Abstract
The etiology for the sepsis-induced leucine (Leu) resistance has not been fully elucidated and the present study investigated various aspects of amino acid activation of the mammalian target of rapamycin (mTOR). Sepsis in adult male rats decreased basal protein synthesis in gastrocnemius, associated with a reduction in mTOR activation as indicated by decreased 4EBP1 and S6K1 phosphorylation. The ability of oral Leu to increase protein synthesis and mTOR kinase after 1 h was largely prevented in sepsis. Sepsis increased CAT1, LAT2 and SNAT2 mRNA content 2- to 4-fold, but only the protein content for CAT1 (20% decrease) was significantly different. Conversely, sepsis decreased the proton-assisted amino acid transporter (PAT)-2 mRNA by 60%, but without a coordinate change in PAT2 protein. There was no sepsis or Leu effect on the protein content for RagA-D, LAMTOR-1 and -2, raptor, Rheb or mTOR in muscle. The binding of mTOR, PRAS40 and RagC to raptor did not differ for control and septic muscle in the basal condition; however, the Leu-induced decrease in PRAS40•raptor and increase in RagC•raptor seen in control muscle was absent in sepsis. The intracellular Leu concentration was increased in septic muscle, compared to basal control conditions, and oral Leu further increased the intracellular Leu concentration similarly in both control and septic rats. Hence, while alterations in select amino acid transporters are not associated with development of sepsis-induced Leu-resistance, the Leu-stimulated binding of raptor with RagC and the recruitment of mTOR/raptor to the endosome-lysosomal compartment may partially explain the inability of Leu to fully active mTOR and muscle protein synthesis.
Keywords: sepsis, protein synthesis, mTOR, amino acid transporters, Rag GTPases, PRAS40, ragulator
Introduction
The erosion of lean body mass (LBM) resulting from prolonged sepsis and inflammation is mediated not only by a reduction in the basal rate of muscle protein synthesis but also an impaired response to many anabolic stimuli, including growth factors (insulin and insulin-like growth factor-I) and nutrients (amino acids, leucine) (Lang and Frost, 2004, Lang and Frost, 2006, Lang et al., 2010). Both of these sepsis-induced defects are in part mediated by an attenuation of the regulatory kinase mTOR (mammalian target of rapamycin) which impairs cap-dependent mRNA translation (Frost and Lang, 2011), although the specific mechanism for this impairment remains to be determined. Regardless of its etiology, the loss of LBM in sepsis remains a clinical concern as it compromises recovery and increases morbidity in the chronically ill patient population (Callahan and Supinski, 2009, Blackburn and Bistrian, 1976).
The ability of the essential amino acid leucine to increase mTOR activity is central to regulating global protein synthesis and maintaining cellular protein homeostasis (Dodd and Tee, 2012). Several mechanisms have been proposed for leucine activation of mTOR (Avruch et al., 2009, Jewell et al., 2013), which could conceivably work independently or in combination to promote anabolic signal transduction. Amino acid transporters have been implicated in the regulation of mTOR by at least two mechanisms. First, the classical transport function, which individually or coupled with other transporters, provides a conduit for extracellular leucine to enter the cell or for the redistribution of leucine within the cell (Verrey, 2003, Hatzoglou et al., 2004). Alternatively or additionally, some transporters [sodium-coupled neutral amino acid transporters (SNAT2/slc38A2) and proton-assisted amino acid transporters (PAT1-4; slc36A)] have been suggested to have a nutrient signaling component thereby functioning as a “transceptor” (Hundal and Taylor, 2009, Ogmundsdottir et al., 2012). However, characterization of sepsis-induced changes in the muscle expression of various amino acid transporters is lacking. Once inside the cell, several models have been proposed whereby leucine stimulates the translocation and activation of mTOR to the late endosome-lysosome where it interacts with a number of proteins, including the Ragulator, vacular (v)-ATPase, and the Rag-GTPases (Sancak et al., 2010, Zoncu et al., 2011). However, again, how sepsis impacts these various components of leucine signal translocation to mTOR has not been fully assessed. Hence, the purpose of the present study was to determine whether the catabolic state produced by sepsis alters various amino acid transporters important for regulating leucine flux, the intracellular leucine concentration, and the protein-protein interaction of the mTOR-Ragulator complex in skeletal muscle.
Materials and methods
Animal protocols
Male specific pathogen-free Sprague-Dawley rats (Charles Rivers Breeding Lab, Cambridge, MA) were quarantined for 1 wk and at the time of surgery weighed 300-325 g (11-12 wks of age). Rats were housed under controlled environmental conditions, 12h light/12h dark, 21-22 C, and 30-70% humidity; no environment enrichment was provided. Rats were housed 3 per cage (wire-bottom) and provided Teklad Global 2019 [calories from protein (23%), fat (22%) and carbohydrate (55%); Harlan Teklad, Boston, MA] and tap water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine (protocol #43186) and adhered to the National Institutes of Health (NIH) guidelines for the use of experimental animals.
Induction of sepsis
Polymicrobial peritonitis was produced by cecal ligation and puncture (CLP), as previously described (Lang and Frost, 2004, Lang and Frost, 2006) in fed animals. This model was selected because it produces a septic state mimicking that seen in humans, and is recognized as an appropriate small animal model of peritonitis. Rats were anesthetized with isoflurane (3% induction +2-3% maintenance; Abbott Laboratories, North Chicago, IL). The abdominal hair was clipped and the skin cleaned with povidone-iodine prior to performing a midline laparotomy. The cecum was isolated, ligated, punctured twice with a 18-gauge needle, and a small amount of cecal material extruded to ensure patency. The cecum was returned to the abdomen, the muscle incision closed with 4-0 surgical suture (Ethicon, Inc., Somerville, NJ), and the skin incision closed with metal wound clips. Lidocaine (Abbott Laboratories), 2-3 drops, was applied to the wound for analgesia. After surgery rats were housed individually in shoe-box cages with corn-cobb bedding. Rats received 10 mL of 0.9% sterile saline (37°C) containing buprenorphine (0.05 mg/kg; Reckitt Benckiser Pharmaceuticals, Richmond, VA) administered subcutaneously every 12 h. Sham control animals experienced the same surgical procedures, but the cecum was not ligated or punctured. Before surgery, animals had unrestricted access to food and water. However, as food consumption in septic rats is nominal during the first 24 h post-CLP, food was withheld from all animals so metabolic differences between groups would be independent of differences in caloric intake.
