Abstract
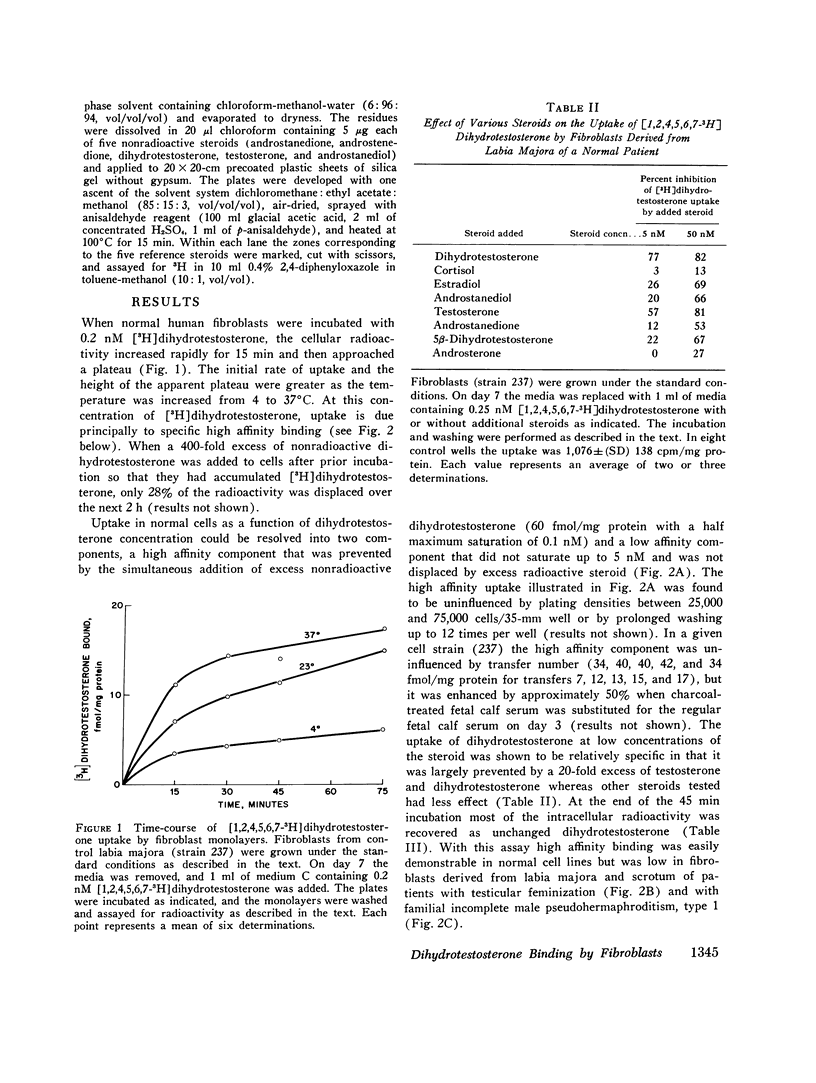
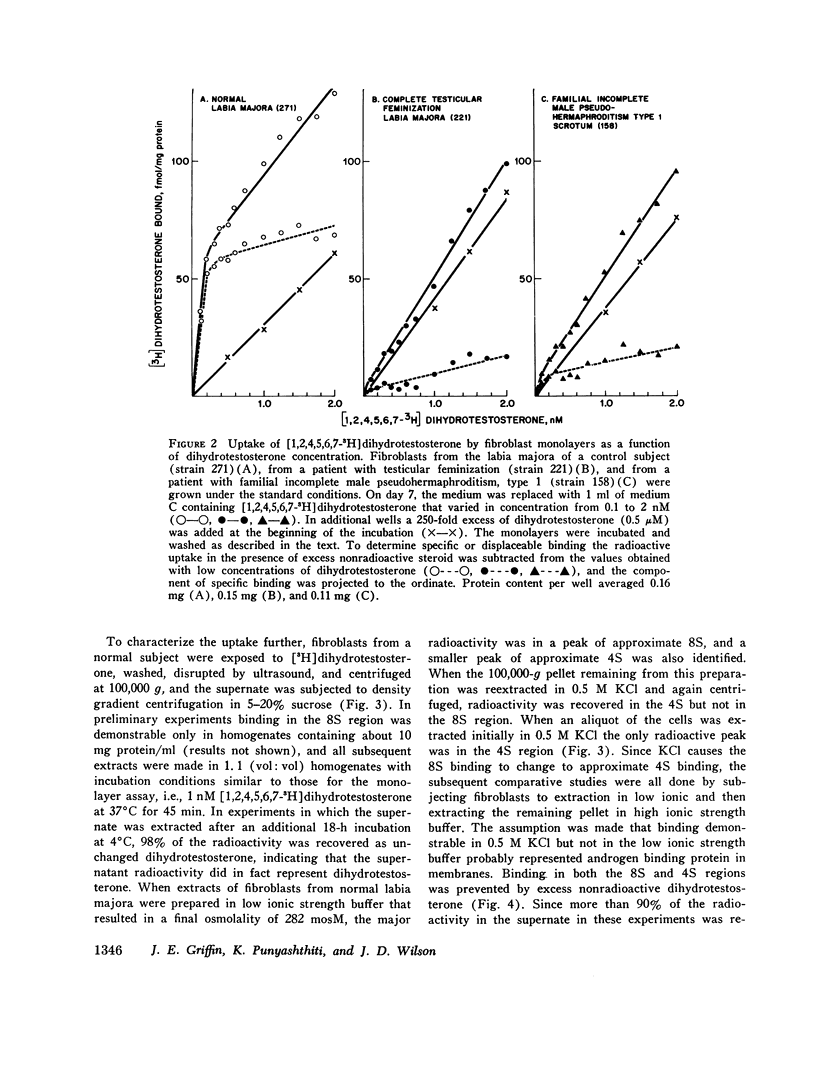
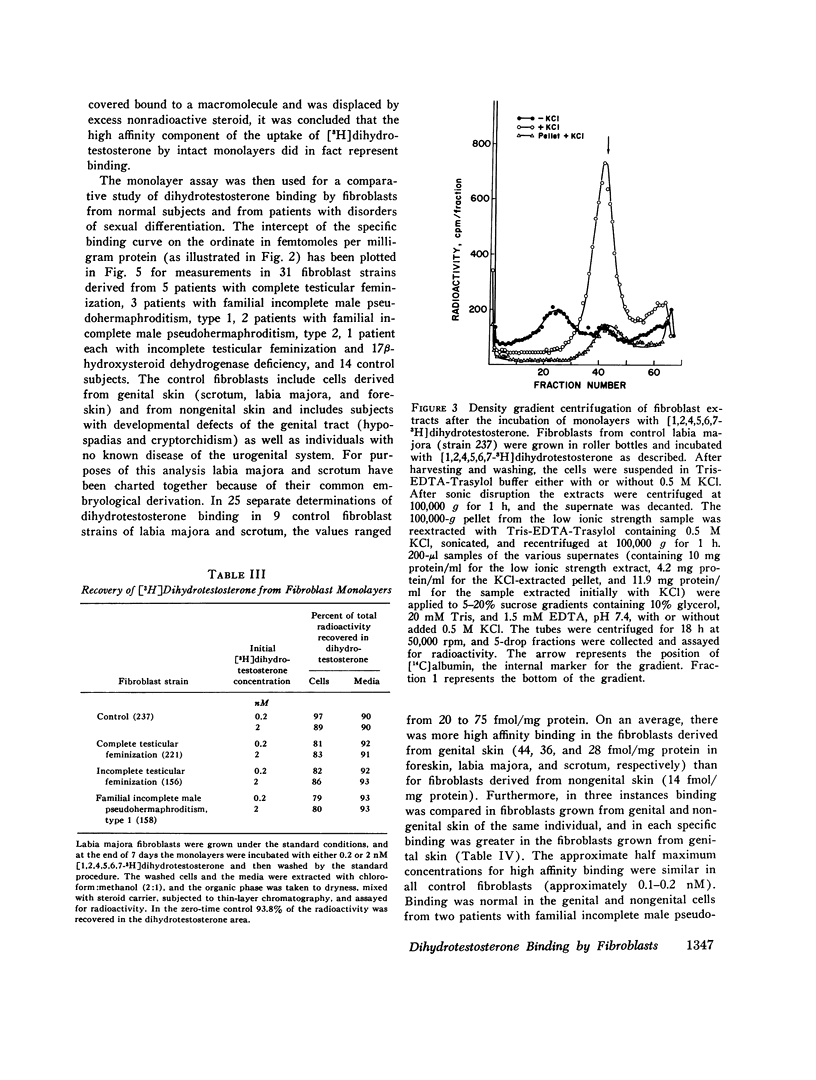
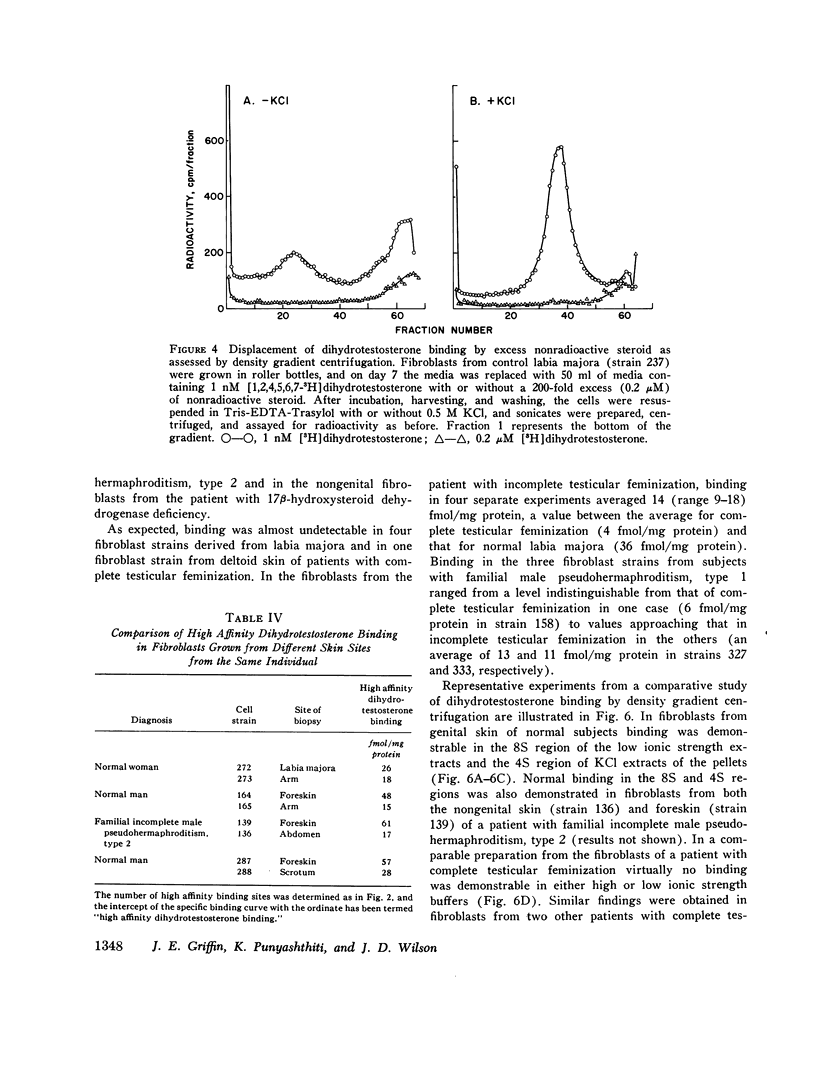
Dihydrotestosterone binding was measured in culture fibroblasts from 14 control subjects and from 12 patients with five different types of hereditary male pseudohermaphroditism. Two assays of binding were used--an intact monolayer assay and density gradient centrifugation of cell extracts. In the intact monolayer assay of normal cells the uptake of [3H]dihydrotestosterone consisted of two components. The first was a high affinity component that exhibited saturation at approximately 1 nM dihydrotestosterone. The second was a low affinity component that was not saturable with concentrations of steroid up to 5 nM. Twice the number of high affinity binding sites were present in fibroblasts grown from genital skin (foreskin, labia majora, and scrotum) as from nongenital sites (37 vs. 14 fmol/mg protein). In the density gradient assay in 5-10% sucrose, the major peak of dihydrotestosterone binding was in the 8S region in low molarity buffer and in the 4S region in 0.5 M KCl. High affinity binding was normal in cells from two patients with familial incomplete male pseudohermaphroditism, type 2, an autosomal recessive defect in which dihydrotestosterone formation is deficient, and in cells from a patient with male pseudohermaphroditism due to 17 beta-hydroxysteroid dehydrogenase deficiency, an autosomal recessive defect of testosterone synthesis. High affinity