Abstract
Half mustard (CEES) and nitrogen mustard (NM) are commonly used surrogates and vesicant analogs of the chemical warfare agent sulfur mustard. In the current study, in situ forming poly(ethylene glycol) (PEG)-based doxycycline hydrogels are developed and evaluated for their wound healing efficacy in CEES and NM exposed rabbit corneas in organ culture. The hydrogels, characterized by UV-Vis spectrophotometry, rheometry, and swelling kinetics, showed that the hydrogels are optically transparent, have good mechanical strength and a relatively low degree of swelling (<7%). In vitro doxycycline release from the hydrogel disks (0.25% w/v) was found to be biphasic with release half times of ~12 and 72 h, respectively, with 80–100% released over a 7-day period. Permeation of doxycycline through vesicant wounded corneas was found to be 2.5 to 3.4 fold higher than non-wounded corneas. Histology and immunofluorescence studies showed a significant reduction of matrix metalloproteinase-9 (MMP-9) and improved healing of vesicant exposed corneas by doxycycline hydrogels compared to a similar dose of doxycycline delivered in phosphate buffered saline (PBS, pH 7.4). In conclusion, the current studies demonstrate that the doxycycline-PEG hydrogels accelerate corneal wound healing after vesicant injury offering a therapeutic option for ocular mustard injuries.
1. Introduction
Sulfur mustard (SM, 2,2-dichlorethyl sulfide) is a potent cytotoxic and mutagenic vesicant that was used as a chemical warfare agent for the first time during World War I and in over 10 subsequent conflicts [1, 2]. In the 1980’s, SM was used in the Iran-Iraq war, affecting not only military personnel but also 100,000 civilians [3, 4]. Exposure to SM causes devastating injuries to the eyes, skin and respiratory system [5, 6], however the eyes are the most sensitive tissue to SM with a threshold of 12 mg.min/m3, compared to 200 mg.min/m3 for the skin. Even low doses of SM induce incapacitation, visual impairment and panic [5]. Although the molecular mechanisms for SM-induced injury are unclear, it is known that the vesicant alkylates DNA, RNA and proteins and causes inflammation, tissue damage and cell death [5]. MMPs are a family of enzymes that enhance the action of many activating factors during inflammatory response and contribute to tissue degradation [7–15]. MMP-9 has been identified as a potential target of therapy for SM damage since it was found that its expression and activation quantitatively increases over time in response to SM exposure [12, 13]. The cornea is clinically impaired by SM exposure exhibiting chronic inflammation and increased MMP activity [16]. Decreased MMP-9 activity in humans has been found to correlate with accelerated wound healing [17, 18]. Hence, intervention targeting of both the inflammatory response and increased protease expression could provide a therapeutic approach for the treatment of SM-induced corneal wounds.
Doxycycline is a long acting semi-synthetic tetracycline, which is well recognized for its therapeutic efficacy in treating MMP mediated ocular surface diseases, such as rosacea, recurrent epithelial erosions and sterile corneal ulcerations.[19–21]. Doxycycline has been found to inhibit MMP-9 activity in vivo in the corneal epithelial cells of experimental dry eye [22] as well as in vitro in human corneal epithelial cells [23, 24]. Treatment with doxycycline has been shown to be beneficial in attenuating acute and delayed ocular injuries caused by SM exposure [25, 26]. The drug is an inexpensive, FDA approved antibiotic that likely promotes wound healing by reducing inflammation and protease activity.
The blood ocular barriers, which include the blood-aqueous and blood-retina barriers protect the eye, but prevent drug distribution to the anterior and posterior chambers, limiting ocular bioavailability [27, 28]. Drug diffusion into the eyes from the systemic circulation is slow and inefficient. Most drugs applied to the eye surface as solutions have ocular bioavailability in the range of about 10% with most of the drug being cleared by local systemic absorption [28, 29]. Solutions are in contact with the eye surface for a very short period of time as the tear film quickly washes them away. The contact time, local drug concentration and thereby duration of action can be prolonged by designing topical formulations with higher viscosities [30].
The ideal drug delivery system for corneal wound repair should be nontoxic, transparent, easy to administer, possess rheological properties to maintain its structural integrity, provide a microbial barrier, release the drug in a controlled and sustained manner and decrease the time of wound healing. There are very few controlled drug delivery systems reported for corneal wound repair applications [31–35]. Although doxycycline is commercially available in a wide variety of dosage formulations including tablets, capsules and suspensions, topical ocular doxycycline eye drop formulations are to this day compounded by a pharmacist. Since there are currently no ocular formulations commercially available for doxycycline, there is a critical need for a controlled release doxycycline delivery system that can be easily applied to the eye to promote wound healing. Earlier, we have shown that pilocarpine-loaded ocular hydrogels provide sustained drug release and increased pharmacological efficacy in comparison to pilocarpine drops [36]. As ocular drug delivery systems, hydrogels provide prolonged corneal contact time, reduced drug loss from the corneal surface, and convenient administration. PEG based hydrogels are used in the current study since PEG is FDA approved, non-toxic, water soluble, easily processable and highly stable to temperature and pH [37–39]. The hydrogels evaluated in the current study are formed in situ, in other words, they are liquids upon instillation and undergo a phase transition at physiological pH to form the hydrogel. This occurs by covalent intermolecular crosslinking of polymer chains through reversible thioester bonds resulting in biodegradable viscoelastic hydrogels. In this study, doxycycline loaded fast forming PEG hydrogels were designed for the treatment of simulated mustard injuries using surrogate vesicants and evaluated in a rabbit corneal organ culture model.
2. Materials and methods
2.1. Materials
The polymers 8-arm-PEG-SH (20 kDa, 83.8% total activity by NMR) and 8-arm-PEG-N-hydroxysuccinimide (PEG-NHS) (20 kDa, 95.1% total activity by NMR) were custom synthesized by NOF Corporation (White Plains, NY). Doxycycline hyclate, agar, ascorbic acid, RPMI 1640 vitamin solution, ciprofloxacin, haematoxylin dye, CEES and NM were obtained from Sigma-Aldrich (St. Louis, MO). CoorsTek spot plates, eosin, Pen-Fix, methanol, acetonitrile, oxalic acid and HPLC grade solvents were obtained from Fisher Scientific (Suwanee, GA). The optical transmission (OT) of the hydrogel was determined using a Milton Roy Spectronic Genesys 5 spectrophotometer (Thermo Fisher Scientific, Pittsburgh, PA). Rheological data were obtained on a SR-2000 rheometer from Rheometric Scientific Inc (Piscataway, NJ) and analyzed by RSI orchestrator software. A Franz diffusion cell apparatus (Permegear, Hellertown, PA) was used for drug release and permeation studies. Polycarbonate membranes used for in vitro release studies were purchased from GE Osmonics Labstore (Minnetonka, MN). Waters HPLC system equipped with a UV detector and an Eclipse XDB-C8 column (Agilent, Zorbax, 4.6 × 150 mm) was used to analyze doxycycline concentration. Rabbit eyes were obtained from Pel-Freeze Biologicals (Rogers, AR). Dulbecco’s modified eagle’s medium (DMEM), High glucose DMEM, MEM-non essential amino acid solution (MEM-NEAA), Alexa-Fluor 488-conjugated goat anti-mouse IgG antibodies, 4′,6-diamidino-2-phenylindole (DAPI) and Prolong Gold were obtained from Invitrogen Corporation (Carlsbad, CA). The cryomolds and Tissue-Tek OCT was obtained from Sakura Finetek (Torrance, CA). Normal goat serum was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Mouse anti MMP-9 monoclonal antibody (catalog no MAB3309) were obtained from Millipore (Billerica, MA). A Microm HM505E cryostat was used for sectioning of cornea. The histology sections were observed using a light microscope (Leica DM-LB, magnification 40x; Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Images were captured and acquired using a ProgRes camera and ProgRes Capture Pro Software 2.6, respectively (Jenoptik Laser, Optik, Systeme GmbH, Jena, Germany).
2.2. Hydrogel formation
Hydrogels (5–22.5, w/v) were prepared by mixing the solutions of 8-arm-PEG-SH and 8-arm-PEG-NHS in PBS (pH 8) and leaving the mixture to stand at room temperature (RT) to form the hydrogel. Hydrogel was considered to be formed when the solution ceased to flow from the inverted tube [40]. For example, to prepare a 5% (1:1) hydrogel, aqueous solutions of PEG-SH and PEG-NHS were separately prepared by dissolving 5.0 mg of each polymer in 100 μL of buffer. The two solutions (total volume = 200μL) were mixed in a glass vial and allowed to stand at RT. Hydrogels were formed in 90 seconds. Similar procedure was used to obtain 7.5, 10, 15, and 22.5% hydrogel with 1:1 and 1:2 stoichiometries. Hydrogels used for optical transmission, degree of swelling, drug loading and in vitro release studies were 200 μL in volume, 9 mm in diameter, and 0.3 mm in thickness.
