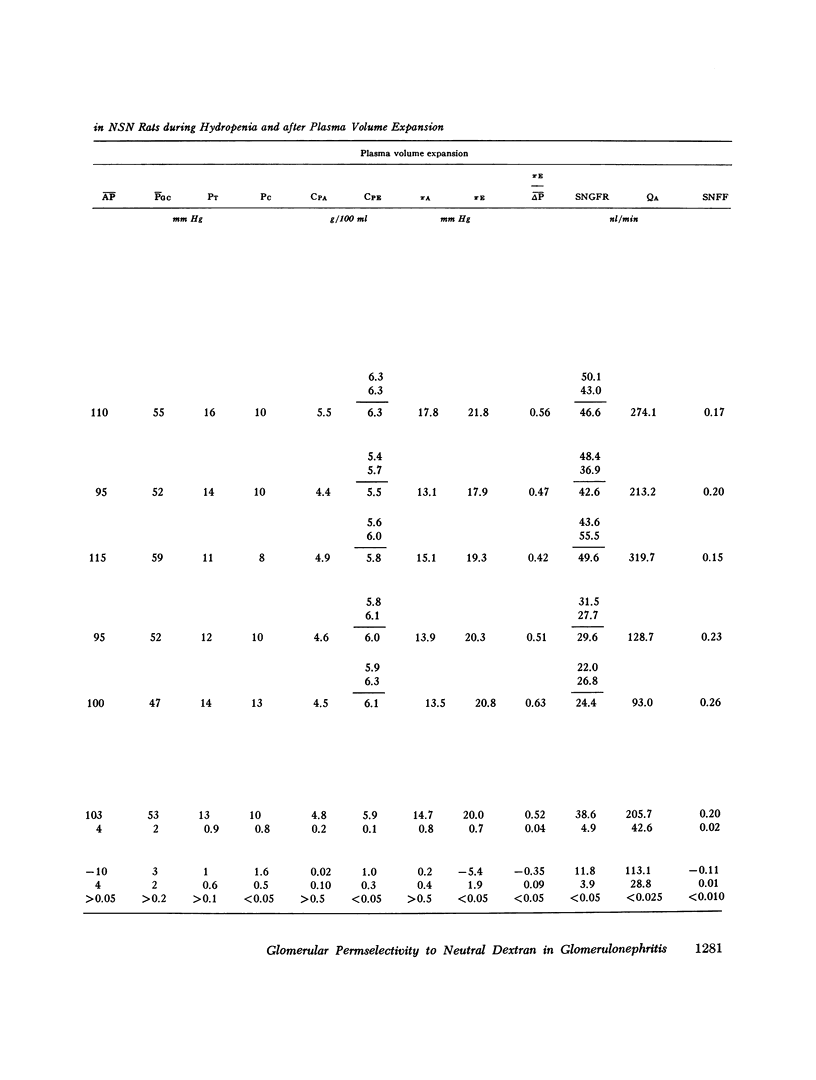
Abstract
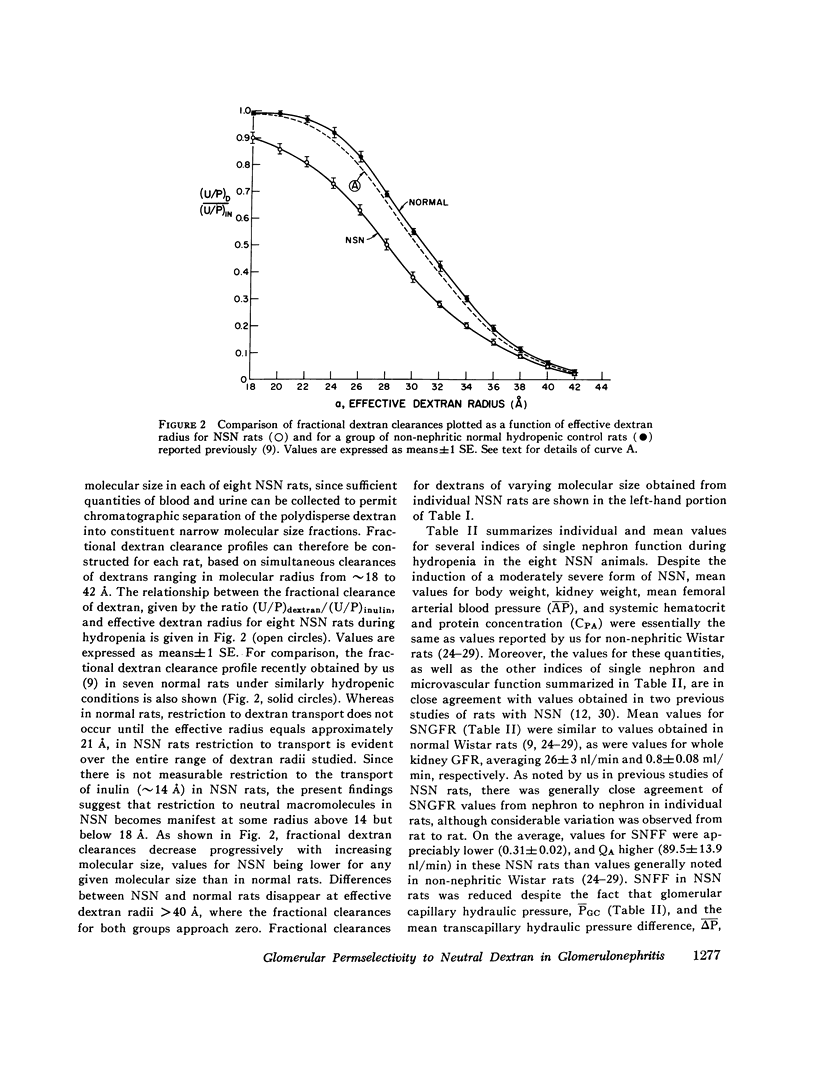
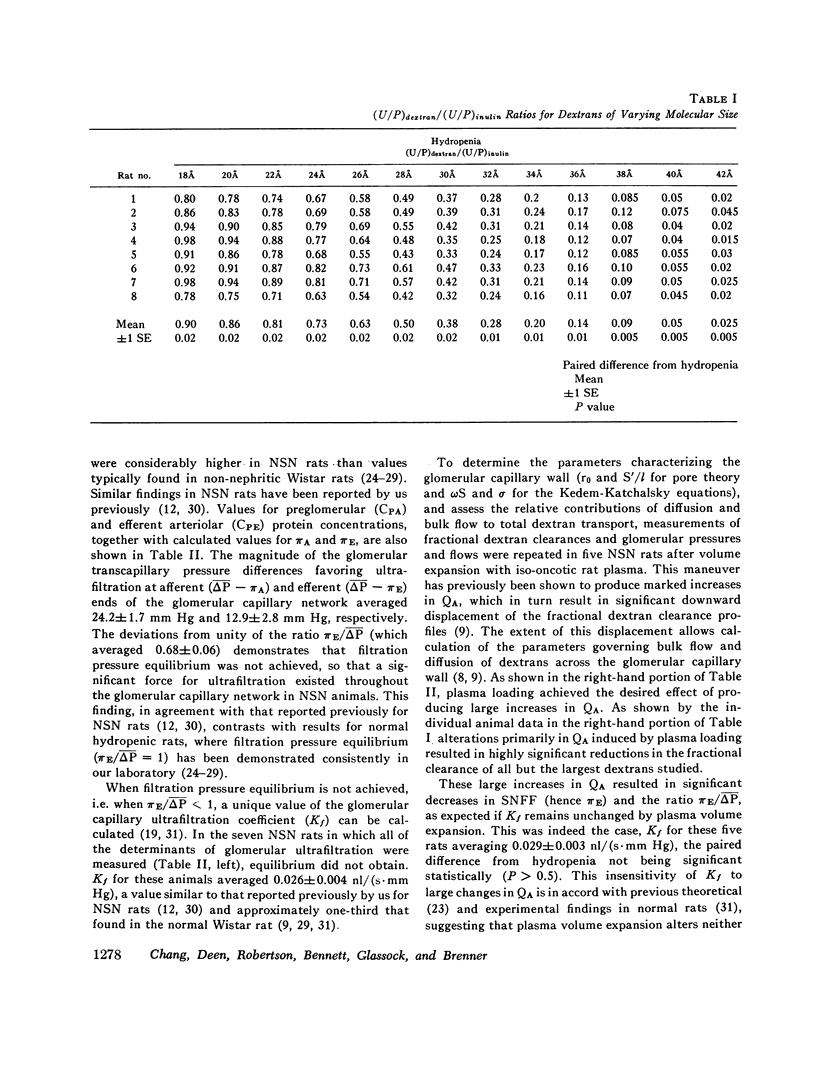
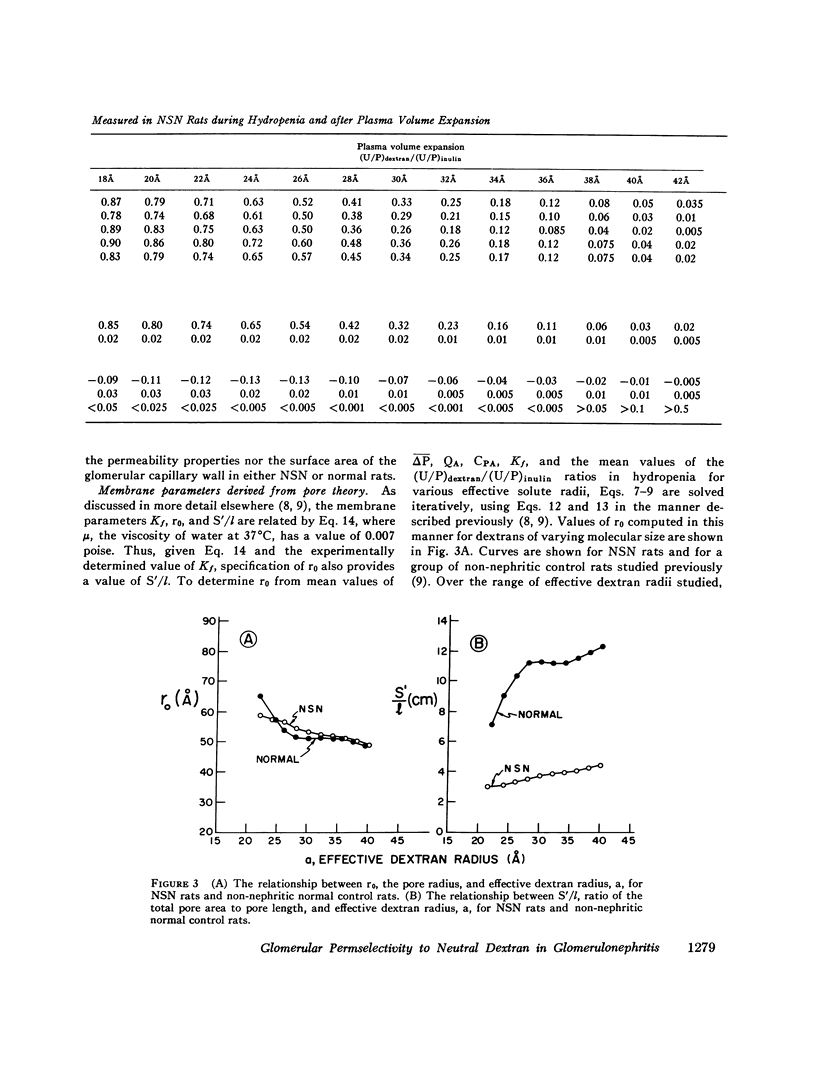
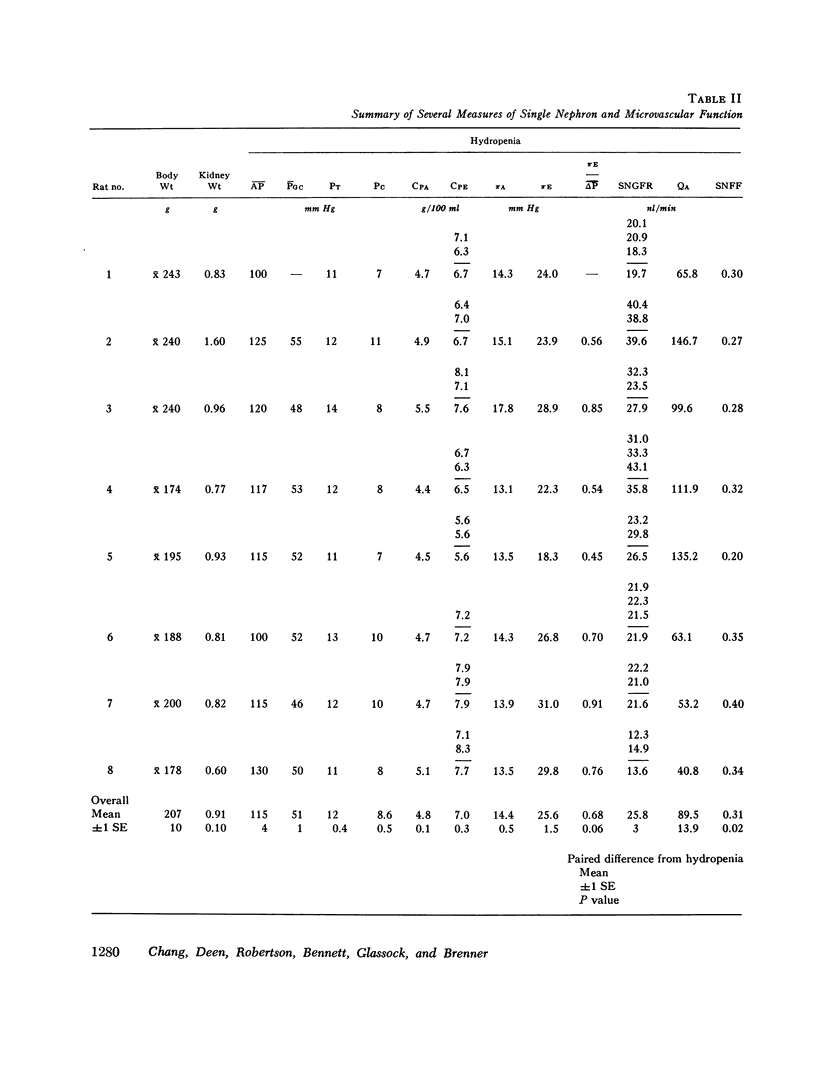
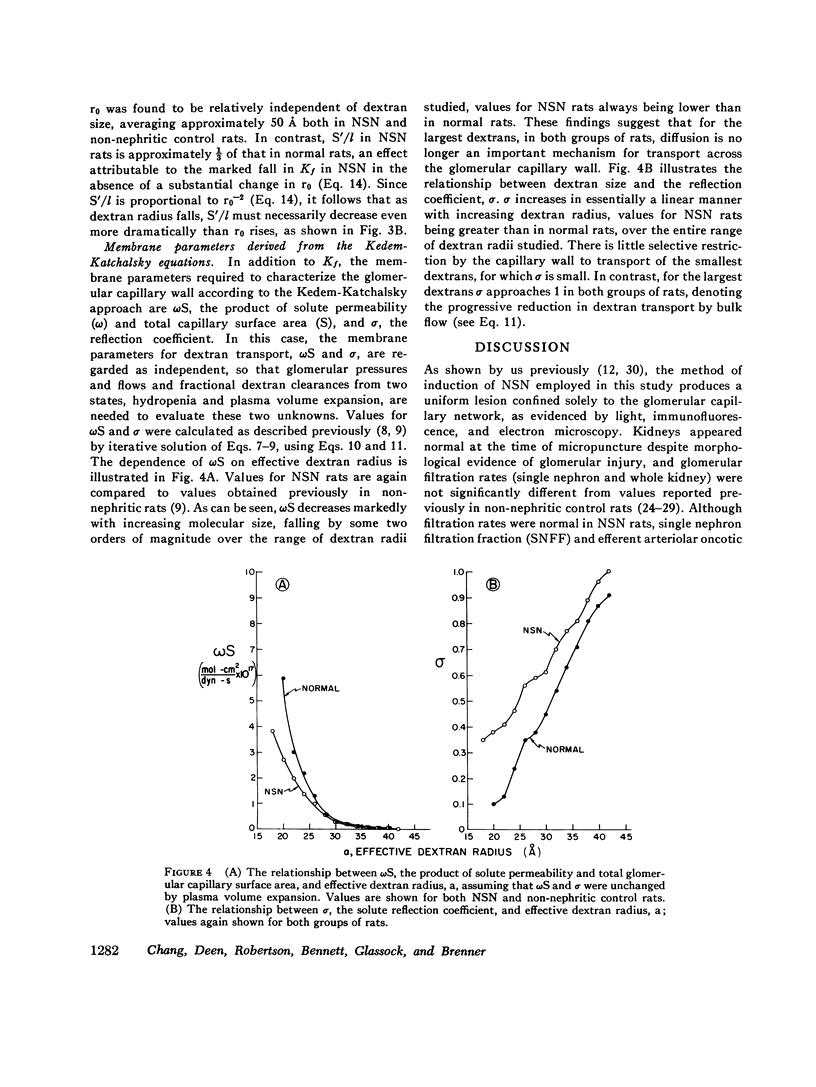
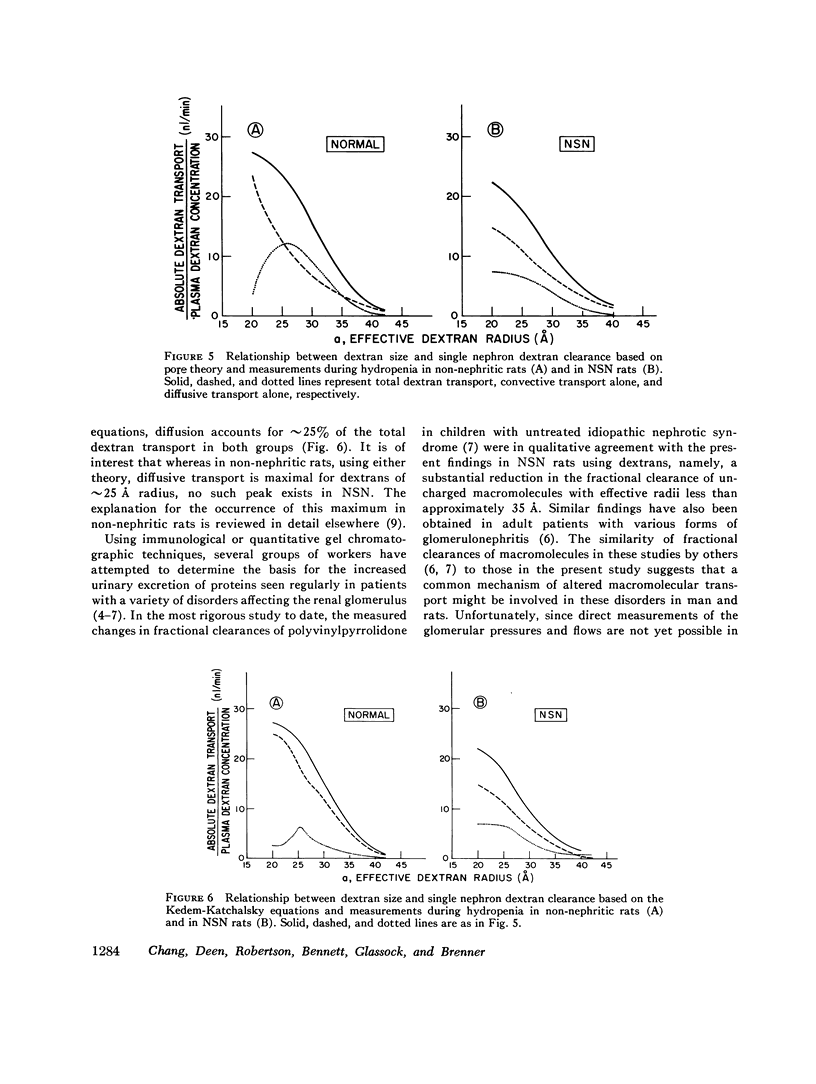
Polydisperse [3h] dextran was infused into eight Munich-Wistar rats in the early autologous phase of nephrotoxic serum nephritis (NSN), thereby permitting direct measurements of pressures and flows in surface glomeruli and fractional clearances for dextrans [(U/P) dextran/(U/P) inulin] ranging in radius from 18 to 42 A. Despite glomerular injury, evidenced morphologically and by a marked reduction in the glomerular capillary ultrafiltration coefficient, the glomerular filtration rate remained normal because of a compensating increase in the mean net ultrafiltration pressure. In NSN rats, as in normal controls, inulin was found to permeate the glomerular capillary wall without measurable