Abstract
Background
Hydrogen, as a novel antioxidant, has been shown to selectively reduce the level of hydroxyl radicals and alleviate acute oxidative stress in many animal experiments. Hydrogen-rich saline provides a high concentration of hydrogen that can be easily and safely applied. Allogeneic hematopoietic stem-cell transplantation (HSCT) has been the most curative therapy for hematological malignancies. However, acute graft-versus-host disease (aGVHD) is the main cause of death in post-transplantation patients. In this study, we examined whether hydrogen-rich saline would show favorable effects on acute GVHD in mice.
Material/Methods
After lethal irradiation, BALB/c mice received bone marrow transplantation from C57BL/6 mice. Hydrogen-rich saline (5 ml/kg) was given to recipient mice in the hydrogen group once a day by intraperitoneal injection, and saline (5 ml/kg) was given to recipient mice in the saline group. Survival rates were monitored, clinical and pathological scores of aGVHD were determined after bone marrow transplantation (BMT), and the serum cytokine levels were examined on the 7th day after BMT.
Result
This study proves that hydrogen-rich saline increased the survival rate, reduced clinical and histopathological scores of aGVHD, promoted the recovery of white blood cells, reduced the serum cytokine levels, and reversed tissue damage after transplantation in mice.
Conclusions
Hydrogen has potential as an effective and safe therapeutic agent in aGVHD.
MeSH Keywords: Adult Stem Cells; Allografts; Transplantation, Homologous
Background
Hematopoietic stem-cell transplantation (HSCT) is probably the most effective treatment of hematologic malignancies and some hereditary abnormalities [1,2]. However, one of the most common and serious complications following allogeneic HSCT is graft-versus-host disease (GVHD). Acute GVHD (aGVHD), which accounts for a substantial portion of early transplant-related morbidity and mortality, is one of the main factors that affect the long-term survival and quality of life in patients following allogeneic HSCT [3,4]. There are 3 stages in the pathophysiology of aGVHD: tissue damage and cellular activation caused by preconditioning; the activation of donor lymphocytes (T cells); and the cellular and inflammatory factors directly attack various host tissues and cause the clinical manifestation of aGVHD [5,6]. However, aGVHD primarily affects the skin, liver, and gastro- intestinal (GI) tract [4].
Recent studies have identified molecular hydrogen (H2) as a novel antioxidant with therapeutic effects in a variety of disease models, including ischemia-reperfusion (I/R) injury, inflammatory disorders, and metabolic syndromes [7–9]. In 2007, Ohsawa et al. [8] discovered that H2 gas has antioxidant and antiapoptotic properties that protect the brain against ischemia-reperfusion injury and stroke by selectively neutralizing hydroxyl and peroxynitrite radicals. This discovery has completely overthrown the traditional view that hydrogen is a physiologically inert gas. Hydrogen is rarely used in the medical field. Since then, H2 has come to the forefront of therapeutic medical gas research. Accumulation of oxidative damage has been demonstrated to restrict the self-renewal capacity of human hematopoietic stem cells [10].
Material and Methods
2 Hydrogen-rich saline production
The hydrogen-rich saline was prepared as described previously [11]. Briefly, hydrogen was dissolved in 0.9% saline for 6 h under high pressure (0.4 MPa) to a supersaturated level by using a self-designed hydrogen-rich saline-producing apparatus provided by the Department of Diving Medicine, the Second Military Medical University, China. The saturated hydrogen-rich saline was stored under atmospheric pressure at 4°C in an aluminum bag with no dead volume, sterilized by gamma radiation, and freshly prepared once a week to ensure that the concentration was maintained at 0.6 mmol/L. Gas chromatography was used to confirm the content of hydrogen in saline by the method described by Ohsawa et al. [8].
Animal preparation
The recipient mice were 8–12-week-old BALB/c female mice, and the donor mice were 8–12-week-old C57BL/6 male mice. All mice were purchased from the Naval General Hospital (Beijing, China) in accordance with the Guide for Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (Publication No. 96-01). These mice were kept for 1 week to let them adapt to the environment. During this period, the mice were fed with acidified water and sterile food.
Graft-versus-host disease model
The GVHD model was performed as previously described [12]. One day after lethal total body irradiation (7.5 Gy with the rate of 170 cGy/min), BALB/c mice received BMT from C57BL/6 mice. BM inoculums were consisted of 2×107 BM cells and 0.5×107 spleen cells of C57BL/6 mice. After transplantation, the recipient mice were treated intraperitoneally with physiologic saline or hydrogen-rich saline (5 ml/kg) every day.