binding was low by both methods in fibroblasts from five patients with complete testicular feminization. Furthermore, binding by both methods was also low in cells from three subjects with familial incomplete male pseudohermaphroditism, type 1, a presumed X-linked recessive disorder of androgen resistance, and in fibroblasts grown from a subject with the incomplete form of testicular feminization. The finding that dihydrotestosterone binding is abnormal in two forms of hereditary androgen resistance in addition to complete testicular feminization suggests either that these disorders are the result of allelic mutations affecting the function of the androgen-binding protein or that normal dihydrotestosterone binding requires the participation of more than one gene product.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi B., Ono S. Cytosol androgen receptor from kidney of normal and testicular feminized (Tfm) mice. Cell. 1974 Aug;2(4):205–212. doi: 10.1016/0092-8674(74)90012-9. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Bullock L. P., Sherins R. J., Mowszowicz I., Blackburn W. R. Androgen metabolism and mechanism of action in male pseudohermaphroditism: a study of testicular feminization. Recent Prog Horm Res. 1973;29:65–109. doi: 10.1016/b978-0-12-571129-6.50006-3. [DOI] [PubMed] [Google Scholar]
- Bullock L. P., Bardin C. W. Androgen receptors in mouse kidney: a study of male, female and androgen-insensitive (tfm-y) mice. Endocrinology. 1974 Mar;94(3):746–756. doi: 10.1210/endo-94-3-746. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. Characterization of a hormone receptor defect in the androgen-insensitivity mutant. Cell. 1974 Sep;3(1):59–64. doi: 10.1016/0092-8674(74)90040-3. [DOI] [PubMed] [Google Scholar]