2.3. Optical Transmission
The hydrogels were screened for their potential application as drug delivery systems for corneal wound repair. Different hydrogel compositions [5% (1:1); 7.5% (1:1); 7.5% (1:2); 10% (1:1); 15% (1:1); 15% (1:2); and 22.5% (1:2)] were analyzed for their OT properties. The hydrogels (200μL) were placed in a quartz cuvette containing distilled water and transmission of light was measured at 480 nm [41]. A cuvette containing only distilled water was used as reference. All OT studies were done in triplicate and the mean ± SEM reported. One way analysis of variance (ANOVA) was used to determine the effect of hydrogel composition on its optical transmission.
2.4. Rheology
The rheological measurements of 5% (1:1), 7.5% (1:1), 7.5% (1:2), 15% (1:1) and 15% (1:2) hydrogels were performed using a rheometer with cone plate geometry at 37 °C (plate diameter: 25 mm, gap: 3 mm, 2° angle) [42, 43]. The hydrogel samples were equilibrated on the plate for 5 min to reach the running temperature before each measurement. Rheological test parameters, storage/elasticity (G′) and loss/viscosity (G″) moduli were obtained under dynamic conditions of non-destructive oscillatory tests. The dynamic strain sweep test was performed at a constant frequency of 1 Hz with percent strain ranging from 10−1 to 102. The dynamic frequency sweep test was carried out at a constant strain of 1% (linear viscoelastic region) with frequency ranging from 10−1 to 101 Hz. All the rheological studies were done in triplicate and the mean ± SEM reported. Two way ANOVA was used to determine the effect of hydrogel composition on its rheological properties.
2.5. Swelling studies
The degree of swelling for different hydrogels [5% (1:1); 7.5% (1:1); 7.5% (1:2); 10% (1:1); 15% (1:1); 15% (1:2); and 22.5% (1:2)] was measured. Hydrogels were placed in a vial and weighed (initial weight) prior to being immersed in PBS (pH 7.4) and placed in an incubator at 37 °C. The degree of swelling of the hydrogels was calculated by weighing the vials after removing the PBS at predetermined time intervals. The buffer was replaced after every measurement and the hydrogels were allowed to swell until equilibrium is reached. The degree of swelling for each hydrogel was determined by using the equation below:
| (1) |
Where Ws is the weight of the swollen hydrogel at time t and W0 is the initial weight. All measurements were made in triplicate for each hydrogel using separate samples and the mean ± SEM reported. Two way ANOVA was used to determine the effect of hydrogel composition on its degree of swelling.
2.6. In vitro doxycycline loading and release
2.6.1. Drug loading efficiency
The 10% (1:1), 15% (1:1), 15% (1:2), and 22.5% (1:2) hydrogels loaded with 0.25% w/v of doxycycline were used for drug loading and release studies. The hydrogels were dissected into small pieces and suspended in 5 mL PBS (pH 7.4). The suspension was sonicated for 30 min to completely extract doxycycline from the hydrogel. The amount of doxycycline extracted was quantified by RP HPLC analysis at a wavelength of 350 nm. 0.01M oxalic acid, acetonitrile and methanol (70:18:12) were used as mobile phase at a flow rate of 1 mL/min. After extraction, suspension containing the hydrogel was stored for several days at 4°C and then reanalyzed to ensure the complete extraction of doxycycline from the hydrogel. Doxycycline was stable under the storage conditions, as determined by HPLC analysis.
2.6.2. In vitro release
In vitro release from the hydrogels were studied on a Franz diffusion cell apparatus with a diameter of 5 mm and a diffusional area of 0.636 cm2. A polycarbonate membrane (0.4 μ) was sandwiched between the lower cell reservoir and the glass cell-top containing the sample for doxycycline release studies. The receiving compartment (volume 5.1mL) was filled with PBS (pH 7.4). The system was maintained at 37°C using a circulating water bath and a jacket surrounding the cell. The receiving medium was continuously stirred (600 rpm) with a magnetic bar to avoid stagnant aqueous diffusion layer effects. 200 μl sample of each hydrogel formulation containing 0.25% w/v doxycycline was prepared and placed in the donor compartment, which was then sealed with parafilm and aluminum foil to prevent evaporation. Aliquots (200 μL) were collected from the receiver compartment at predetermined intervals and replaced with equal volume of PBS to maintain sink conditions throughout the study. The concentration of doxycycline in the release medium was determined using RP HPLC. The cumulative amount of doxycycline released from the hydrogel was determined using a calibration curve. All release experiments were done in quadruplicate and the results were reported as mean ± SEM. The release data were fitted using a two-phase exponential association equation in GraphPad Prism 4 software. Two way ANOVA was used to determine the effect of hydrogel composition on the in vitro doxycycline release.
2.7. Ex vivo evaluation using Rabbit Cornea Organ Culture Model
A rabbit cornea organ culture model system adapted from Foreman, et al [44] was used to evaluate healing after exposure to model vesicants CEES and NM, followed by subsequent treatment with doxycycline drops or doxycycline hydrogels. Rabbit eyes were stored in DMEM (with penicillin, streptomycin, amphotericin B and gentamicin) and transported to the laboratory on ice. Corneas with a surrounding 2 mm scleral rim were dissected from the eye and placed with the epithelial side facing down into spot plates containing a small amount of DMEM to prevent drying of the epithelium. The corneal endothelial concavity was then filled with DMEM containing 0.75% agar at 50°C and this mixture was allowed to set (usually within 1 min). Corneas were then inverted and transferred to 60 mm sterile tissue culture dishes and cultured at 37°C in a humidified 5% CO2 incubator in the presence of medium (500 mL high glucose DMEM, 5 mg ciprofloxacin, 5 mL of 100x MEM-NEAA, 5 mL RPMI 1640 vitamin solution and 50 mg ascorbic acid). To moisten the epithelium, 500 μL of medium was added drop wise to the surface of the corneal epithelium every 8 h. The level of medium in dishes was allowed to rise only to the corneal-scleral rim. All agents were added drop wise to the central cornea. Either 200 nmoles CEES (dissolved first in absolute ethanol and then DMEM) or 100 nmoles NM (dissolved first in saline and then DMEM) were applied onto the cornea and allowed to remain there unwashed for 2 h. The 2 h time period approximately simulates the time that would pass before an exposure is recognized (based on the delayed times for tearing and pain) and medical help is secured.
2.7.1. Permeation of doxycycline through vesicant exposed rabbit’s cornea
Permeability studies were performed in a Franz diffusion cell apparatus to evaluate the effect of vesicants on permeability barrier properties of cornea. Corneas in the organ culture were treated with either 100 nmoles CEES or 200 nmoles CEES or 100 nmoles NM and put into designated incubator at 37°C for 2 h. Corneas untreated with either of the above vesicants were used as controls. After 2 h, the corneas were placed horizontally on the receptor compartment with the endothelial surface facing the receiver compartment of the Franz diffusion cell set up. The donor half cell was carefully placed on top of the receptor half cell and clamped. 200 μL sample of 15% (1:2) hydrogel encapsulating 0.25% w/v doxycycline was placed in the donor compartment. Aliquots (200 μL) were collected from the receiver compartment at predetermined intervals and replaced with equal volume of PBS to maintain sink conditions through out the study. The concentration of doxycycline in the release medium was determined using a RP HPLC as described above. The cumulative amount of doxycycline permeated through the corneas was determined using a calibration curve. All permeation experiments were done in triplicate and the results reported as mean ± SEM. Two way ANOVA was used to determine the statistical significance of permeation between different treatment groups.
2.7.2. Wound healing efficacy of doxycycline PEG hydrogels on corneas exposed to CEES and NM
The corneas in organ culture were exposed to either 200 nmoles CEES or 100 nmoles NM and incubated for 2 h at 37°C. Medium was replaced with fresh medium after 2 h and then each cornea was treated with doxycycline. Doxycycline solution (2M in 50 μl) was added drop wise to the central cornea 3 times over the subsequent 24 h, whereas 15% (1:2) doxycycline hydrogel (6M in 50 μl) was applied once. After 24 h, the corneas were put in cryomolds containing Tissue-Tek O.C.T. compound with the epithelial side facing down and placed on ice for 15 min before snap freezing them in liquid nitrogen. Corneas were stored at −80°C until sectioned for histology and immunofluorescence (IF) analysis. The 10 μm corneal sections were stained using a modified Hematoxylin & Eosin (H & E) staining method. The corneal sections were fixed in a Pen-Fix solution for 60 s, stained with H & E, dehydrated through graded alcohols, immersed in xylene and covered with a cover slip. Digital images were captured with a light microscope at 40x magnification.