Survival assays
For the evaluation of the therapeutic effect of hydrogen, mice were returned to the animal facility and received routine care for 30 days after BMT. Survival was checked and scored daily for 30 days.
Assessment of the score of aGVHD
The recipient mice were examined daily from the start of conditioning until the indicated sampling day. Animals were evaluated for 5 clinical symptoms of aGVHD: weight loss, posture, activity, fur texture, and skin integrity as described elsewhere [12,13], and all those symptoms were scored on the criteria provided in Table 1.
Table 1.
Clinical scoring criteria of aGVHD in mice.
| Score index | 0 | 1 | 2 |
|---|---|---|---|
| Weight loss | <10% | 10–25% | >25% |
| Posture | Normal | Hunched post ar rest | Severly hunched posture affecting activies |
| Activity | Normal | Mild to moderate reduction in activity | No activity under stimulation |
| Fur change | Normal | Mild to moderate hair loss | Severe hair loss |
| Skin integrity | Normal | Local lesion at the grasping place | A large scale of lesion |
Histopathology
To confirm aGVHD, histopathological evaluation was performed as described previously [12]. Tissue samples were fixed in neutral buffered formalin for 24 h, transferred to 70% ethanol, dehydrated, and embedded in paraffin according to standard procedures. Sections of 4 um were prepared and stained by hematoxylin and eosin for histopathological evaluation. And the score of histopathology was assessed according to the criteria provided in Table 2.
Table 2.
Histopathological scoring criteria of the skin, liver and gut tissue in mice.
| Score index | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Skin | Normal | Vacuolar degeneration of epidermal basal cells | Spongiosis and eosinophilic degeneration of scatterd individual cells | Separation of the dermo-epidermal junction | Extensive destruction of epidermis |
| Liver | Normal | Epithelial damage and destruction of <25% of small bile ducts | 25–49% | 50–74% | >75% |
| Gut | Normal | Single cell apoptosis od crypt or gland epithelium | Destruction of single crypts or glands | Focal mucosal ulceration | Diffuse mucosal ulceration with denudation |
Analysis of cytokine secretion
Mice in the 2 groups were bled on day 7 after BMT and analyzed for the specified cytokines (IL-2, IL-6, IL-1β, and TNF-α). These cytokines were measured with a commercial enzyme-linked immunosorbent assay (ELISA) kit according to the instructions of the manufacturer.
Leukocyte counts
To evaluate the therapeutic effect of hydrogen, the peripheral blood cell count was determined regularly from the tail vein of mice on different days after BMT, as described previously [14].
Statistical analysis
Survival curves were conducted using the Kaplan-Meier product limit estimator and analyzed using the log-rank rest. Other results are expressed as mean ±SD and analyzed by the 2-sample Student’s T test for differences in means. P<0.05 was deemed to be significant in all experiments.
Results
The effects of hydrogen on survival rate of recipient mice after transplantation
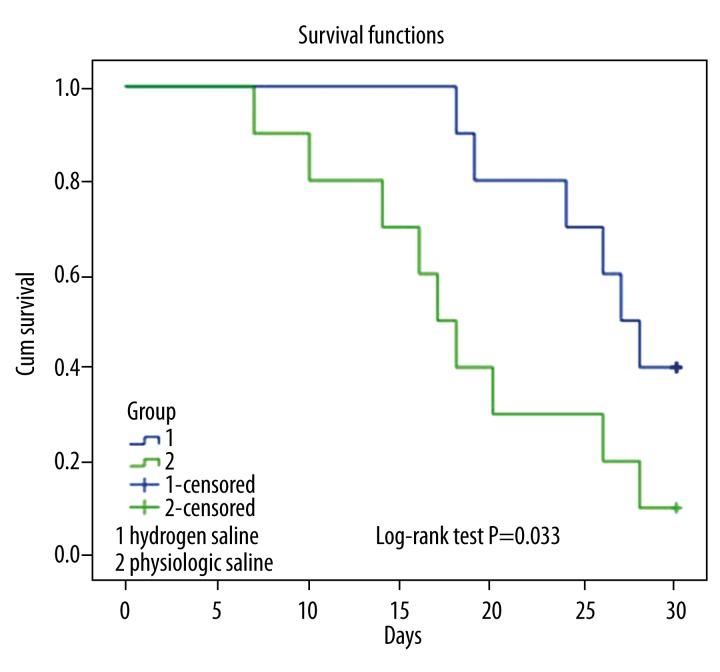
All the mice without transplantation after lethal total body irradiation died within 12 days. Forty percent of aGVHD mice with physiologic saline treatment survived to the 18th day after BMT, and 90% of the mice with hydrogen-rich saline had survived (Figure 1).This result suggests that hydrogen-rich saline can protect mice from lethal aGVHD.