- Goebelsmann U., Horton R., Mestman J. H., Arce J. J., Nagata Y., Nakamura R. M., Thorneycroft I. H., Mishell D. R., Jr Male pseudohermaphroditism due to testicular 17 -hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1973 May;36(5):867–879. doi: 10.1210/jcem-36-5-867. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Guerrero L., Gautier T., Peterson R. E. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974 Dec 27;186(4170):1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- Jung I., Baulieu E. E. Neo-nuclear androgen receptor in rat ventral prostate. Biochimie. 1971;53(6):807–817. doi: 10.1016/s0300-9084(71)80122-0. [DOI] [PubMed] [Google Scholar]
- Keenan B. S., Meyer W. J., 3rd, Hadjian A. J., Jones H. W., Migeon C. J. Syndrome of androgen insensitivity in man: absence of 5 alpha-dihydrotestosterone binding protein in skin fibroblasts. J Clin Endocrinol Metab. 1974 Jun;38(6):1143–1146. doi: 10.1210/jcem-38-6-1143. [DOI] [PubMed] [Google Scholar]
- Keenan B. S., Meyer W. J., Hadjian A. J., Migeon C. J. Androgen receptor in human skin fibroblasts. Characterization of a specific 17beta-hydroxy-5alpha-androstan-3-one-protein complex in cell sonicates and nuclei. Steroids. 