2.7.3. Detection of MMP-9 in vesicant exposed and treated corneas by immunofluorescence
The sectioned corneas (10 μm) were fixed in 100% methanol for 10 min at −20°C. After rinsing with PBS nonspecific binding was blocked with 5% normal goat serum for 1 h. The blocking agent was removed and the sections were incubated with primary mouse anti human MMP-9 monoclonal antibodies (1:400) overnight at 4°C. Sections were blotted and washed four times with PBS/Tween and incubated for 1 h at RT in dark with Alexa-Fluor 488-conjugated goat anti-mouse IgG secondary antibodies (1:1000). The sections were washed with PBS/Tween, counterstained with DAPI for 5 min, mounted with Prolong gold and cover slipped. Negative controls replaced primary antibodies with PBS. Digital epifluorescent images were captured from a light microscope at 494 nm excitation and 517 nm emission and acquired at 10x magnification.
3. Results and Discussion
3.1. Mechanism of hydrogel formation
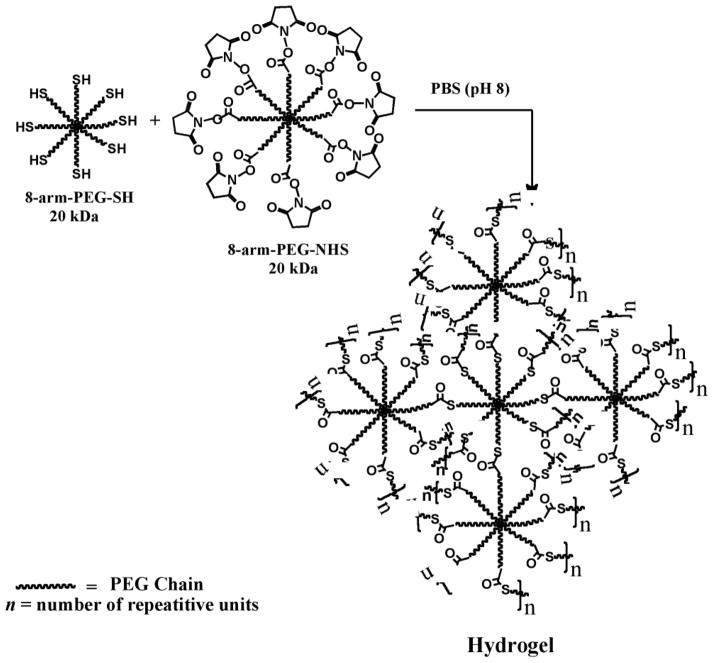
Hydrogels were formed by the intermolecular crosslinking of polymer chains, resulting from the reaction of eight-arm PEG polymers containing the thiol groups (8-arm-PEG-SH) with another eight-arm polymer containing the N-hydroxysuccinimidyl ester groups (8-arm-PEG-NHS) at RT in aqueous buffer (PBS, pH 8) (Scheme 1). The thiol groups are known to react with active esters under neutral to slightly alkaline pH to give thioester bonds (Scheme 2). The thioester bonds are hydrolytically labile and therefore impart in vivo biodegradability to the hydrogel network under physiological conditions [45].
Scheme 1.
Hydrogel formation by intermolecular crosslinking of PEG polymers containing mutually reactive thiol (8-arm-PEG-SH) and N-hydroxysuccinimidyl ester (8-arm-PEG-NHS) groups at room temperature in buffer (pH 8). The hydrogel networks are produced by the formation of thioester bonds.
Scheme 2.
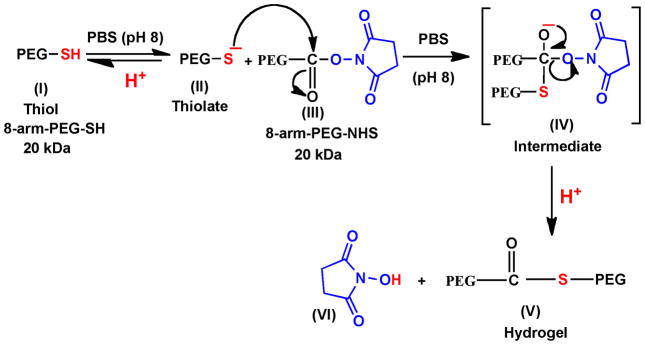
Mechanism of formation of thioester bonds by reaction of thiol group with N-hydroxysuccinimidyl ester. The 8-arm-PEG-SH (I) exists as a thiolate (II) in PBS (pH 8), which acts as a nucleophile and attacks the carbonyl carbon of 8-arm-PEG-NHS (III) to form the intermediate (IV) leading to the formation of thioester bonds accompanied by the cleavage of N-hydroxysuccinimide.
Hydrogels of different compositions (5–22.5% w/v) were formed by varying the concentration and ratios of the two polymers (Table 1). Hydrogels were not formed when polymer concentrations were less than 5%, possibly due to insufficient intermolecular crosslinking. The 5% (1:1), 7.5% (1:1), 7.5% (1:2), 10% (1:1), 15% (1:1), 15% (1:2), 22.5% (1:2) hydrogels were formed in 90, 85, 75, 65, 55, 45 and 30 s, respectively. Thus, an increase in polymer concentration results in faster hydrogel formation due to more efficient crosslinking reaction. The faster hydrogel formation with 1:2 stoichiometries is due to the fact that the NHS ester has a half life of ~1 h at room temperature at pH ~8 [46]. Consequently, when the NHS ester is present only in equimolar concentrations, there is a possibility of incomplete reaction. The presence of NHS ester in excess (1:2) excludes this possibility and also explains the reason behind faster hydrogel formation using 1:2 stoichiometries.
Table 1.
Composition of 0.25% w/v doxycycline PEG hydrogel formulations evaluated in the current study
| Polymer ratios | Gelation time (s) | Hydrogel Composition
|
||
|---|---|---|---|---|
| 8-arm-PEG-SH (% w/v) | 8-arm-PEG-NHS (% w/v) | Total weight of polymers (% w/v) | ||
| (1:1) | 90 | 5 | 5 | 5 |
| (1:1) | 85 | 7.5 | 7.5 | 7.5 |
| (1:2) | 75 | 5 | 10 | 7.5 |
| (1:1) | 65 | 10 | 10 | 10 |
| (1:1) | 55 | 15 | 15 | 15 |
| (1:2) | 45 | 10 | 20 | 15 |
| (1:2) | 30 | 15 | 30 | 22.5 |
3.2. Optical Transmission (OT)
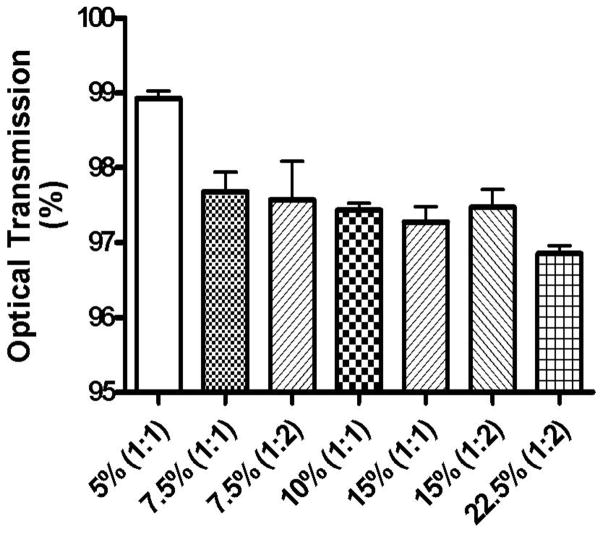
Formulations developed for the eye should ideally be transparent and therefore OT measurements were carried out at 480 nm using a UV-Vis spectrophotometer. Hydrogels with OT ≥90% were classified as transparent, those in the 10–90% range were classified as translucent, and those ≤10% as opaque [41]. The % OT of various hydrogels is shown in Fig. 1 and as can be seen from the figure, all hydrogels used in this study are transparent. It was also observed that a change in hydrogel composition produces a statistically significant effect (p<0.05) on their optical transmission properties. An increase in the concentration of 8-arm-PEG-SH and/or 8-arm-PEG-NHS resulted into a slight decrease in the transparency of the hydrogels. The transparent characteristic of these hydrogels could be beneficial for their use as ocular drug delivery systems.