Figure 1.
Survival curves by Kaplan-Meier analysis of mice in hydrogen-rich saline group and physiologic saline group. After transplantation, the two group mice were treated intraperitoneally with physiologic saline or hydrogen-rich saline every day and survival was checked daily for 30 days. Survival, P<0.05 for hydrogen-rich saline group (1) versus physiologic saline group (2).
The effects of hydrogen on serum cytokine levels in mice with aGVHD
We examined the serum cytokine levels (TNF-α, IL-1β, IL-2, IL-6) to determine whether hydrogen could alleviate the degree of aGVHD in mice. All the serum cytokine levels in the hydrogen-rich saline group were lower compared to the physiologic saline group (P<0.05) as shown in Table 3.
Table 3.
Serum IL-1β, IL-2, IL-6 and TNF-α concentrations in mice treated with hydrogen-rich saline or physiologic saline. Data are expressed as means ±SD. P<0.05 for hydrogen-rich saline group versus physiologic saline group.
| Group | IL-1β | IL-2 | IL-6 | TNF-α |
|---|---|---|---|---|
| Hydrogen-rich saline | 58.976±5.571 | 13.450±1.426 | 31.593±1.788 | 63.954±5.660 |
| Physiologic saline | 105.556±7.598 | 30.138±3.157 | 42.371±2.006 | 160.688±4.815 |
The effects of hydrogen on aGVHD score
We determined the aGVHD score on the 7th and 15th day after BMT. As shown in Table 4, hydrogen can significantly reduce the aGVHD score (P<0.05).
Table 4.
The clinical score of aGVHD in mice treated with hydrogen-rich saline or physiologic saline. Data are expressed as means ±SD. P<0.05 for hydrogen-rich saline group versus physiologic saline group.
| Group | 7th day | 15th day |
|---|---|---|
| Hydrogen-rich saline | 1.8±0.249 | 3.9±0.526 |
| Physiologic saline | 3.9±0.314 | 5.9±0.433 |
The effects of hydrogen on leukocyte counts
After lethal total body irradiation and transplantation, leukocyte counts declined rapidly, and then increased from the 7th day after BMT. We analyzed the leukocyte counts on day 0, 7, 14, 21, and 28 after BMT. The leukocyte counts on day 0 and day 7 were not different between the 2 groups, and the leukocyte counts on day 14, 21, and 28 in the hydrogen-rich saline group were higher than in the physiologic saline group (P<0.05) as shown in Figure 2.
Figure 2.
The change of leukocyte counts in mice treated with hydrogen-rich saline or physiologic saline after transplantation. After lethal total body irradiation and transplantation, leukocyte counts declined rapidly, and they would elevate from the 7th day after transplantation. The leukocyte counts on day 0 and day 7 were not different between two groups, and the leukocyte counts on day 14, 21 and 28 in hydrogen-rich saline group were higher than that in physiologic saline group (P<0.05).
The effects of hydrogen on histopathology
Histopathology scoring was conducted on the basis of the criteria provided previously (liver, skin, and gut). Hydrogen-rich saline significantly reversed the damage, and the histopathology grade was lower in the hydrogen-rich saline group compared with the physiologic saline group (p<0.05) as shown in Table 5 and Figure 3. The normal spleen structure was destroyed in receptors and the number of lymphocyte cells in splenic white pulp was reduced (Figure 3H) in the physiologic saline group, and the change in spleen was reversed in the hydrogen-rich saline group (Figure 3G).
Table 5.
The tissue injury evaluated by histopathological scoring system in mice treated with hydrogen-rich saline or physiologic saline. Data are expressed as means ±SD. P<0.05 for hydrogen-rich saline group versus physiologic saline group.
| Group | Liver | Skin | Gut |
|---|---|---|---|
| Hydrogen-rich saline | 1.0±0.333 | 1.1±0.233 | 1.1±0.233 |
| Physiologic saline | 2.3±0.335 | 2.6±0.221 | 1.7±0.153 |
Figure 3.