A separate group of control and septic rats were administered an oral gavage of either leucine (1.35 g/kg BW) or saline, and skeletal muscle excised 60 min thereafter. The dose of leucine was selected based on prior studies demonstrating maximal stimulation of muscle protein synthesis and phosphorylation of 4E-BP1 and S6K1 (Crozier et al., 2005). Timing of the muscle sample after leucine administration was selected based on previously studies indicating leucine increases the mRNA expression of several amino acid transporters (Drummond et al., 2012, Drummond et al., 2010). This model of sepsis has been previously demonstrated to decrease basal and leucine-stimulated muscle protein synthesis (Kazi et al., 2011, Lang and Frost, 2004, Lang et al., 2005, Lang and Frost, 2006). In vivo studies were repeated on three separate occasions with each study including all four experimental groups.
In vivo protein synthesis
The in vivo rate of protein synthesis in the gastrocnemius and plantaris complex (hereafter referred to as muscle) was determined ~24 h after induction of sepsis using the flooding-dose technique, as detailed previously (Vary and Lang, 2008). Rats were anesthetized with isoflurane as described above and a catheter inserted in the carotid artery. Subsequently, L-[2,3,4,5,6-3H]phenylalanine (Phe; 150 mM, 30 μCi/ml; 1 ml/100 g body weight [BW]) was administered as a bolus injection into the jugular vein. Serial arterial blood samples were drawn at 2, 6 and 10 min after Phe injection for measurement of Phe concentration and radioactivity. Immediately after the final blood sample, skeletal muscle was excised and a portion freeze-clamped; remaining fresh muscle was directly homogenized. Blood was centrifuged and plasma was collected. All tissue and plasma samples were stored at −80° C. A portion of the frozen muscle was used to estimate the global rate of incorporation of [3H]Phe into protein, exactly as previously described (Vary and Lang, 2008).
Western blot analysis and immunoprecipitation
Fresh tissue was homogenized (Kinematic Polytron; Brinkmann, Westbury, NY) in ice-cold homogenization buffer consisting of (in mmol/L): 20 HEPES (pH 7.4), 2 EGTA, 50 sodium fluoride, 100 potassium chloride, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 phenyl-methane-sulphonylfluoride, 1 benzamidine, and 0.5 sodium vanadate. The protein concentration was quantified using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) and equal amounts of total protein per sample were subjected to standard SDS-PAGE (Kazi et al., 2011, Lang and Frost, 2004, Lang and Frost, 2006, Lang et al., 2010, Lang et al., 2005). All antibodies were purchased from Cell Signaling Technology (Beverly, MA) unless otherwise noted. As an index of mTOR kinase activity, Western blot analysis was performed for total and phosphorylated (T37/46) 4E-BP1 (Bethyl Laboratories, Montgomery, TX) as well as total and phosphorylated (T389) S6K1 which are downstream mTOR substrates. To assess activation of 4E-BP1, the amount of eIF4G bound to immunoprecipitated eIF4E was quantitated; activation of S6K1 was determined by immunoblotting for total and phosphorylated ribosomal protein S6 (S240/244). Total and Ser473 phosphorylated AKT as well as total and Thr246 phosphorylated PRAS40 (proline-rich AKT substrate 40) were assessed as upstream regulators of PRAS40 and its binding to raptor. The relatively expression of other proteins was determined by antibodies for CAT1 (cationic amino acid transporter 1; SLC7A1), LAT2 (L-type amino acid transporter 2; SLC7A5) and SNAT2 (sodium-coupled neutral amino acid transporter 2; SLC38A2) (Santa Cruz Biotechnology, Dallas, TX). Also, PAT-1 and PAT-4 (Santa Cruz Biotechnology) and PAT2 (Millipore, Billerica, MA) as well as LAMTOR (late endosomal/lysosomal adaptor, MAPK and mTOR activator) 1 and 2, mTOR, raptor and Rheb were determined by Western blotting. All lanes were normalized for protein loading and blotted for tubulin or other appropriate loading control. Blots were developed with enhanced chemiluminescence Western blotting reagents (Supersignal Pico, Pierce Chemical, Rockford, IL). Dried blots were exposed to x-ray film to achieve a signal within the linear range and film was then scanned (Microtek ScanMaker IV; Cerritos,CA) and quantified using Scion Image 3b software (Scion Corporation, Frederick, MD).
The eIF4E•eIF4G complexes were quantified by immunoprecipitation (Kazi et al., 2011). Briefly, eIF4E was immunoprecipitated from aliquots of supernatants using an anti-eIF4E monoclonal antibody. Antibody-antigen complexes were collected using magnetic beads, subjected to SDS-PAGE, and finally transferred to a PVDF membrane. Blots were incubated with a mouse anti-human eIF4E antibody, rabbit anti-rat 4E-BP1 antibody, or rabbit anti-eIF4G antibody. Also, to investigate protein-protein interactions within mTOR complex 1 (mTORC1), fresh muscle was also homogenized in CHAPS buffer (pH 7.5) composed of (in mM): 40 HEPES, 120 NaCl, 1 EDTA, 10 pyrophosphate, 10 β-glycerophosphate, 50 NaF, 1.5 sodium vanadate, 0.3% CHAPS and 1 protease inhibitor cocktail tablet. The homogenate was clarified by centrifugation and an aliquot of the supernatant was combined with anti-Raptor antibody and immune complexes were isolated with goat anti-rabbit BioMag IgG (PerSeptive Diagnostics, Boston, MA) beads. The beads were collected, washed with CHAPS buffer, precipitated by centrifugation and subjected to SDS-PAGE as described above. All blots were then developed with ECL and the autoradiographs were quantified as above and previously described (Kazi et al., 2011, Lang and Frost, 2004, Lang and Frost, 2006).
