Abstract
Previous studies suggest that multiple corticolimbic and hypothalamic structures are involved in glucocorticoid-mediated feedback inhibition of the hypothalamic-pituitary-adrenal (HPA) axis, including the dorsomedial hypothalamus (DMH), but a potential role of the DMH has not been directly tested. To investigate the role of the DMH in glucocorticoid-mediated negative feedback, adult male Sprague Dawley rats were implanted with jugular cannulae and bilateral guide cannulae directed at the DMH, and finally were either adrenalectomized (ADX) or were subjected to sham-ADX. Adrenalectomized rats received CORT replacement in the drinking water (25 µg/ml), which, based on initial studies, restored a rhythm of plasma CORT concentrations in ADX rats that was similar in period and amplitude to the diurnal rhythm of plasma CORT concentrations in sham-ADX rats, but with a significant phase delay. Following recovery from surgery, rats received microinjections of either CORT (10 ng, 0.5 µL, 0.25 µL/min, per side) or vehicle (aCSF containing 0.2% EtOH), bilaterally, directly into the DMH, prior to a 40 min period of restraint stress. In sham-ADX rats, bilateral intra-DMH microinjections of CORT, relative to bilateral intra-DMH microinjections of vehicle, decreased restraint stress-induced elevation of endogenous plasma CORT concentrations 60 minutes after the onset of intra-DMH injections. Intra-DMH CORT decreased the overall area under the curve for plasma CORT concentrations during the intermediate time frame of glucocorticoid negative feedback, from 0.5–2 h following injection. These data are consistent with the hypothesis that the DMH is involved in feedback inhibition of HPA axis activity at the intermediate time frame.
Keywords: 5-HT1A, corticosterone, dorsomedial hypothalamus, HPA axis, negative feedback, automated blood sampling, serotonin
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is an important component of adaptive physiological responses that allow organisms to react rapidly to stressful or threatening stimuli. However, persistent activation of the stress response can lead to impaired physiological or behavioral function (McEwen, 2007). Thus, efficient termination of stress responses is also essential for adaptation to aversive stimuli. However, the rapid transient actions of corticosteroids that facilitate organismal survival and initiate negative feedback mechanisms are poorly understood.
Negative feedback inhibition of HPA axis activity may involve multiple physiological mechanisms operating in different temporal domains. Negative feedback inhibition of HPA axis activity by glucocorticoids has been proposed to operate in three distinct timeframes (Keller-Wood and Dallman, 1984). These include fast feedback (0–10 min onset, lasting 1–15 min), intermediate feedback (0.5–2 h onset, lasting ~6–12 h), and slow feedback (12 h onset with constant glucocorticoid stimulation, lasting days) (Dallman et al., 1987;Keller-Wood and Dallman, 1984). Glucocorticoids are thought to act through non-genomic mechanisms to mediate fast feedback inhibition of the HPA axis and by altering gene transcription to mediate intermediate and slow feedback (Hinz and Hirschelmann, 2000;Keller-Wood and Dallman, 1984). The mechanisms underlying glucocorticoid-mediated negative feedback, particularly fast and intermediate negative feedback, control of the HPA axis are not clear.
The dorsomedial hypothalamus (DMH) is a target for the rapid actions of corticosteroids (Feng et al., 2009;Gasser et al., 2006;Lowry et al., 2001;Thrivikraman et al., 2000), and appears to be involved in integrating stress-related signals with neuroendocrine, autonomic, and behavioral output (Bernardis and Bellinger, 1998;DiMicco et al., 2002;Herman et al., 2002). Stress-induced activation of DMH neurons is also associated with increased behavioral arousal and anxiety (Shekhar and Katner, 1995). Conversely, inhibition of DMH activity produces autonomic and behavioral responses consistent with decreased anxiety, fear, and arousal (Shekhar et al., 2002). The DMH has both glutamatergic and GABAergic projections to the parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVN), so DMH activity can elicit either excitatory or inhibitory responses in the PVN, which, in turn, regulate pituitary release of adrenocorticotropic hormone (ACTH) (Boudaba et al., 1996).
Taken together, these studies implicate the DMH as a site for actions of corticosteroids that play a critical role in control of HPA axis function and suggest that the DMH may participate in negative feedback control of the HPA axis during a stress response. The current experiment was designed to test the hypothesis that glucocorticoid effects on the DMH contribute to the negative feedback control of HPA axis activity.