Fig. 1.
Optical transmission of 5% (1:1), 7.5% (1:1), 7.5% (1:2), 10% (1:1), 15% (1:2), 15% (1:1), and 22.5% (1:2) hydrogels. All the hydrogels were found to be transparent. A change in hydrogel composition resulted in a statistically significant effect (p<0.05) on their optical transmission properties. A slight decrease in transparency of the hydrogels was observed with an increase in the polymer concentration and their crosslinking ratios.
3.3. Rheology
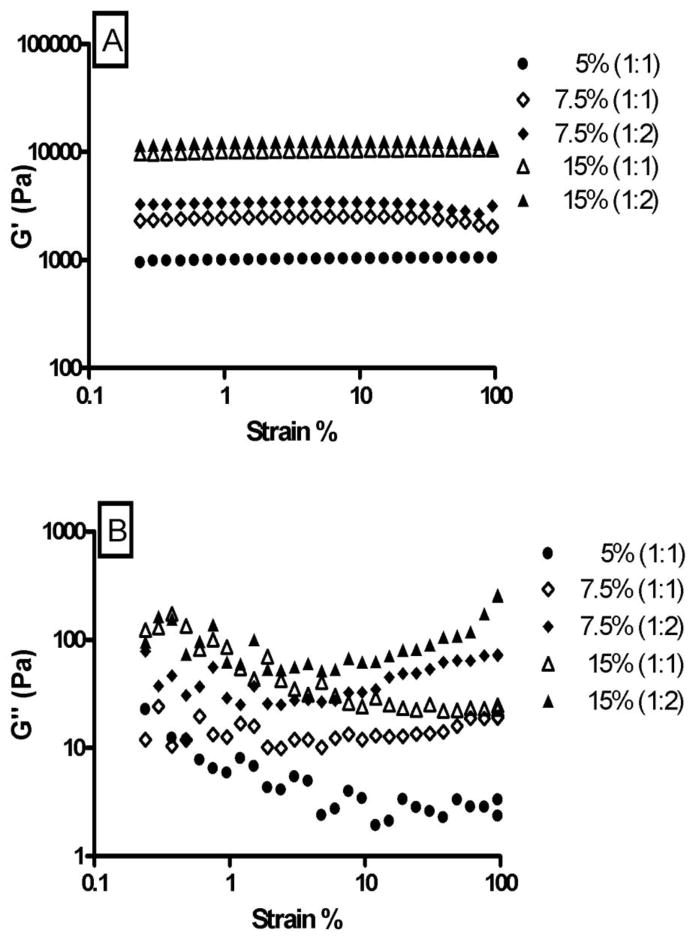
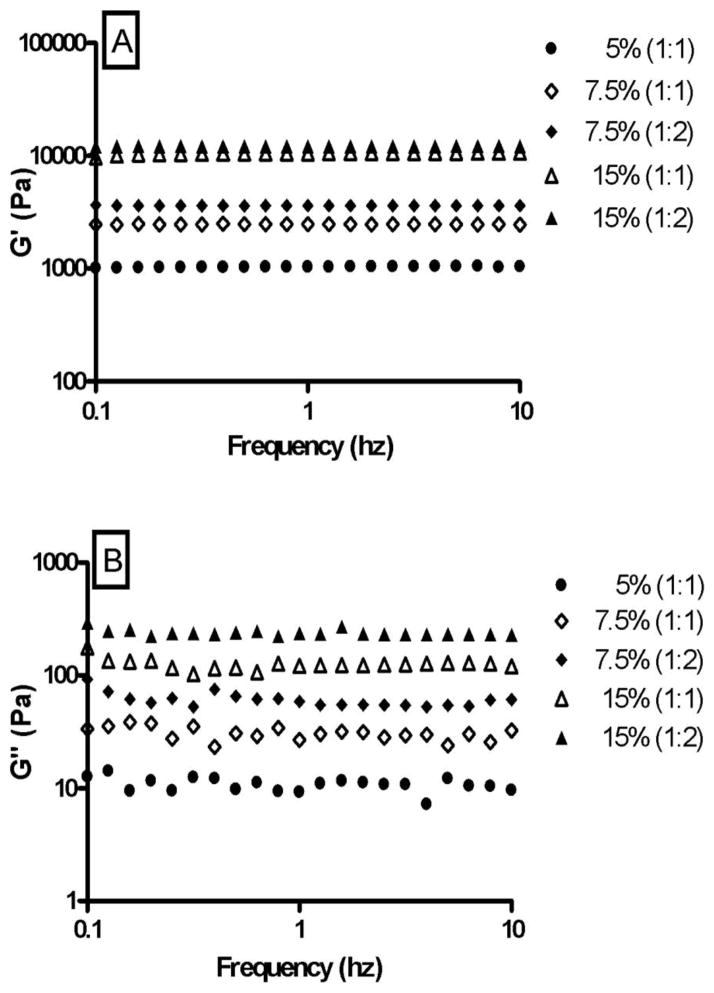
The retention behavior and physical integrity of hydrogels can be assessed in vivo by measuring their mechanical strength and viscoelastic properties [42, 43]. Hydrogels with good mechanical strength are expected to maintain their integrity and help prevent physical drug loss from blinking in vivo [47]. The viscoelastic properties of the hydrogels were evaluated by strain sweep test (Fig. 2) and frequency sweep test (Fig. 3). The strain sweep test allows the determination of linear viscoelasticity (LVE) range and the subsequent choice of strain value to be used in the frequency sweep test. The frequency sweep test provides a ‘fingerprint’ of the viscoelastic system under non destructive conditions [48, 49]. Both the strain sweep test and frequency sweep test are used to obtain the rheological parameters G′ (storage/elastic modulus), G″ (loss/viscous modulus) and loss tangent/phase angle (tan δ = G″/G′). G′ represents the elastic storage of energy and is a measure of how well-structured a hydrogel is. G″ represents the viscous energy dissipation and changes depending on the viscosity of the hydrogel. The strain sweep test results suggest that G′ dominates in both the formulations and this is supported by the results obtained from the frequency sweep test. Since G′ was one order higher than G″, the hydrogels are more elastic than viscous in the investigated frequency range. Fig’s 2 and 3 also show that G′ is independent of frequency and strain whereas G″ is weakly dependent on both. The hydrogels crosslinked in a 1:2 ratio have slightly higher G′ and G″ than hydrogels crosslinked in a 1:1 ratio. This can be attributed to the formation of denser and stronger crosslinking networks in 1:2 hydrogels.
Fig. 2.
Influence of strain on G′ (A) and G″ (B) of 5% (1:1), 7.5% (1:1), 7.5% (1:2), 15% (1:1), and 15% (1:2) hydrogels. The strain sweep test establishes the range of linear viscoelasticity (LVE) for the hydrogels.
Fig. 3.
Influence of frequency on G′ (A) and G″ (B) of 5% (1:1), 7.5% (1:1), 7.5% (1:2), 15% (1:1), and 15% (1:2) hydrogels. The frequency sweep test shows that the hydrogels are more elastic than viscous and that they have the ability to resist structural changes under strain. A change in hydrogel composition resulted in a statistically significant effect (p<0.001) on the viscoelasticity of the hydrogels.
A change in hydrogel composition resulted in a statistically significant effect (p<0.001) on the mechanical strength of the hydrogels. The hydrogels containing higher concentrations of polymers [15% (1:1) and (1:2)] showed a higher and constant G′ under increasing frequency, suggesting that the hydrogels have the ability to resist structural changes under strain. The small tan δ values indicate that G′ is the dominant feature in all the hydrogels and that variations in hydrogel composition do not result in extreme variations in rheological parameters. The rheological data show that the hydrogels have good viscoelastic properties, which might help prolong their ocular residence time and prevent structural breakage. An increased contact time in turn may lead to an increased duration of pharmacological response.