RNA extraction and real-time quantitative PCR
Total RNA was extracted using Tri-reagent (Molecular Research Center, Inc., Cincinnati, OH) and RNeasy mini kit (Qiagen, Valencia, CA) following manufacturers’ protocols. Tissue was homogenized in Tri-reagent followed by phenol/chloroform extraction according to the manufacturer's instruction. An equal volume of 70% ethanol was added to the aqueous phase and the mixture was loaded on a Qiagen mini-spin column. The Qiagen mini-kit protocol was followed from this step onwards including the on-column DNase I treatment to remove residual DNA contamination. RNA was eluted from the column with RNase-free water and an aliquot was used for quantitation (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA). Quality of the RNA was analyzed on a 1% agarose gel. Total RNA (1 μg) was reversed transcribed to cDNA using superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) in a total reaction volume of 20 μl following instructions from the manufacturer. Real-time quantitative PCR was performed on 1-2 μl of the reversed transcribed reaction mix in a StepOnePlus system using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) for the following: CAT-1 (slc7a1, NM_013111.2); SNAT-2 (slc38a2, NM_181090.2); LAT-2 (slc3a2, NM_019283.1); PAT-1 (slc36a1, NM_130415.1); PAT-2 (slc36a2, NM_139339.1); PAT4 (slc36a4, NM_001108127.1); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NM_017008.3). The comparative quantitation method 2-ΔΔCt was used in presenting gene expression of target genes in reference to the endogenous control.
Plasma concentrations
The plasma insulin concentration was determined by ELISA (Alpco; Salem, NH) and the leucine and glutamine concentrations in plasma and muscle were determined using reverse-phase high-pressure liquid chromatography after precolumn derivatization of amino acids with phenylisothiocyanate (Vary and Lang, 2008).
Statistics
Data for each condition are summarized as means ± standard error of the mean (SEM) where the number of rats per treatment is indicated in the legend to the figure or table. Statistical evaluation of the data was performed using 2-way ANOVA with post-hoc Student-Neuman-Keuls test when the interaction was significant. Differences between groups were considered significant at P < 0.05. GraphPad Prism version 5.0 (GraphPad software, La Jolla, CA) was used for statistical analysis.
Results
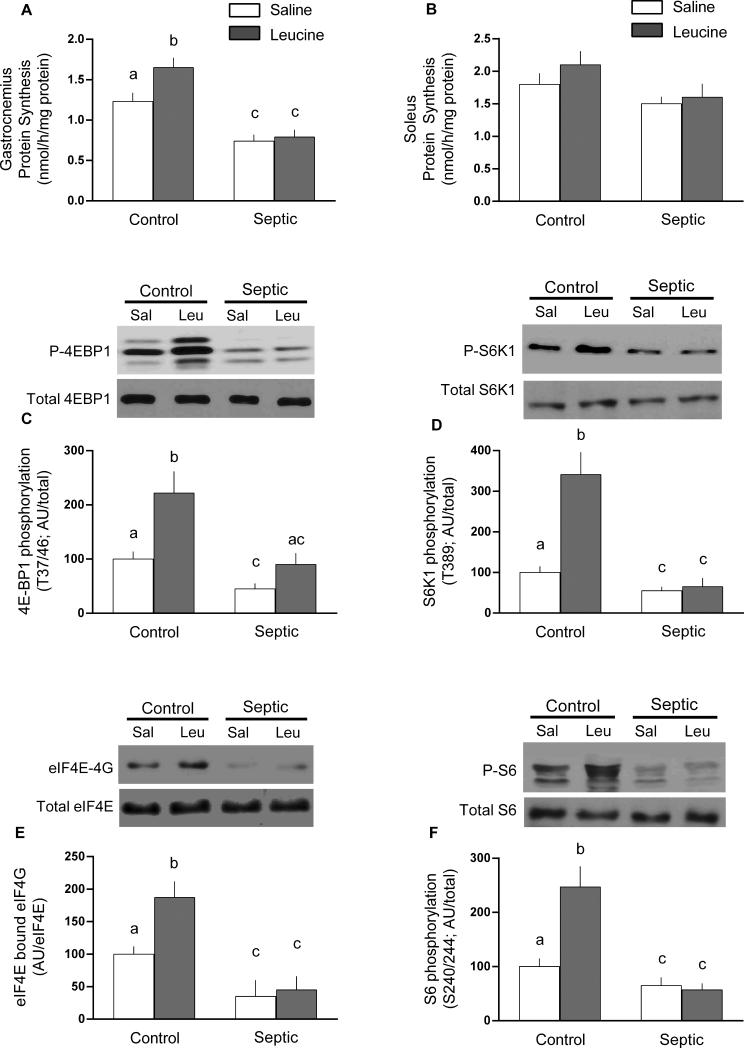
Figure 1 provides data related to sepsis-induced changes in protein synthesis under the basal condition and following leucine stimulation. Sepsis decreased global protein synthesis 35% in gastrocnemius, but not soleus (Figure 1A and 1B, respectively). This sepsis-induced decrease was accompanied by an impaired mTOR activity as indicated by the 45-55% reduction in phosphorylation of 4E-BP1 and S6K1, both downstream substrates for mTOR (Figure 1C and 1D, respectively). As the formation of the active eIF4E•eIF4G complex is proportional to the extent of 4E-BP1 phosphorylation, the sepsis-induced reduction in eIF4E•eIF4G binding (65%; Figure 1E) is consistent with the observed reduction in phosphorylated 4E-BP1. Likewise, the sepsis-induced reduction in the phosphorylation of S6 (35%; Figure 1F) is consistent with the above mentioned decreased S6K1 activity. In nonseptic control rats, leucine increased protein synthesis in gastrocnemius (but not soleus) and this was associate with increased phosphorylation of 4E-BP1, S6K1 and S6 as well as an increased formation of the eIF4E•eIF4G complex (Figure 1). This typical anabolic response to leucine was essentially absent in gastrocnemius from septic rats.