Materials and methods
Animals
Experiments were conducted using adult male Sprague Dawley rats (Cat No. 002, Harlan, Indianapolis, IN, USA) weighing approximately 280 g at the time of surgery. Rats were housed in pairs in cages (23 cm × 38 cm × 11 cm tall, 1292N, Tecniplast, Buguggiate, Italy) using standard cage bedding (Teklad Laboratory Grade Aspen Bedding, Harlan) and allowed to acclimate for 1 week prior to beginning the surgeries. Rats were kept on a 12 h light:12 h dark schedule (~230 lux, lights on at 0700 h) at 22 °C. Both food (Cat. No. 2018, Teklad Global 18% Protein Rodent Diet, Harlan) and tap water, unless otherwise indicated, were provided ad libitum for the duration of the experiment. All animal experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, D.C., 2011) and were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Experimental design
Experiment 1. Validation of the restraint stress model using automated blood sampling
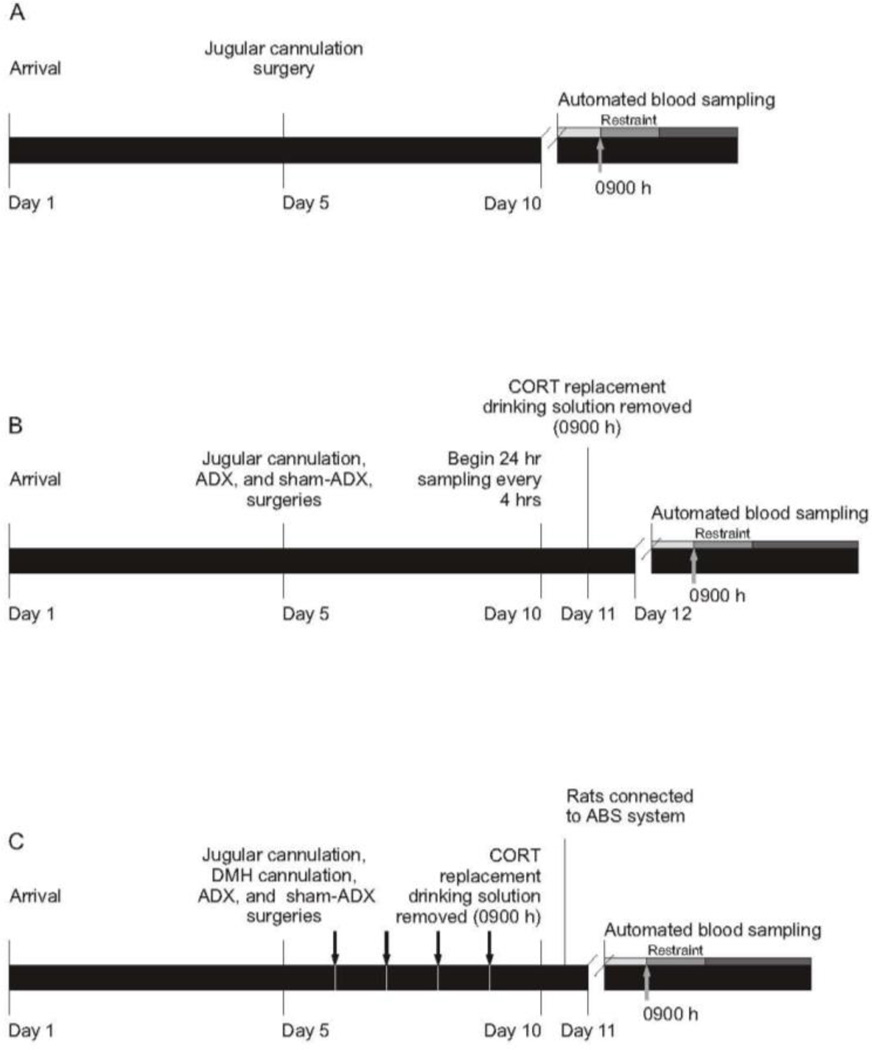
In order to validate the use of an automated blood sampling system to characterize the HPA axis response to restraint stress we exposed adrenal-intact rats to a 40 minute period of restraint and sampled blood at 10 minute intervals from 30 minutes prior to restraint until 50 minutes following the end of restraint (n = 8) (Figure 1A). Rats underwent jugular cannulation surgery as described in the General procedures section. Once the rats recovered from anesthesia following surgery, they were singly housed in the automated blood sampling (ABS) procedure room. A metal spring protecting the jugular cannula was connected to a liquid swivel to allow free movement around the cage. At 0830 h on the day of the experiment the ABS system was programed to collect ~450 µl of diluted blood (sufficient to measure both ACTH and corticosterone (CORT)) every 10 minutes for a total of 12 samples (3 baseline samples prior to restraint, 4 samples during the 40 min restraint period, and 5 samples following restraint; Figure 1A). Automated blood sampling, restraint, spectrophotometric blood dilution assays, and CORT assays were conducted as described in the General procedures section.
Figure 1.