1975 Apr;25(4):535–552. doi: 10.1016/0039-128x(75)90030-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madden J. D., Walsh P. C., MacDonald P. C., Wilson J. D. Clinical and endocrinologic characterization of a patients with the syndrome of incomplete testicular feminization. J Clin Endocrinol Metab. 1975 Oct;41(4):751–760. doi: 10.1210/jcem-41-4-751. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969 Dec;45(4):531–541. doi: 10.1677/joe.0.0450531. [DOI] [PubMed] [Google Scholar]
- Meyer W. J., 3rd, Migeon B. R., Migeon C. J. Locus on human X chromosome for dihydrotestosterone receptor and androgen insensitivity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1469–1472. doi: 10.1073/pnas.72.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhail G., Wu C. H., Ferin M., Vande Wiele R. L. Radioimmunoassay of plasma estrone and estradiol. Steroids. 1970 Mar;15(3):333–352. doi: 10.1016/s0039-128x(70)80053-8. [DOI] [PubMed] [Google Scholar]
- Milewich L., Gomez-Sanchez C., MacDonald P., Siiteri P. K. Radioimmunoassay of androstenedione: the steroid molecule as a probe for antibody specificity. J Steroid Biochem. 1975 Oct;6(10):1381–1387. doi: 10.1016/0022-4731(75)90073-4. [DOI] [PubMed] [Google Scholar]
- Moore R. J., Griffin J. E., Wilson J. D. Diminished 5alpha-reductase activity in extracts of fibroblasts cultured from patients with familial incomplete male pseudohermaphroditism, type 2. J Biol Chem. 1975 Sep 25;250(18):7168–7172. [PubMed] [Google Scholar]