Histopathological changes of the liver, gut, skin and spleen tissue in mice treated with hydrogen-rich saline or physiologic saline. Tissue stained by the hematoxylin and eosin. (A) liver in hydrogen-rich saline group(100×), (B) liver in physiologic saline group (100×), (C) gut in hydrogen-rich saline group (100×), (D) gut in physiologic saline group (100×), (E) skin in hydrogen-rich saline group (200×), (F) skin in physiologic saline group (200×), (G) spleen in hydrogen-rich saline group (40×), (H) spleen in physiologic saline group (40×). Apparent histopathological differences between mice treated with hydrogen-rich saline and physiologic saline were observed. Hydrogen-rich saline could significantly attenuate the tissue injury induced by aGVHD.
Discussion
In many studies, examination of survival rate provides a criterion standard determination for experimental GVHD [15]. In our first study of hydrogen in mice with allogeneic hematopoietic stem-cell transplantation, as in the model of mixed bone marrow transplantation in mice [6], hydrogen-rich saline was shown to protect mice from lethal aGVHD. It could improve clinic grade of aGVHD, promote the recovery of white blood cells, and increase the survival rate of recipient mice.
GVHD is characterized by immune damage and is the result of an attack on the target organs of the host and the actions of related cytokines by activated donor T cells characterized by immune damage [16]. During all 3 phases of GVHD, recipient conditioning regimen damages patient tissues and causes release of inflammatory cytokines. The T helper cell type 1 (Th1) cytokines, such as TL-2 and TL-6, could increase the risk of worsening aGVHD [17,18], and those cytotoxic molecules directly assault various host tissues and underlie the clinical manifestations of aGVHD [5,6]. Those activated cells produce free radicals, which can result in severe cell damage and play an important role in the development of aGVHD. Hydrogen as a kind of selective antioxidant [19] and has great potential in the prevention and treatment of many illnesses, with effects of anti-oxidation [20–23], anti-inflammation [20,23–25], anti-apoptosis [23], and signaling pathways regulation [26–28].
Several reports have shown that tissue damage due to conditioning is important in triggering and development of GVHD [29,30]. It is not clear why some tissues (e.g., liver and skin) are more vulnerable to GVHD damage [31]. In the animal model with GVHD, mice may show clinical symptoms such as hunched posture, hair loss, and diarrhea [32]. With those clinical symptoms, the tissue damage could not be ignored. Our findings show that hydrogen-rich saline can significantly reduce the grading of tissue histopathology, including skin, liver, and gut. There were no criteria provided in the grading of spleen histopathology in the GVHD model, but some studies have reported that in the GVHD model normal spleen structure disappeared, and the number of lymphocytes in white pulp decreased [32–37]. Our study shows that hydrogen treatment can reduce spleen damage.
Conclusions
From the results obtained, we know that hydrogen can reduce the levels of cytokines and reverse tissues damage in the aGVHD model, suggesting that, due to its high diffusibility, hydrogen could be used clinically as a novel therapeutic drug.
Footnotes
Conflicts of interest statement
None declared.
Source of support: Innovation Promoted Foundation of Navy General Hospital (Grant no. CXPY201309)
References
- 1.Thomas ED. Bone marrow transplantation: a review. Semin Hematol. 1999;36(4 Suppl 7):95–103. [PubMed] [Google Scholar]
- 2.Schmitz N, Beksac M, Hasenclever D, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100(3):761–67. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 3.Couriel D, Caldera H, Champlin R, et al. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101(9):1936–46. doi: 10.1002/cncr.20613. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 6.Hill GR, Teshima T, Gerbite A, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–67. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda K, Asoh S, Ishikawa M, et al. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–74. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 8.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Met. 2007;13:688–94. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida K, Sano M, Ohsawa I, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 10.Yahata T, Takanashi T, Muguruma Y, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118(11):2941–50. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wang C, Zhang JH, et al. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–61. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 12.Sadeghi B, Aghdami N, Hassan Z, et al. GVHD after chemotherapy conditioning in allogeneic transplanted mice. Bone Marrow Transplant. 2008;42(12):807–18. doi: 10.1038/bmt.2008.261. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald KP, Rowe V, Filippich C, et al. Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood. 2003;101:2033–42. doi: 10.1182/blood-2002-05-1529. [DOI] [PubMed] [Google Scholar]