3.4. Swelling studies
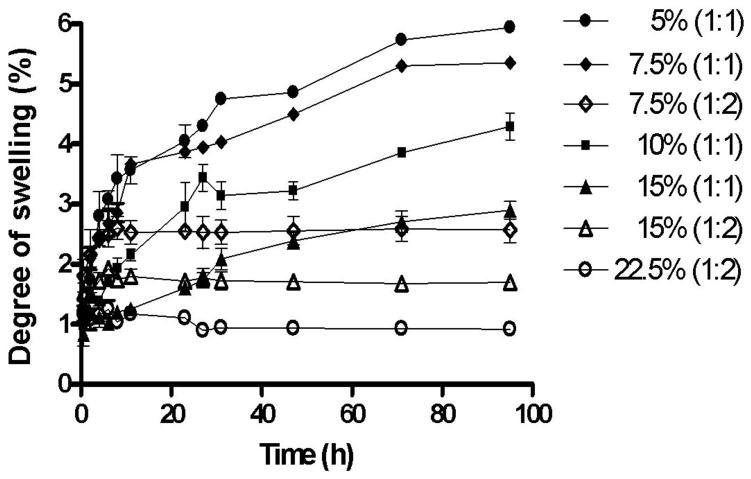
Hydrogels are swelling controlled systems and the degree of swelling is a measure of the crosslinking density of hydrogels, which is also important for regulating their pore size. The equilibrium degree of swelling of a hydrogel directly influences the rate of water sorption, the permeability to drugs and the mechanical strength of the hydrogel. Therefore, the effect of concentration of the polymers on the degree of swelling was determined. Fig. 4 shows the degree of swelling expressed as percent swelling plotted against time for 5% (1:1), 7.5% (1:1), 7.5% (1:2), 10% (1:1), 15% (1:1), 15% (1:2) and 22.5% (1:2) hydrogels. The hydrogels in this study showed a relatively lower degree of swelling (<7%) when compared to other hydrophilic hydrogels reported in the literature [50, 51]. The hydrogels crosslinked in a 1:1 ratio initially swelled rapidly, and then gradually reached equilibrium. Furthermore, the hydrogels crosslinked in a 1:2 ratio showed a much lower degree of swelling (<3%) than hydrogels crosslinked in a 1:1 ratio (<7%). A change in hydrogel composition resulted in a statistically significant effect (p<0.001) on the degree of swelling. Hence, a smaller pore size of the hydrogels obtained from increasing the polymer concentration or crosslinking ratio results in a lower degree of hydrogel swelling [52, 53].
Fig. 4.
Effect of polymer concentration and crosslinking density on the swelling kinetics of 5% (1:1), 7.5% (1:1), 7.5% (1:2), 10% (1:1), 15% (1:1), 15% (1:2), and 22.5% (1:2) hydrogels. The higher the concentration of polymers and crosslinking density, the lower is the degree of swelling.
3.5. In vitro doxycycline loading and release
Doxycycline loading efficiency results show that 22.5% (1:2), 15% (1:2), 15% (1:1) and 10% (1:1) hydrogels resulted in doxycycline loading efficiencies of 44.7, 47.5, 51.4 and 48.2%, respectively. Higher drug loading efficiency was observed when equivalent ratios of the polymers were used.
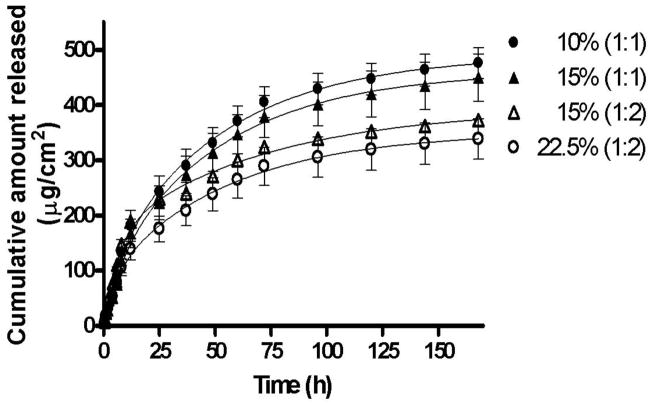
The doxycycline release profiles from different hydrogels were studied in vitro using a Franz diffusion cell apparatus. A plot of cumulative amount of doxycycline released (μg/cm2) as a function of time (h) (Fig. 5) demonstrates that doxycycline entrapped in the hydrogel shows sustained drug release for about 7 days (168 h) with 80 to 100% of doxycycline being released from different formulations. From Fig. 5, it appears that as the total concentration of the polymers increased in the hydrogels, the release of doxycycline was sustained. Also, as the crosslinking density increases from 1:1 to 1:2 in the hydrogels, a slower sustained doxycycline release was observed. A statistically significant (p<0.001) decrease in drug release was observed as the polymer concentration and crosslinking density increased in the hydrogels. The formation of a well-defined crosslinked network contributes to the decreased pore size and slower drug release from the hydrogels. The release data were fitted using two-phase exponential association equation in GraphPad Prism 4 software. The goodness of fit for the different hydrogels varied from 0.87 to 0.99.
Fig. 5.
Cumulative amount of doxycycline released as a function of time for hydrogels: 10% (1:1), 15% (1:2), 15% (1:1), and 22.5% (1:2). The release data were fitted using a two-phase exponential association equation in GraphPad Prism 4 software. The goodness of fit varied from 0.87 to 0.99. The release mechanism is non-Fickian or anomalous involving both diffusion and polymer relaxation (0.5<n<1).
The relative influence of diffusion and polymer relaxation on the mechanism of doxycycline release was determined by fitting the experimental data (first 60% of the total amount released) to the Ritger-Peppas equation [54].
| (2) |
In Equation 2, Mt/M∞ is the fractional release of the drug, k is the proportionality constant, n is the diffusional exponent and t is the time. The diffusion exponent was calculated from the slope of the natural logarithmic values (ln) of the fractional release as a function of time (Table 2). The release mechanism for all of the hydrogels was found to be Non-Fickian or Anomalous suggesting that the rates of diffusion and polymer relaxation are comparable (0.5<n<1). Doxycycline release was dependent on water migration into the hydrogel and drug diffusion through continuously swelling hydrogels.
Table 2.
Estimation of flux and diffusion exponent (n) for various hydrogel formulations
| % w/v hydrogels (PEG-SH:PEG-NHS) | Flux (J) (μg/cm2 sec−1) × 10−6 | Diffusion exponent (n) |
|---|---|---|
| 20 % (1:1) | 4.34 | 0.95 |
| 30 % (1:1) | 3.94 | 0.92 |
| 30 % (1:2) | 3.85 | 0.81 |
| 45 % (1:2) | 3.22 | 0.74 |
Table 2 shows the flux and diffusion exponent (n) values for various hydrogel formulations. Flux was calculated from the slope of the linear portion of the cumulative amount of doxycycline released (μg/cm2) as a function of time (h) plot. The order of flux and diffusional exponent for the hydrogels is 10% (1:1) > 15% (1:1) > 15% (1:2) > 22.5% (1:2). The flux values decreased with increasing polymer concentration and crosslinking density. This can be attributed to the smaller pore size of the hydrogels resulting in a lower degree of swelling and slower drug release. The in vitro release studies show that by changing the concentration of the polymers and crosslinking density, drug release from hydrogels can be tailored.
3.6. Ex vivo evaluation using Cornea Organ Culture Model
3.6.1. Permeability studies
Permeability studies were performed to evaluate the barrier function (transcorneal drug permeability) of vesicant-exposed corneas. The corneal epithelium is generally the rate limiting barrier to ocular penetration of topically applied drugs [55, 56]. The barrier is primarily due to the presence of annular tight junctions (zonula occludens), which completely surround and effectively seal the corneal epithelial cells, thus providing a diffusional barrier to drug absorption into the anterior chamber of the eye [57]. The permeability properties of vesicant-exposed corneas were expected to increase by disrupting the zonula occludens. Since the absorption-controlling biological membrane (i.e., cornea) would no longer be functional, the hydrogel delivery system would have to provide the rate controlling properties.
OT, rheology, degree of swelling and in vitro drug release properties of the hydrogel were considered for selection of the appropriate composition of hydrogel to be applied on the cornea. The 15% (1:2) hydrogel showed high OT (>97%), high mechanical strength (G′ >10000 Pa), low degree of swelling (<2%), slow sustained drug release (89% doxycycline released in 168 h) and therefore it was chosen for further studies including evaluation of corneal permeation and wound healing efficacy. Even though the 22.5% (1:2) hydrogel showed desirable physicochemical properties (OT>96%, <2% degree of swelling and 80% doxycycline released in 168 h), the 15% (1:2) hydrogel was preferred since it satisfied the requirements for an ideal ocular drug delivery system at lower polymer concentrations.
The permeation profiles of doxycycline through CEES and NM exposed corneas were evaluated for 24 h using a Franz diffusion cell apparatus. Fig. 6 shows a plot of the cumulative amount of doxycycline permeated (μg/cm2) as a function of time (h). Table 3 shows the lag times and permeability coefficients of doxycycline permeation through CEES and NM-exposed cornea. The lag time for doxycycline permeation was determined by extrapolating the linear portion of the permeation curve to x-axis. Flux (J) was obtained from the slope of the linear portion of the permeation curve and permeability coefficient (P) was calculated from flux using the equations below.