Fig 1.
Protein synthesis and mTOR signaling in gastrocnemius (panel A) and soleus (panel B) from control and septic rats under basal conditions or 1 h after oral administration of leucine (Leu) or saline (Sal). Panels C and D, representative Western blots for phosphorylated and total 4E-BP1 and S6K1, endpoints of mTOR signaling in gastrocnemius. Panel E, representative blot for binding of eIF4E with eIF4G, which is an indicator 4E-BP1 phosphorylation, determined on immunoprecipitated eIF4E. Panel F, representative immunoblot for ribosomal protein S6, a downstream target of S6K1. For all immunoblots, data were quantitated for all rats and presented in bar graphs as means ± SEM; n = 8, 9, 9 and 9, respectively for the four experimental groups. Values having a different superscript letter (a versus b versus c) are statistically different (P < 0.05); values with the same letter are not significantly different
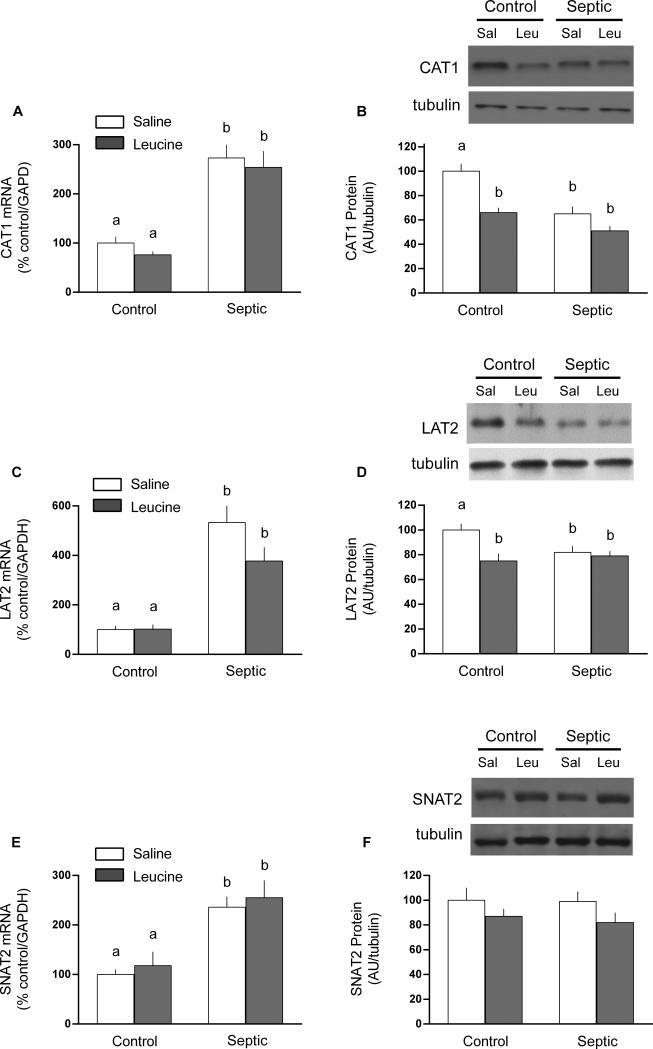
Amino acid transporters are important for regulating the intracellular concentration of leucine and other amino acids; hence, we assessed the mRNA and protein content for various transporters in gastrocnemius. In general, there was considerable discordance between mRNA and protein content determined for these transport proteins. For example, in the basal condition, sepsis increased CAT1 (2.7-fold), LAT2 (5.3-fold) and SNAT2 (2.4-fold) mRNA content (Figure 2A, 2C, and 2E). However, no coordinate up-regulation of these transporters at the protein level was detected (Figure 2B, 2D, 2F). In contradistinction to the mRNA data, Western analysis indicated sepsis decreased muscle CAT1 and LAT2 protein content under basal conditions (25% and 32%, respectively), but did not alter SNAT2 protein content. The mRNA expression for CAT1, LAT2 and SNAT2 did not differ between control and septic rats 1 h after oral leucine administration. However, leucine decreased the protein content 25-30% for CAT1 and LAT2 in muscle from controls but not septic rats.
Fig 2.