- Saez J. M., De Peretti E., Morera A. M., David M., Bertrand J. Familial male pseudohermaphroditism with gynecomastia due to a testicular 17-ketosteroid reductase defect. I. Studies in vivo. J Clin Endocrinol Metab. 1971 May;32(5):604–610. doi: 10.1210/jcem-32-5-604. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., Schwarz B. E., Moriyama I., Ashby R., Linkie D., MacDonald P. C. Estrogen binding in the rat and human. Adv Exp Med Biol. 1973;36(0):97–112. doi: 10.1007/978-1-4684-3237-4_6. [DOI] [PubMed] [Google Scholar]
- Walker A. C., Stack E. M., Horsfall W. A. Familial male pseudohermaphroditism. Med J Aust. 1970 Jan 24;1(4):156–160. doi: 10.5694/j.1326-5377.1970.tb77782.x. [DOI] [PubMed] [Google Scholar]
- Walsh P. C., Madden J. D., Harrod M. J., Goldstein J. L., MacDonald P. C., Wilson J. D. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974 Oct 31;291(18):944–949. doi: 10.1056/NEJM197410312911806. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Dihydrotestosterone formation in cultured human fibroblasts. Comparison of cells from normal subjects and patients with familial incomplete male pseudohermaphroditism, Type 2. J Biol Chem. 1975 May 10;250(9):3498–3504. [PubMed] [Google Scholar]
- Wilson J. D., Goldstein J. L. Classification of hereditary disorders of sexual development. Birth Defects Orig Artic Ser. 1975;11(4):1–16. [PubMed] [Google Scholar]
- Wilson J. D., Harrod M. J., Goldstein J. L., Hemsell D. L., MacDonald P. C. Familial incomplete male pseudohermaphroditism, type 1. Evidence for androgen resistance and variable clinical manifestations in a family with the Reifenstein syndrome. N Engl J Med. 1974 May 16;290(20):1097–1103. doi: 10.1056/NEJM197405162902001. [DOI] [PubMed] [Google Scholar]