restriction, and dextrans were shown to be neither secreted nor reabsorbed. For dextran radii of 18, 22, 26, 30, 34, 38, and 42 A, (U/P) dextran/(U/P) inulin in NSN and control rats, respectively, averaged 0.90 vs. 0.99, 0.81 vs. 0.97, 0.63 vs. 0.83, 0.38 vs 0.55, 0.20 vs. 0.30, 0.08 vs. 0.11, and 0.02 vs. 0.03. Using a theory based on macromolecular transport through pores, the results indicate that in NSN rats, effective pore radius is the same as in controls, approximately 50 A. In NSN, however, the ratio of total pore surface area to pore length, a measure of the number of pores, is reduced to approximately 1/3 that of control, probably due to a reduction in capillary surface area. These results suggest that proteinuria in glomerular disease is not due simply to increases in effective pore radius or number of pores, as previously believed. Using a second theoretical approach, based on the Kedem-Katchalsky flux equations, dextran permeability across glomerular capillaries was found to be slightly lower, and reflection coefficient slightly higher in NSN than in control rats.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arturson G., Groth T., Grotte G. Human glomerular membrane porosity and filtration pressure: dextran clearance data analysed by theoretical models. Clin Sci. 1971 Feb;40(2):137–158. doi: 10.1042/cs0400137. [DOI] [PubMed] [Google Scholar]
- Bennett C. M., Glassock R. J., Chang R. L., Deen W. M., Robertson C. R., Brenner B. M., Troy J. L., ueki I. R., Rasmussen B. Permselectivity of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using dextran sulfate. J Clin Invest. 1976 May;57(5):1287–1294. doi: 10.1172/JCI108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., MacInnes R. M. Quantitative importance of changes in postglomerular colloid osmotic pressure in mediating glomerulotubular balance in the rat. J Clin Invest. 1973 Jan;52(1):190–197. doi: 10.1172/JCI107164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. Pressures in cortical structures of the rat kidney. Am J Physiol. 