Fig. 6.
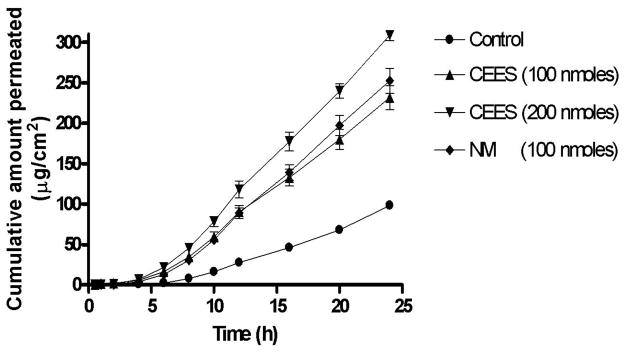
Cumulative amount of doxycycline permeated as a function of time through cornea exposed to different concentrations of CEES and NM. The permeability of doxycycline through CEES and NM-exposed corneas was significantly higher than the controls (p<0.0001) by 2.5 to 3.3 fold.
Table 3.
Estimation of lag time and permeation coefficient of doxycycline through vesicant-exposed corneas
| Cornea exposed to vesicants | Permeation coefficient (P) (cm h−1) × 10−4 | Lag time (h) |
|---|---|---|
| Unexposed (Control) | 15.11 ± 0.8217 | 3.339 |
| CEES (100 nmoles) | 36.76 ± 1.744 | 2.496 |
| CEES (200 nmoles) | 50.46 ± 1.838 | 2.665 |
| NM (100 nmoles) | 41.15 ± 1.849 | 2.925 |
| (3) |
| (4) |
Where J indicates the steady state flux, dQ the amount of drug permeated, A the corneal area exposed, dt the time of permeation and C0 represents the initial drug concentration in the donor compartment.
The permeability of doxycycline through CEES and NM exposed corneas was significantly higher than untreated corneas (p<0.0001) by 2.5 to 3.3 fold. The cumulative amount of doxycycline permeated through vesicant-exposed corneas is almost equal to the cumulative amount released in 24 h, which verifies that corneal epithelium no longer acts as a barrier for permeation of drugs after exposure.
The two major factors that determine ocular drug absorption to the anterior ocular tissues via transcorneal absorption are ocular contact time of the delivery system and drug permeability in the cornea [58]. The contact time of most conventional ocular solutions ranges between 5–25 minutes due to eye blinking and tear drainage that promote rapid clearance and reduced bioavailability, resulting in a short duration of pharmacological response in the eye [59–62]. In our previous report [36] we showed that hydrogel drug delivery system can improve pharmacological efficacy by increasing the ocular residence time. Since the corneal epithelial barrier is compromised when exposed to vesicants, the current hydrogel system can be expected to promote wound healing not only by prolonging ocular contact time but also by providing a continuous drug release at the injury in a sustained manner.
3.6.2. Wound healing efficacy of doxycycline loaded hydrogels on corneas exposed to CEES and NM
CEES (half mustard, 2-chloroethyl ethyl sulfide) and NM (nitrogen mustard, mechlorethamine hydrochloride) are structural analogs of SM and have been used widely as surrogates to simulate SM injury in the eyes, lung and skin without the need for a specialized containment facility [63–69]. Four corneas were evaluated for wound healing efficacy in each treatment group and representative H & E stained histological sections are shown in Fig. 7. The doxycycline solution was applied drop wise three times over the 24 h time period (every 8 h). The doxycycline 15% (1:2) hydrogel was applied as a solution that gelled in a few seconds after instillation onto the cornea. The hydrogel formed a thin transparent film, and likely because of its high water content, the hydrogel was retained in place for the entire duration of the study (24 h). This adherence is analogous to a contact lens where the attraction between the lens polymer and the tear film on the cornea holds the lens in place [70, 71].
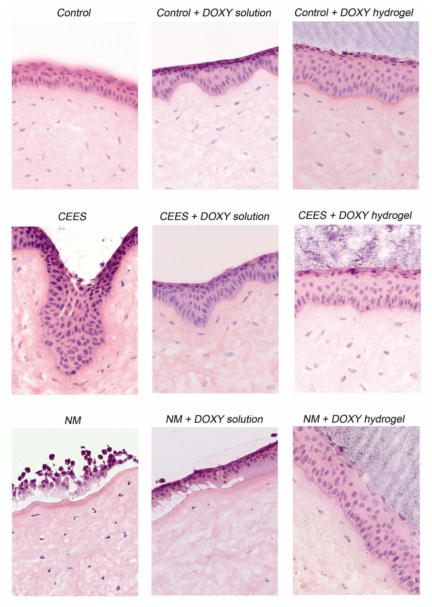
Fig. 7.
H & E staining to visualize the histology of CEES and NM-exposed corneas treated for 24 h with doxycycline in solution or in a hydrogel. The damaged area is where the epithelium meets the stroma. The wound healing efficacy of doxycycline solution was close to the doxycycline hydrogel for CEES exposed corneas, as the extent of damage was comparatively mild. However, a superior wound healing efficacy was observed with hydrogels over solutions when harshly damaged NM-exposed corneas were treated with doxycycline.
The histology of the control cornea showed an epithelium with normal thickness and an intact stroma with corneal keratocytes separated by extracellular matrix. The controls treated with hydrogel (not shown) and doxycycline hydrogel were very similar to the controls demonstrating that the hydrogel did not cause damage to the cornea. The CEES-exposed corneas exhibited a loss of distinctness of the epithelial-stromal border with frequent dipping into the stroma (also known as pitting). This visible damage, seen in foci throughout the cornea at the epithelial-stromal junction was expected, since this is the known target area of vesicants. In addition, the cells of the anterior stroma were swollen. CEES-exposed corneas treated with doxycycline in solution have an epithelial-stromal border that appears more normal when compared to those without treatment. The epithelial cell layer demonstrated less pitting, looking more like control tissues. CEES-exposed corneas treated with doxycycline hydrogel were similar to those treated with doxycycline solution, and thus were also much more normal in appearance than CEES-exposed corneas. The flattening of the epithelial-stromal border suggests that these corneas are perhaps more like controls than the CEES-exposed corneas treated with doxycycline solution. CEES causes mild damage, and therefore the difference in corneal wound healing efficacy between doxycycline solution and the doxycycline hydrogel would be expected to be minimal. The most significant differences that were seen indicated that the hydrogel ameliorated pitting. Fig. S1 of the Supplementary data shows the histology of the cornea 5 h after exposure to CEES and NM without any further treatment. The micrographs confirm the data from Fig. 7 showing that vesicant exposure damages the cornea and doxycycline treatment acts to maintain the basement membrane zone integrity.
Severe damage to the epithelium with NM causes epithelial cell sloughing, epithelial cell dissociation and pitting. The epithelium is detached from the stroma and the epithelial cells are separated, apparently having lost their cell to cell junctions. Where the epithelium is still attached, the basal cell nuclei appear to be more distant from the stroma than in controls. When treated with doxycycline in solution for 24 h after NM exposure, the epithelial-stromal border is somewhat improved. However there are still many areas where the epithelium is detached from the stroma and in areas where the epithelium and stroma are still attached, the basal cell nuclei were more distant from the stroma than in controls. For NM-exposed samples treated with doxycycline hydrogel, the epithelium remains attached to the basement membrane in most areas and shows a significant improvement in the appearance of the epithelial-stromal border. Doxycycline in solution probably did not show superior efficacy because drop wise application on a curved surface would favor a low retention time and only a small percentage of the doxycycline would be expected to remain in the wound area. The PEG doxycycline hydrogel on the other hand showed a great improvement over the NM exposed corneas. In a sense, it acted as a bandage, improving the doxycycline-cornea contact time and preventing epithelial sloughing. The higher wound healing efficacy of doxycycline loaded hydrogels compared to doxycycline in solution most likely is due to the increased contact time and sustained doxycycline release.