Protein and mRNA content for amino acid transporters CAT1, LAT2 and SNAT2 in gastrocnemius from control and septic rats under basal conditions or 1 h after oral administration of leucine (Leu) or saline (Sal). For bar graphs, values are means ± SEM; n = 8, 9, 9 and 9, respectively for the four experimental groups. The mRNA content is expressed as percent of control normalized for GAPDH, where the value for the nonseptic control value was arbitrarily set at 100%. Values having a different superscript letter (a versus b) are statistically different (P < 0.05); values with the same letter are not significantly different. Representative Western blots are provided for each experimental group and for each amino acid transporter protein
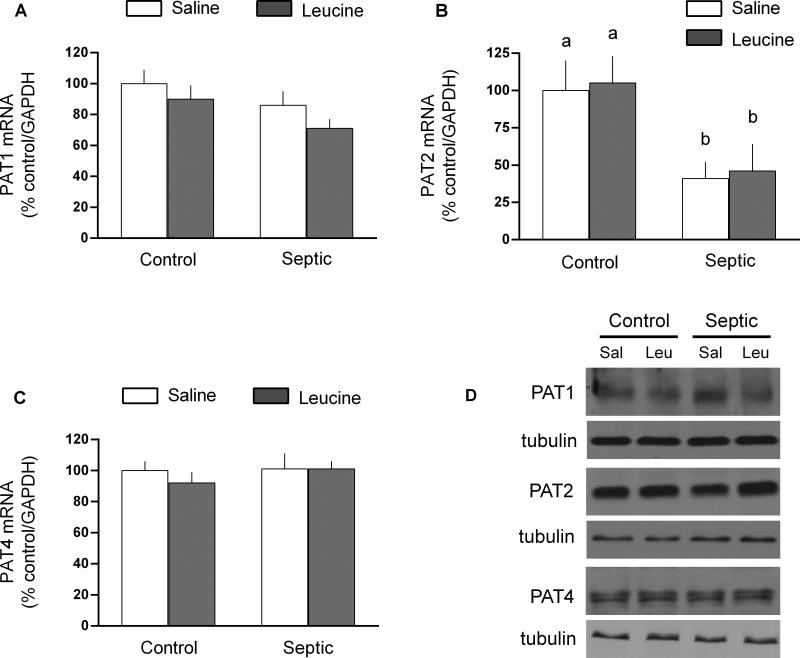
The proton-assisted amino acid symporters-1, −2 and −4 were also assessed in gastrocnemius (Figure 3). There was no sepsis and/or leucine effect on the mRNA or protein content for PAT-1 and PAT4. In contrast, while sepsis decreased PAT2 mRNA expression by 60%, the PAT2 protein content was unchanged. Furthermore, leucine did not alter PAT2 mRNA or protein in muscle from either control or septic rats.
Fig 3.
Protein and mRNA content for proton-assisted amino acid transporter (PAT)-1, −2 and −4 in gastrocnemius from control and septic rats under basal conditions or 1 h after oral administration of leucine (Leu) or saline (Sal). For bar graphs, values are means ± SEM; n = 8, 9, 9 and 9, respectively for the four experimental groups. The mRNA content is expressed as percent of control normalized for GAPDH, where the value for the nonseptic control value was arbitrarily set at 100%. Values having a different superscript letter (a versus b) are statistically different (P < 0.05); values with the same letter are not significantly different. Representative Western blots are provided for each experimental group and for each protein; no statistically significant sepsis or leucine effect was detected for PAT-1, −2 or −4 (data not shown)
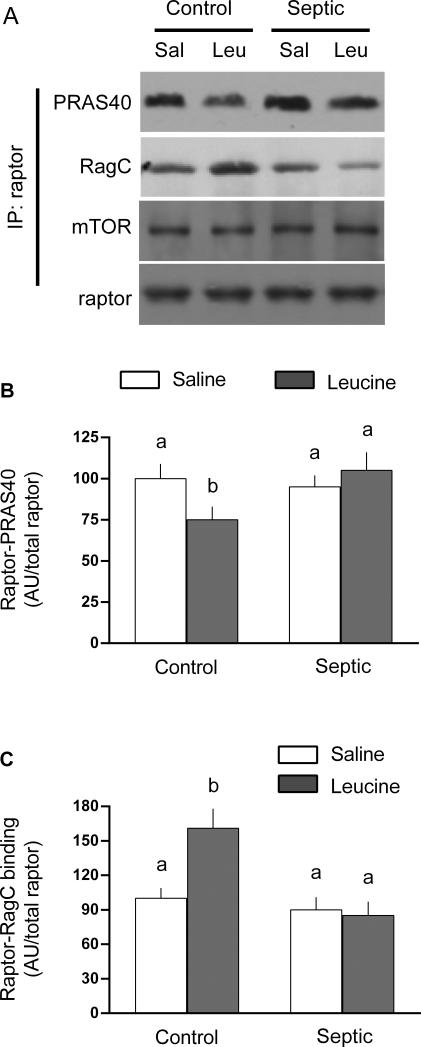
There was no sepsis and/or leucine effect for total mTOR, raptor, Rheb, RagA, RagB, RagC, RagD, LAMTOR1 or LAMTOR2 in the whole muscle homogenate (data not shown). Next, raptor was immunoprecipitated and the binding of several interacting proteins assessed (Figure 4). There was no sepsis- or leucine-induced change in the binding of mTOR to raptor (Figure 4A). Likewise, in the basal condition the binding of PRAS40 and RagC to raptor did not differ in muscle from control and septic rats. However, the binding of PRAS40 to raptor was decreased ~20% (Figure 4B) and the binding of RagC to raptor increased ~50% (Figure 4C) in control muscle. These reciprocal responses to leucine were not detected in septic rats.
Fig 4.
Binding of raptor with PRAS40 and RagC in gastrocnemius from control and septic rats under basal conditions or 1 h after oral administration of leucine (Leu) or saline (Sal). For bar graphs, values are means ± SEM; n = 8, 9, 9 and 9, respectively for the four experimental groups. Values having a different superscript letter (a versus b) are statistically different (P < 0.05); values with the same letter are not significantly different. Representative Western blots from the raptor immunoprecipitate (IP) are provided for each experimental group and for each protein
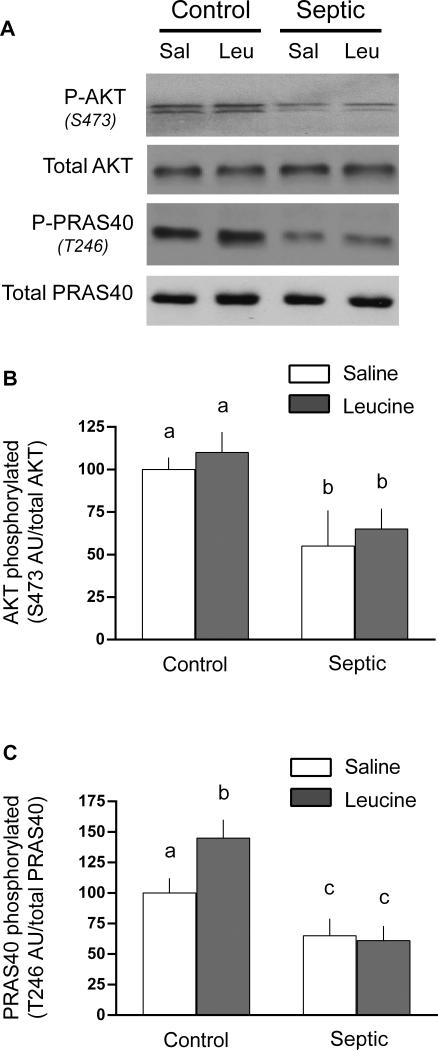
As PRAS40 is a downstream substrate of AKT and phosphorylation of PRAS40 releases its binding and inhibitory effect on raptor, we next examined total and phosphorylated AKT and PRAS40. Sepsis decreased Ser473 phosphorylated AKT by 45% and this change was independent of a change in total AKT (Figure 5A and 5B). Leucine gavage did not alter AKT phosphorylation in muscle of either control or septic rats. Sepsis also decreased Thr246 phosphorylated PRAS40 by 35-40%, compared to control values, in the basal condition (Figure 5A and 5C). In contrast, oral leucine increased PRAS40 phosphorylation in muscle from control rats by ~30%, but no increase was detected in septic rats.