1972 Feb;222(2):246–251. doi: 10.1152/ajplegacy.1972.222.2.246. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971 Aug;50(8):1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Ueki I. F., Troy J. L., Deen W. M., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. II. Experimental studies in rats using neutral dextran. Biophys J. 1975 Sep;15(9):887–906. doi: 10.1016/S0006-3495(75)85863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Robertson C. R., Deen W. M., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. I. Theoretical considerations. Biophys J. 1975 Sep;15(9):861–886. doi: 10.1016/S0006-3495(75)85862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Mercer P. F., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. V. Response to ischemic injury. J Clin Invest. 1974 Jan;53(1):105–116. doi: 10.1172/JCI107527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Maddox D. A., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VII. Response to reduced renal mass. Am J Physiol. 1974 Sep;227(3):556–562. doi: 10.1152/ajplegacy.1974.227.3.556. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. Concentration polarization in an ultrafiltering capillary. Biophys J. 1974 May;14(5):412–431. doi: 10.1016/S0006-3495(74)85924-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois R., Decoodt P., Gassée J. P., Verniory A., Lambert P. P. Determination of glomerular intracapillary and transcapillary pressure gradients from sieving data. I. A mathematical model. Pflugers Arch. 1975;356(4):299–316. doi: 10.1007/BF00580004. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Berliner R. W. Hydrostatic pressures in peritubular capillaries and tubules in the rat kidney. Am J Physiol. 1971 May;220(5):1422–1426. doi: 10.1152/ajplegacy.1971.220.5.1422. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Baer P. G., Chirito E., Dirks J. H. Composition of mammalian glomerular filtrate. Am J Physiol. 1974 Oct;227(4):972–976. doi: 10.1152/ajplegacy.1974.227.4.972. [DOI] [PubMed] [Google Scholar]