3.6.3. Detection of MMP-9 in vesicant exposed and treated corneas by immunofluorescence (IF)
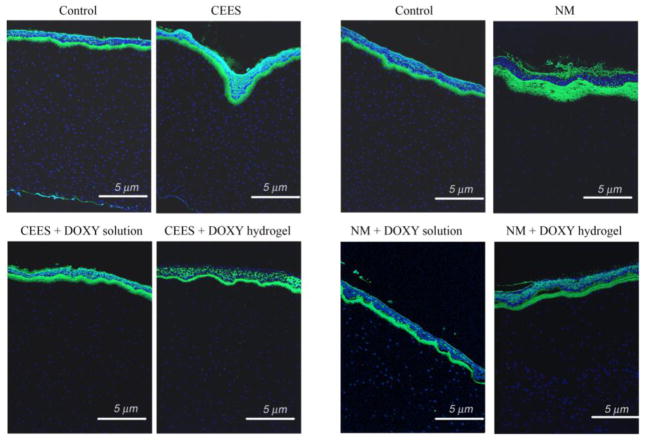
MMP-9 is a corneal epithelial product upregulated by wounding [16] and is specifically localized at the edge of the migrating epithelial sheet. MMP-9 spreads distally throughout the wound site, in a timely manner correlating with remodeling at the basement membrane zone. Very low amounts are seen in unwounded corneas [10, 72]. The IF staining of corneas exposed to CEES and NM, and subsequently treated with doxycycline either in solution or hydrogel for 24 h are shown in Fig. 8. In the controls, a very small amount of MMP-9 staining (green) is seen under the basal epithelial cells in the basement membrane zone. The staining at the apical epithelial cells is typical of the corneal epithelium’s auto-fluorescence. Others have verified that staining in the basal epithelial cells in the basement membrane is real MMP-9 IF [10, 72]. Nuclei were stained blue. Higher magnification pictures of Fig. 8 are shown in Fig. S2 in Supplementary data.
Fig. 8.
Immunofluorescent staining of corneas exposed to CEES and NM and subsequently treated with doxycycline either in solution or hydrogel. The intensity of MMP-9 staining, increased from exposure to CEES and NM, was significantly decreased by doxycycline both in solution and hydrogel. However, the doxycycline hydrogel also improved the attachment between the epithelium and stroma after NM exposure. This demonstrated that the doxycycline hydrogel formulation was more effective than doxycycline in solution.
For CEES exposed corneas, there is a moderate increase in MMP-9 staining observed in the basement membrane zone, apical cells and a small amount throughout the epithelium. For CEES-exposed corneas subsequently treated with doxycycline in solution, there is a slight, if any, decrease in staining. However, applying a doxycycline hydrogel after CEES exposure reduces immuno reactivity in the basement membrane zone and returning the sub epithelial expression to its original low MMP-9 levels.
For NM-exposed cornea, a drastic increase in MMP-9 staining was observed at the basement membrane zone, reflecting the greater wounding by of NM. The corneas exposed to NM, then treated with doxycycline in solution showed a less intense level of fluorescence. However, in this treatment group, there remained many areas where the epithelial cells were totally detached from the stroma. In this case there were few epithelial cells to secrete MMP-9 and thus there was a lower intensity of fluorescence in those areas. For NM-exposed samples treated with doxycycline hydrogel, the fluorescence was significantly less intense than NM exposure without treatment. Most areas show the epithelium to be in contact with the stroma. Hence intervention of MMP-9 activity with doxycycline hydrogels should be pursued as a potential treatment option for healing of mustard injuries in the eye.
4. Conclusions
Mustard injuries to the cornea have been shown to upregulate MMP-9. Thus agents that inhibit MMPs such as doxycycline are being evaluated as potential therapeutics. In the current study, the results suggest that doxycycline delivered by the hydrogel will be more effective for treatment of mustard injuries than doxycycline applied in solution. The in situ PEG hydrogels prepared and evaluated in the current study are biodegradable and optically transparent. They show resistance to external forces and provide sustained doxycycline release for up to 7 days. These hydrogel formulations can be administered as a solution which rapidly forms a hydrogel capable of withstanding shear forces in the eye. As expected, the permeability studies show that the barrier property of the cornea is compromised when exposed to vesicants, further allowing drug access to the cornea. The ex vivo histology and immunofluorescence results show that the hydrogel formulation provides a superior wound healing response compared to a similar dose of drug in solution. This is likely due to prolonged corneal contact time over the curve of the corneal surface. Overall, the results support the rationale behind using PEG-based hydrogels as ocular drug delivery systems.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health Counter ACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award #U54AR055073) and by NIH EY009056 to M.K.G.
Footnotes
The contents here in are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanc PD. The legacy of war gas. Am J Med. 1999;106(6):689–690. doi: 10.1016/s0002-9343(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 2.Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clin Exp Dermatol. 2006;31(1):1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- 3.Borak J, Sidell FR. Agents of chemical warfare: sulfur mustard. Ann Emerg Med. 1992;21(3):303–308. doi: 10.1016/s0196-0644(05)80892-3. [DOI] [PubMed] [Google Scholar]
- 4.Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: a review. Inhal Toxicol. 2007;19(5):451–456. doi: 10.1080/08958370601174990. [DOI] [PubMed] [Google Scholar]
- 5.Papirmeister B. In: Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Papirmeister B, Feister AJ, Robinson SI, Ford RD, editors. Boca Raton, Fl: CRC Press; 1991. [Google Scholar]
- 6.Smith WJ, Dunn MA. Medical defense against blistering chemical warfare agents. Arch Dermatol. 1991;127(8):1207–1213. [PubMed] [Google Scholar]
- 7.Gu Q, Wang D, Gao Y, Zhou J, Peng R, Cui Y, et al. Expression of MMP1 in surgical and radiation-impaired wound healing and its effects on the healing process. J Environ Pathol Toxicol Oncol. 2002;21(1):71–78. [PubMed] [Google Scholar]
- 8.Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114(Pt 1):131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holleran WM, Galardy RE, Gao WN, Levy D, Tang PC, Elias PM. Matrix metalloproteinase inhibitors reduce phorbol ester-induced cutaneous inflammation and hyperplasia. Arch Dermatol Res. 1997;289(3):138–144. doi: 10.1007/s004030050169. [DOI] [PubMed] [Google Scholar]
- 10.Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277(3):2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- 11.Paquet P, Nusgens BV, Pierard GE, Lapiere CM. Gelatinases in drug-induced toxic epidermal necrolysis. Eur J Clin Invest. 1998;28(7):528–532. doi: 10.1046/j.1365-2362.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 12.Sabourin CL, Danne MM, Buxton KL, Casillas RP, Schlager JJ. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. J Biochem Mol Toxicol. 2002;16(6):263–272. doi: 10.1002/jbt.10050. [DOI] [PubMed] [Google Scholar]
- 13.Shakarjian MP, Bhatt P, Gordon MK, Chang YC, Casbohm SL, Rudge TL, et al. Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure. J Appl Toxicol. 2006;26(3):239–246. doi: 10.1002/jat.1134. [DOI] [PubMed] [Google Scholar]
- 14.Wormser U, Brodsky B, Reich R. Topical treatment with povidone iodine reduces nitrogen mustard-induced skin collagenolytic activity. Arch Toxicol. 2002;76(2):119–121. doi: 10.1007/s00204-001-0307-5. [DOI] [PubMed] [Google Scholar]
- 15.Yancey KB. The pathophysiology of autoimmune blistering diseases. J Clin Invest. 2005;115(4):825–828. doi: 10.1172/JCI24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, et al. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263(1):59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Gerecke DR, Chen M, Isukapalli SS, Gordon MK, Chang YC, Tong W, et al. Differential gene expression profiling of mouse skin after sulfur mustard exposure: Extended time response and inhibitor effect. Toxicol Appl Pharmacol. 2009;234(2):156–165. doi: 10.1016/j.taap.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarlton JF, Vickery CJ, Leaper DJ, Bailey AJ. Postsurgical wound progression monitored by temporal changes in the expression of matrix metalloproteinase-9. Br J Dermatol. 1997;137(4):506–516. doi: 10.1111/j.1365-2133.1997.tb03779.x. [DOI] [PubMed] [Google Scholar]
- 19.Akpek EK, Merchant A, Pinar V, Foster CS. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104(11):1863–1867. [PubMed] [Google Scholar]
- 20.Dursun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132(1):8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 21.Seedor JA, Perry HD, McNamara TF, Golub LM, Buxton DF, Guthrie DS. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Arch Ophthalmol. 1987;105(2):268–271. doi: 10.1001/archopht.1987.01060020122043. [DOI] [PubMed] [Google Scholar]
- 22.De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 24.Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(12):4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 25.Amir A, Turetz J, Brandeis R, Dachir S, Cohen L, Cohen M, et al. Medical Defense Bioscience Review. Vol. 2004 Baltimore, MD: 2004. Evaluation of Protease Inhibitors in Sulfur Mustard Ocular Injuries. [Google Scholar]