Fig 5.
Total and phosphorylation of AKT and PRAS40 in gastrocnemius from control and septic rats under basal conditions or 1 h after oral administration of leucine (Leu) or saline (Sal). For bar graphs, values are means ± SEM; n = 8, 9, 9 and 9, respectively for the four experimental groups. Values having a different superscript letter (a versus b) are statistically different (P < 0.05); values with the same letter are not significantly different. Representative Western blots are provided for each experimental group and for each protein
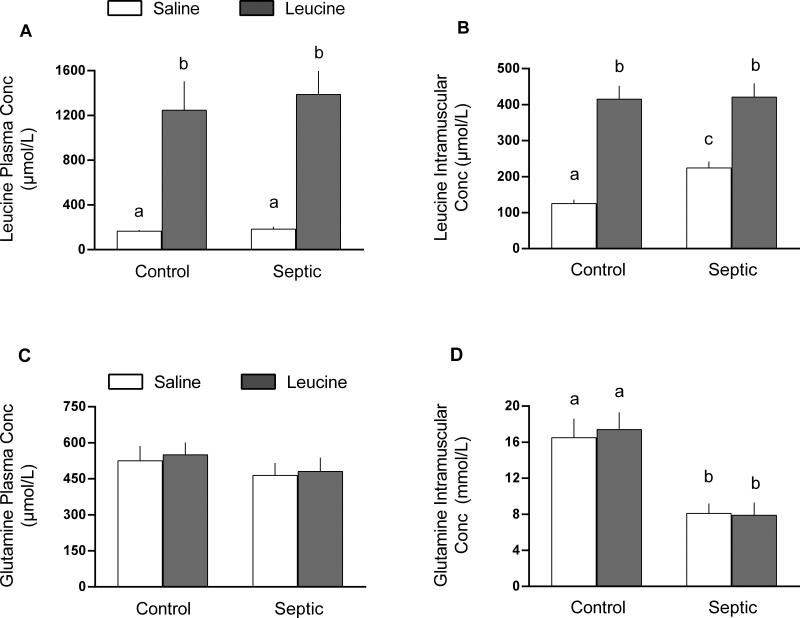
The plasma leucine concentration did not differ between control and septic rats in the basal condition or 1 h after oral administration of leucine; however, septic and control animals both exhibited ~8-fold increase in plasma leucine in response to leucine gavage (Figure 6A). The intracellular leucine concentration was elevated 75% in muscle from septic rats in the basal condition, compared to control values (Figure 6B). Leucine administration increased the intracellular leucine concentration in muscle of control and septic rats to the same level. Plasma glutamine concentrations did not differ in control and septic rats either under basal conditions or 1 h after leucine (Figure 6C). The intramuscular glutamine concentration was reduced ~50% by sepsis in both saline- and leucine-treated groups (Figure 6D).
Fig 6.
Concentration of leucine and glutamine in plasma and gastrocnemius in control and septic rats under basal conditions or 1 h after oral administration of leucine (Leu) or saline (Sal). Values are means ± SEM; n = 7 per group. Values having a different superscript letter (a versus b versus c) are statistically different (P < 0.05); values with the same letter are not significantly different
Finally, as leucine is a known insulin secretague, the plasma insulin concentration was also assessed. The basal plasma insulin did not differ between control and septic rats (105 ± 11 vs 92 ± 12 pmol/L, respectively; P > 0.05). Moreover, the plasma insulin concentration 1 h after oral leucine also did not differ between control or septic rats (112 ± 15 vs 108 ± 14 pmol/L, respectively; P > 0.05).
Discussion
The present study investigated potential regulatory steps by which sepsis might impair leucine activation of mTOR-dependent muscle protein synthesis. As amino acid transporters facilitate the import of extracellular leucine into the cell as well as potentially function as nutrient sensors (Hatzoglou et al., 2004, Hundal and Taylor, 2009, Ogmundsdottir et al., 2012), we first examined amino transporters implicated in leucine homeostasis. Although CAT1 does not transport leucine per se, it is ubiquitously expressed (except liver) and transports both arginine, important of synthesis of nitric oxide, and ornithine from which glutamine is synthesized (Hatzoglou et al., 2004), the latter of which may indirectly affect leucine transport via the LAT1 leucine/glutamine symporter. Increased CAT1 mRNA is seen in response to elevated glucocorticoids and amino acid starvation and results from increased transcription and mRNA stability (Liu and Hatzoglou, 1998, Fernandez et al., 2002). However, in contrast to these conditions where CAT1 mRNA and protein were concomitantly increased, we detected a sepsis-induced decrease in CAT1 protein despite more than doubling of CAT1 mRNA expression. In general terms our data indicate that CAT1 translation is impaired, which is consistent with the sepsis-induced decrease in translational efficiency previously reported in skeletal muscle (Kazi et al., 2011, Lang et al., 2010) and which is mediated in part by increased systemic and local concentrations of proinflammatory cytokines and glucocorticoids (Lang and Frost, 2006, Lang and Frost, 2007, Lang et al., 1996). A similar discordance between CAT1 transporter mRNA and protein content in muscle has been reported in the catabolic condition of extended bed rest (Drummond et al., 2012). At least for sepsis, this discrepancy between mRNA and protein may be due to the absence of an increase in eIF2α phosphorylation in muscle (Lang, unpublished observations), which was central to the increased translation of CAT1 in response to amino acid deprivation (Fernandez et al., 2002).