- 14.Chuai Y, Shen J, Qian L, et al. Hydrogen-rich saline protects spermatogenesis and hematopoiesis in irradiated BALB/c mice. Med Sci Monit. 2012;18(3):BR89–94. doi: 10.12659/MSM.882513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, Ota A, Hori T, et al. Early expression of plasma CCL8 closely correlates with survival rate of acute graft-vs.-host disease in mice. Exp Hematol. 2011;39(11):1101–12. doi: 10.1016/j.exphem.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris AC, Ferrara JL, Levine JE. Advances in predicting acute GVHD. Br J Haematol. 2013;160(3):288–302. doi: 10.1111/bjh.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy J, Blazar BR, Ochs L, et al. The tissue expression of cytokines in human acute cutaneous graft-versus-host disease. Transplantation. 1995;60(4):343–47. doi: 10.1097/00007890-199508270-00008. [DOI] [PubMed] [Google Scholar]
- 19.Buchholz BM, Kaczorowski DJ, Sugimoto R, et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8(10):2015–24. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Kang Z, Cai J, et al. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med. 2009;234(10):1212–19. doi: 10.3181/0812-RM-349. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Sun Q, He B, et al. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148(1):91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura T, Huang CS, Peng X, et al. The effect of donor treatment with hydrogen on lung allograft function in rats. Surgery. 2011;150(2):240–49. doi: 10.1016/j.surg.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70(1):71–86. doi: 10.1093/bmb/ldh025. Erratum in: Br Med Bull, 2005; 73–74: 139. [DOI] [PubMed] [Google Scholar]
- 24.Buchholz BM, Masutani K, Kawamura T, et al. Hydrogen-enriched preservation protects the isogeneic intestinal graft and amends recipient gastric function during transplantation. Transplantation. 2011;92(9):985–92. doi: 10.1097/TP.0b013e318230159d. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Chen Q, Mao Y, et al. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem Res. 2010;35(7):1111–18. doi: 10.1007/s11064-010-0162-y. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Li J, Liu Q, et al. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci Lett. 2011;491(2):127–32. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Shen WF, Sun HY, et al. Hydrogen – rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. 2010;30(7):958–68. doi: 10.1111/j.1478-3231.2010.02254.x. [DOI] [PubMed] [Google Scholar]
- 28.Holler E, Kolb HJ, Mittermuller J, et al. Modulation of acute graft-versus-host disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F) Blood. 1995;86:890–99. [PubMed] [Google Scholar]
- 29.Perez-Simon JA, Diez-Campelo M, Maritino R, et al. Influence of the intensity of the conditioning regimen on the characteristics of acute and chronic graft-versus-host disease after allogeneic transplantation. Br J Haematol. 2005;130:394–403. doi: 10.1111/j.1365-2141.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 30.Teshima T, Ferrara JL. Understanding the alloresponse: new approaches to graft-versus-disease prevention. Semin Hematol. 2002;39:15–22. doi: 10.1053/shem.2002.29246. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Zeng Y, Wang Q, et al. Establishment of mouse allogeneic bone marrow transplantation-induced acute graft-versus-host disease (GVHD) model. Chinese Journal of Pathophysiology. 2004;20(10):1947–49. [Google Scholar]
- 32.Lin X, Gao R, Ni G, et al. Establishment and Application of T Cells and Mice Model in Screening Drugs for Prevention and Treatment of Graft Versus Host Disease. Journal of Zhejiang Chinese Medical University. 2003;37(8):945–50. 955. [Google Scholar]
- 33.Qian LR, Fu W, Shen JL. Agents for refractory/relapsed acute lymphocytic leukemia in adults. Eur Rev Med Pharmacol Sci. 2014;18(17):2465–74. [PubMed] [Google Scholar]
- 34.Qian L, Mei K, Shen J, Cai J. Administration of Hydrogen-Rich Saline Protects Mice From Lethal Acute Graft-Versus-Host Disease (aGVHD) Transplantation. 2013;95(5):658–62. doi: 10.1097/TP.0b013e31827e6b23. [DOI] [PubMed] [Google Scholar]
- 35.Qian L, Wu Z, Shen J. Advances in the treatment of acute graft-versus-host disease. J Cell Mol Med. 2013;17(8):966–75. doi: 10.1111/jcmm.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian L, Shen J, Chuai Y, Cai J. Hydrogen as a New Class of Radioprotective Agent. Int J Biol Sci. 2013;9(9):887–94. doi: 10.7150/ijbs.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian L, Shen J. Hydrogen therapy may be an effective and specific novel treatment for Acute Graft-versus-host disease (GVHD) J Cell Mol Med. 2013;17(8):1059–63. doi: 10.1111/jcmm.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]