- 26.Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, et al. Ocular injuries following sulfur mustard exposure-Pathological mechanism and potential therapy. Toxicology. 2008 doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Maurice DM, Mishima S. Ocular pharmacokinetics. In: Sears ML, editor. Handbook of experimental pharmacology. Berlin-Heidelberg: Springer Verlag; 1984. [Google Scholar]
- 28.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Urtti A, Pipkin JD, Rork GS, Sendo T, Finne U, Repta AJ. Controlled drug delivery devices for experimental ocular studies with timolol. 2. Ocular and systemic absorption in rabbits. Int J Pharm. 1990;61:241–249. [Google Scholar]
- 30.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60(2):207–225. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Grinstaff MW. Designing hydrogel adhesives for corneal wound repair. Biomaterials. 2007;28(35):5205–5214. doi: 10.1016/j.biomaterials.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hori K, Sotozono C, Hamuro J, Yamasaki K, Kimura Y, Ozeki M, et al. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release. 2007;118(2):169–176. doi: 10.1016/j.jconrel.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Palmer RM, McDonald MB. A corneal lens/shield system to promote postoperative corneal epithelial healing. J Cataract Refract Surg. 1995;21(2):125–126. doi: 10.1016/s0886-3350(13)80497-x. [DOI] [PubMed] [Google Scholar]
- 34.Pratoomsoot C, Tanioka H, Hori K, Kawasaki S, Kinoshita S, Tighe PJ, et al. A thermoreversible hydrogel as a biosynthetic bandage for corneal wound repair. Biomaterials. 2008;29(3):272–281. doi: 10.1016/j.biomaterials.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Sheardown H, Clark H, Wedge C, Apel R, Rootman D, Cheng YL. A semi-solid drug delivery system for epidermal growth factor in corneal epithelial wound healing. Curr Eye Res. 1997;16(3):183–190. doi: 10.1076/ceyr.16.3.183.15403. [DOI] [PubMed] [Google Scholar]
- 36.Anumolu SS, Singh Y, Gao D, Stein S, Sinko PJ. Design and evaluation of novel fast forming pilocarpine-loaded ocular hydrogels for sustained pharmacological response. J Control Release. 2009;137(2):152–159. doi: 10.1016/j.jconrel.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JH, Ju YM, Kim DM. Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of PEO-containing block copolymers. Biomaterials. 2000;21(7):683–691. doi: 10.1016/s0142-9612(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 38.Park KD, Kim YS, Han DK, Kim YH, Lee EH, Suh H, et al. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials. 1998;19(7–9):851–859. doi: 10.1016/s0142-9612(97)00245-7. [DOI] [PubMed] [Google Scholar]
- 39.Shalaby SW, Burg KJL. Polyethylene glycol-based copolyesters: Absorbable and biodegradable polymers. Boca Raton, FL: CRC Press; 2004. [Google Scholar]
- 40.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Murthy SK, Ravi N. Hydrogels as potential probes for investigating the mechanism of lenticular presbyopia. Curr Eye Res. 2001;22(5):384–393. doi: 10.1076/ceyr.22.5.384.5493. [DOI] [PubMed] [Google Scholar]
- 42.Rudraraju VS, Wyandt CM. Rheology of Microcrystalline Cellulose and Sodiumcarboxymethyl Cellulose hydrogels using a controlled stress rheometer: part II. Int J Pharm. 2005;292(1–2):63–73. doi: 10.1016/j.ijpharm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Xu Z. Physical characterization of a chitosan-based hydrogel delivery system. J Pharm Sci. 2002;91(7):1669–1677. doi: 10.1002/jps.10157. [DOI] [PubMed] [Google Scholar]
- 44.Foreman DM, Pancholi S, Jarvis-Evans J, McLeod D, Boulton ME. A simple organ culture model for assessing the effects of growth factors on corneal re-epithelialization. Exp Eye Res. 1996;62(5):555–564. doi: 10.1006/exer.1996.0065. [DOI] [PubMed] [Google Scholar]
- 45.Lendlein A, Langer R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science. 2002;296(5573):1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 46.Hermanson G. Bioconjugate techniques. 2. Burlington, MA: Elsevier Science & Technology Books; 2008. [Google Scholar]
- 47.Carlfors J, Edsman K, Petersson R, Jornving K. Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur J Pharm Sci. 1998;6(2):113–119. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 48.Lippacher A, Muller RH, Mader K. Investigation on the viscoelastic properties of lipid based colloidal drug carriers. Int J Pharm. 2000;196(2):227–230. doi: 10.1016/s0378-5173(99)00428-7. [DOI] [PubMed] [Google Scholar]
- 49.Lippacher A, Muller RH, Mader K. Liquid and semisolid SLN dispersions for topical application: rheological characterization. Eur J Pharm Biopharm. 2004;58(3):561–567. doi: 10.1016/j.ejpb.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 50.de Souza Costa E, Junior, Pereira MM, Mansur HS. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J Mater Sci Mater Med. 2009;20(2):553–561. doi: 10.1007/s10856-008-3627-7. [DOI] [PubMed] [Google Scholar]
- 51.Liu SQ, Ee PL, Ke CY, Hedrick JL, Yang YY. Biodegradable poly(ethylene glycol)-peptide hydrogels with well-defined structure and properties for cell delivery. Biomaterials. 2009;30(8):1453–1461. doi: 10.1016/j.biomaterials.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 52.Park H, Park K, Kim D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J Biomed Mater Res A. 2006;76(1):144–150. doi: 10.1002/jbm.a.30533. [DOI] [PubMed] [Google Scholar]
- 53.Tan H, Chu CR, Payne KA, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serra L, Domenech J, Peppas NA. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials. 2006;27(31):5440–5451. doi: 10.1016/j.biomaterials.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, et al. Enhancement of ocular drug penetration. Crit Rev Ther Drug Carrier Syst. 1999;16(1):85–146. [PubMed] [Google Scholar]
- 57.Klyce SD. Electrical profiles in the corneal epithelium. J Physiol. 1972;226(2):407–429. doi: 10.1113/jphysiol.1972.sp009991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58(11):1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Chrai SS, Robinson JR. Corneal permeation of topical pilocarpine nitrate in the rabbit. Am J Ophthalmol. 1974;77(5):735–739. doi: 10.1016/0002-9394(74)90541-8. [DOI] [PubMed] [Google Scholar]
- 60.Maurice DM. In: Ophthalmic drug delivery: biopharmaceutical, technological, and clinical aspects. Saettone MF, Bucci M, Speiser P, editors. Padova: Liviana press; 1987. [Google Scholar]
- 61.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122(2):119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102(1):23–38. doi: 10.1016/j.jconrel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J Pharmacol Exp Ther. 2009;328(3):732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jowsey PA, Williams FM, Blain PG. DNA damage, signalling and repair after exposure of cells to the sulphur mustard analogue 2-chloroethyl ethyl sulphide. Toxicology. 2009;257(3):105–112. doi: 10.1016/j.tox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Karacsonyi C, Shanmugam N, Kagan E. A clinically relevant in vitro model for evaluating the effects of aerosolized vesicants. Toxicol Lett. 2009;185(1):38–44. doi: 10.1016/j.toxlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Kenar L, Karayilanoglu T, Yuksel A, Gunhan O, Kose S, Kurt B. Evaluation of protective ointments used against dermal effects of nitrogen mustard, a vesicant warfare agent. Mil Med. 2005;170(1):1–6. doi: 10.7205/milmed.170.1.1. [DOI] [PubMed] [Google Scholar]
- 67.Morad Y, Banin E, Averbukh E, Berenshtein E, Obolensky A, Chevion M. Treatment of ocular tissues exposed to nitrogen mustard: beneficial effect of zinc desferrioxamine combined with steroids. Invest Ophthalmol Vis Sci. 2005;46(5):1640–1646. doi: 10.1167/iovs.04-1165. [DOI] [PubMed] [Google Scholar]
- 68.Tewari-Singh N, Rana S, Gu M, Pal A, Orlicky DJ, White CW, et al. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicol Sci. 2009;108(1):194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang GQ, Xia ZF. Tissue injury by hot fluid containing nitrogen mustard. Burns. 2007;33(7):923–926. doi: 10.1016/j.burns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Campbell R, Caroline P. Lens Adhesion and RGP Daily Wear. Contact Lens Spectrum. 1996 Jan;(1):1–2. [Google Scholar]
- 71.Eiden S, Cristina S. Adherence of Daily Wear RGP Contact Lenses. Contact Lens Spectrum. 1996 Feb;(1):1–6. [Google Scholar]
- 72.Gordon GM, Ledee DR, Feuer WJ, Fini ME. Cytokines and signaling pathways regulating matrix metalloproteinase-9 (MMP-9) expression in corneal epithelial cells. J Cell Physiol. 2009;221(2):402–411. doi: 10.1002/jcp.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.