Transporters in the SLC38 family (System A) control influx of various small neutral amino acids, including glutamine, and comprise the various SNAT isoforms (Mackenzie and Erickson, 2004). Of these, SNAT2 is the most widely expressed, is present in skeletal muscle, and regulated by growth factors, amino acid supply, and various stressors (Hyde et al., 2007, Hyde et al., 2005). The SNAT symporters regulate the uphill flux of amino acids into the cell coupled with the inward movement of Na+ down its electrochemical gradient. In turn, the intracellular glutamine can then be exported in exchange for leucine and other branched-chain amino acids via the System L transporters LAT1 and 2 (Hundal and Taylor, 2009). Inhibition or knockdown of SNAT2 in cultured myotubes decreases intracellular amino acids, including leucine and impairs mTOR activity and protein synthesis (Evans et al., 2007). Our results indicate that while sepsis increased SNAT2 mRNA, the SNAT2 protein content in muscle did not differ between control and septic rats. These data are consistent with the lack of change SNAT2 seen in humans following extended bed rest which also produces muscle atrophy (Drummond et al., 2012). Additionally, the oral leucine load did not alter mRNA or protein content in control muscle for any of the amino acid transporters examined, confirming a previous report (Suryawan et al., 2013). Our data extend these findings to muscle from septic rats. Although this lack of effect for both control and septic muscle might be explained by the relatively short duration of leucine stimulation, other in vivo studies have reported increased expression of one or more transporter in a similar time frame (Drummond et al., 2012, Drummond et al., 2010, Suryawan et al., 2013).
Our data indicate sepsis decreases the intracellular glutamine concentration, independent of a change in the circulating concentration of this amino acid, a finding which has been previously reported (Roth et al., 1982, Askanazi et al., 1980). This result is consistent with the above mentioned sepsis-induced reduction in CAT1 protein which imports ornithine which can then be converted to glutamine intracellularly. In contrast, the sepsis-induced increase in intracellular leucine was paradoxically associated with a decrease in LAT2 protein. This discordance might be due more to the mismatch between the increased rate of leucine appearance from protein breakdown and the increased rate of leucine oxidation by muscle (Wolfe et al., 1989), than any change in plasma membrane amino acid transporter content. Furthermore, both the plasma and intracellular leucine concentrations were increased to a comparable magnitude in control and septic rats gavaged with leucine. While the leucine-induced increase in intracellular leucine has been reported after 1-3 hours in normal subjects (Drummond et al., 2010), a comparable increase in septic muscle has not been previously reported. Hence, the diminished anabolic response to leucine in septic muscle appears independent of differences in either the circulating or intracellular leucine concentration, and cannot be fully explained by changes in the protein content for selected amino acid transporters.
The proton-assisted amino acid transporters are responsible for transport of small amino acids. While the PATs do not transport leucine per se, they have been reported to have characteristics of intracellular amino acid sensors (Goberdhan et al., 2009). Knockdown of PAT1 and PAT4 in cultured cells impairs mTOR kinase signaling and the normal rapid anabolic response of starved cells to amino acid refeeding (Heublein et al., 2010, Ogmundsdottir et al., 2012). Moreover, PAT1 is an mTOR binding partner and interacts in vivo with the RagGTPase at the surface of the late endosome/lysosome (Ogmundsdottir et al., 2012). There appear to be no reports of sepsis- or trauma-induced changes in this family of amino acid transporters. Our data indicate sepsis did not alter the mRNA content for PAT1 or PAT4, although a decreased PAT2 mRNA in muscle was noted. Moreover, the protein content for PAT1, -2 and -4 in muscle did not differ between control and septic rats. Further, there was no detectable change for either mRNA or protein for the PATs in response to acute leucine administration, and this finding is consistent with the previously reported inability of hyperaminoacidemia to alter PAT1 and PAT2 mRNA content in muscle (Suryawan et al., 2013). Collectively, these data suggest a difference in total PAT protein content is an unlikely mediator of the sepsis-induced decrease in muscle protein synthesis. However, we cannot exclude the possibility that sepsis alters the intracellular localization of one or more of the PATs and its subsequent association with the Rag proteins and mTORC1, as reported for the developmentally regulated decrease in mTOR activity (Suryawan et al., 2013).