- Hulme B., Hardwicke J. Human glomerular permeability to macromolecules in health and disease. Clin Sci. 1968 Jun;34(3):515–529. [PubMed] [Google Scholar]
- JOACHIM G. R., CAMERON J. S., SCHWARTZ M., BECKER E. L. SELECTIVITY OF PROTEIN EXCRETION IN PATIENTS WITH THE NEPHROTIC SYNDROME. J Clin Invest. 1964 Dec;43:2332–2346. doi: 10.1172/JCI105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert P. P., Verniory A., Gassee J. P., Ficheroulle P. Sieving equations and effective glomerular filtration pressure. Kidney Int. 1972 Sep;2(3):131–146. doi: 10.1038/ki.1972.83. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Brenner B. M. Control of proximal tubule fluid reabsorption in experimental glomerulonephritis. J Clin Invest. 1975 Jun;55(6):1315–1325. doi: 10.1172/JCI108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Daugharty T. M., Brenner B. M. Determinants of glomerular filtration in experimental glomerulonephritis in the rat. J Clin Invest. 1975 Feb;55(2):305–318. doi: 10.1172/JCI107934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VIII. Effects of hematocrit. Circ Res. 1975 Mar;36(3):425–435. doi: 10.1161/01.res.36.3.425. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R. Passage of molecules through capillary wals. Physiol Rev. 1953 Jul;33(3):387–423. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- Robson A. M., Giangiacomo J., Kienstra R. A., Naqvi S. T., Ingelfinger J. R. Normal glomerular permeability and its modification by minimal change nephrotic syndrmone. J Clin Invest. 1974 Nov;54(5):1190–1199. doi: 10.1172/JCI107862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEDERHIELM C. A., WOODBURY J. W., KIRK S., RUSHMER R. F. PULSATILE PRESSURES IN THE MICROCIRCULATION OF FROG'S MESENTERY. Am J Physiol. 1964 Jul;207:173–176. doi: 10.1152/ajplegacy.1964.207.1.173. [DOI] [PubMed] [Google Scholar]