The raptor protein functions as a scaffold recruiting select substrates to mTORC1 (Kim et al., 2002). As previously reported (Kazi et al., 2011), and confirmed herein, we did not detect a sepsis- and/or leucine-induced change in the total amount of mTOR or raptor, or the interaction of these two proteins which might be expected to alter kinase activity (Kim et al., 2002). Raptor also binds the Rag GTPases which facilitates the recruitment of mTORC1 to the lysosome via a multiprotein complex identified as the Regulator, which functions as a guanine nucleotide exchange factor activating the Rags and thus is central to amino acid sensing (Bar-Peled et al., 2012, Sancak et al., 2010). The present study indicated the total amounts of several mTORC1-interacting proteins (e.g., Rheb, LAMTOR1 [p18], LAMTOR2 [p14] and Rag A-D) within the whole tissue homogenate did not differ between control and septic rats and were not changed acutely by leucine. Other proteins (e.g., HBXIP and C7orf59) are present in the Ragulator (Bar-Peled et al., 2012, Sancak et al., 2010), but were not investigated. Furthermore, the amount of PRAS40 and RagC (e.g., negative and positive mTORC1 regulators, respectively) bound to the raptor immunoprecipitate did not differ between control and septic muscle in the basal condition. In contrast, it is noteworthy that the leucine-induced decrease in PRAS40•raptor binding and the increase in RagC•raptor binding seen in control muscle was not observed in septic rats. These results support previous studies in cultured cells and perfused liver which reported similar changes in the association of PRAS40 and RagC with mTORC1 in response to insulin and nutrients (Dennis et al., 2011, Sancak et al., 2007) and extend them to intact skeletal muscle. Also, the inability of leucine to decrease PRAS40•raptor and increase RagC•raptor in septic rats is consistent with the observed leucine resistance in these animals. The leucine-induced decrease in PRAS40 binding to raptor in control rats is consistent with the concomitant increase in PRAS40 phosphorylation. While these data stress the potential importance of changes within mTORC1 related to the binding of PRAS40 and RagC to raptor as a mediator for the leucine resistance, other potential regulators of the Rag GTPase, such as MAP4K3, v-ATPase, leucyl-tRNA synthetase, and SH3BP4 (Zoncu et al., 2011, Findlay et al., 2007, Han et al., 2012), remain to be elucidated.
In summary, our results suggest the sepsis-induced leucine resistance observed in vivo in skeletal muscle occurs in the presence of elevated intracellular leucine, which cannot be explained by a change in the abundance of the glutamine/leucine antiporter LAT2. While the content of the various proteins within mTORC1 and the Ragulator were unchanged in sepsis, the normal leucine-induced decrease in raptor•PRAS40 and increase in raptor•RagC was absent in muscle from septic rats suggesting a putative mechanism for the development of anabolic resistance in this catabolic condition.
Acknowledgments
This work was supported by National Institutes of Health grant GM38032.
Footnotes
Conflict of interest
The authors state that they have no conflict of interest to declare.
References
- Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg. 1980;192:78–85. doi: 10.1097/00000658-198007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn GL, Bistrian BR. Nutritional care of the injured and/or septic patient. Surg Clin North Am. 1976;56:1195–1224. doi: 10.1016/s0039-6109(16)41038-8. [DOI] [PubMed] [Google Scholar]
- Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37:S354–367. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287–8296. doi: 10.1074/jbc.M110.209171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab. 2012;302:E1329–1342. doi: 10.1152/ajpendo.00525.2011. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol. 2007;18:1426–1436. doi: 10.1681/ASN.2006091014. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Yaman I, Merrick WC, Koromilas A, Wek RC, Sood R, Hensold J, Hatzoglou M. Regulation of internal ribosome entry site-mediated translation by eukaryotic initiation factor-2alpha phosphorylation and translation of a small upstream open reading frame. J Biol Chem. 2002;277:2050–2058. doi: 10.1074/jbc.M109199200. [DOI] [PubMed] [Google Scholar]
- Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 2011;26:83–96. doi: 10.1152/physiol.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Ogmundsdottir MH, Kazi S, Reynolds B, Visvalingam SM, Wilson C, Boyd CA. Amino acid sensing and mTOR regulation: inside or out? Biochem Soc Trans. 2009;37:248–252. doi: 10.1042/BST0370248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hatzoglou M, Fernandez J, Yaman I, Closs E. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr. 2004;24:377–399. doi: 10.1146/annurev.nutr.23.011702.073120. [DOI] [PubMed] [Google Scholar]
- Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem. 2007;282:19788–19798. doi: 10.1074/jbc.M611520200. [DOI] [PubMed] [Google Scholar]
- Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi AA, Pruznak AM, Frost RA, Lang CH. Sepsis-induced alterations in protein-protein interactions within mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. Shock. 2011;35:117–125. doi: 10.1097/SHK.0b013e3181ecb57c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Lang CH, Fan J, Cooney R, Vary TC. IL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesis. Am J Physiol. 1996;270:E430–437. doi: 10.1152/ajpendo.1996.270.3.E430. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab. 2004;287:E721–730. doi: 10.1152/ajpendo.00132.2004. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–299. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism. 2007;56:49–57. doi: 10.1016/j.metabol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Bronson SK, Lynch CJ, Vary TC. Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. Am J Physiol Endocrinol Metab. 2010;298:E1283–1294. doi: 10.1152/ajpendo.00676.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Pruznak AM, Frost RA. TNFalpha mediates sepsis-induced impairment of basal and leucine-stimulated signaling via S6K1 and eIF4E in cardiac muscle. J Cell Biochem. 2005;94:419–431. doi: 10.1002/jcb.20311. [DOI] [PubMed] [Google Scholar]
- Liu J, Hatzoglou M. Control of expression of the gene for the arginine transporter Cat-1 in rat liver cells by glucocorticoids and insulin. Amino Acids. 1998;15:321–337. doi: 10.1007/BF01320897. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Ogmundsdottir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One. 2012;7:e36616. doi: 10.1371/journal.pone.0036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth E, Funovics J, Muhlbacher F, Schemper M, Mauritz W, Sporn P, Fritsch A. Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr. 1982;1:25–41. doi: 10.1016/0261-5614(82)90004-8. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Nguyen HV, Almonaci RD, Davis TA. Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids. 2013;45:523–530. doi: 10.1007/s00726-012-1326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary TC, Lang CH. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol. 2008;447:343–355. doi: 10.1007/978-1-59745-242-7_22. [DOI] [PubMed] [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Jahoor F, Hartl WH. Protein and amino acid metabolism after injury. Diabetes Metab Rev. 1989;5:149–164. doi: 10.1002/dmr.5610050205. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]