Timelines for Experiments 1, 2, and 3. (A) Experiment 1. Experiment 1 was designed to determine the amplitude and time course of restraint-induced activation of the hypothalamic-pituitary-adrenal axis using the automated blood sampling system. Automated blood sampling was started at 0830 h on Day 10 and the shaded bars represent the 3 baseline (light gray), 4 restraint (gray), and 5 post-restraint (dark gray) samples. The gray arrow indicates that restraint started at 0900 h. (B) Experiment 2. Experiment 2 was designed to determine the pattern of plasma corticosterone (CORT) concentrations in adrenalectomized (ADX) rats drinking CORT-replacement solution (25 µg/ml CORT and 0.45% HBC in 0.9% saline), relative to diurnal rhythm of plasma CORT concentrations in adrenal-intact rats (sham-ADX), drinking tap water containing 0.45% HBC, as well as the rate of clearance of exogenous CORT in ADX rats once CORT-replacement solution was removed. Automated blood sampling was initiated at 0900 h on Day 10. Corticosterone-replacement solution was removed at 0900 h on Day 11, 24 hours before the onset of restraint. The shaded bars on Day 12 represent the 3 baseline (light gray), 4 restraint (gray), and 8 post-restraint (dark gray) samples, respectively. The gray arrow indicates that restraint started at 0900 h. (C) Experiment 3. Experiment 3 was designed to determine the effects of intra-dorsomedial hypothalamus (DMH) CORT infusion on restraint stress-induced HPA axis activity. Rats were habituated to insertion of injection cannulae and jugular cannulae were flushed with heparinized saline daily at 0900 h starting on Day 5 and ending on Day 10 (represented by black arrows). Corticosterone-replacement solution was removed from ADX rats 24 hours prior to the beginning of sampling and replaced with 0.9% saline containing 0.45% HBC. The shaded bars on Day 11 represent the 3 baseline samples (light gray), 2 samples during intra-DMH injection and placement of rats into restraint and 4 samples during (gray), and 7 post-restraint samples (dark gray), respectively. The gray arrow indicates that restraint started at 0900 h. Abbreviations: ADX, adrenalectomized; CORT, corticosterone; DMH, dorsomedial hypothalamus; HBC, hydroxypropyl-beta-cyclodextrin.
Experiment 2. Characterization of plasma CORT concentrations in adrenalectomized rats with CORT replacement
We conducted a second study using the ABS system in order to ensure that the concentration of CORT added to the CORT-replacement solution (25 µg/ml CORT, Cat. No. C2504, Sigma-Aldrich, St. Louis, MO, USA, in 0.9% saline and 0.45% hydroxypropyl-beta-cyclodextrin, HBC; Cat. No. 332607, Sigma-Aldrich) was effective in restoring a rhythm of CORT in ADX rats (n = 8) that was comparable to the endogenous diurnal rhythm in adrenal intact rats drinking tap water with 0.45% HBC (n = 8; Figure 1B). This study was also designed to determine if replacing the CORT solution with 0.9% saline and 0.45% HBC in tap water 24 hours prior to the beginning of restraint allowed sufficient time for the exogenous CORT to clear from ADX rats. Rats underwent jugular cannulation surgery followed immediately by either ADX or sham-ADX surgery as described in the General procedures. After surgery, rats were allowed to recover as described for Experiment 1. For ADX rats, CORT-replacement solution was provided to mimic the diurnal rhythm of plasma CORT concentrations. Bottles were wrapped in tin foil to minimize degradation of CORT. Sham-ADX rats were given tap water with 0.45% HBC. Drinking bottles were refilled every 2 days. The ABS system was programmed to begin sampling ~50 µl of diluted blood (sufficient to measure CORT) every 4 hours for 48 h prior to the experimental day (Figure 1B). At 0900 h, 24 h prior to the experimental day, the CORT solution was replaced with 0.9% saline and 0.45% HBC for the ADX rats. At 0830 h on the experimental day the sampling protocol was changed so that the ABS system was programmed to collect ~450 µl of diluted blood (for measurements of ACTH and CORT every 10 minutes for a total of 15 samples (3 baseline, 4 restraint, and 8 post-restraint samples; Figure 1B). Automated blood sampling, restraint, spectrophotometric blood dilution assays, and CORT assays were conducted as described in the General procedures section.
Experiment 3. Effects of intra-DMH CORT microinjection on plasma CORT concentrations during restraint stress
In order to examine neural mechanisms underlying feedback inhibition of the HPA axis, we administered bilateral microinjections of CORT into the DMH immediately prior to restraint and assessed HPA axis activity using ABS procedures (Figure 1C). Rats were assigned to 4 different treatment groups, 1) sham-ADX/intra-DMH vehicle (n = 12), 2) sham-ADX/intra-DMH CORT (n = 12), 3) ADX/intra-DMH vehicle (n = 11), 4) ADX/intra-DMH CORT (n = 13). The rats underwent jugular cannulation, intracranial guide cannula implantation, and sham-ADX or ADX surgeries successively. Rats were allowed to recover in a warm cage and ADX rats were provided with CORT-replacement solution as described in Experiment 2. For ADX rats, CORT-replacement solution was replaced with 0.9% saline containing 0.45% HBC 24 h before microinjections (0900 h). At 0830 h on the day of the experiment, the ABS system was programed to collect ~450 µl of diluted blood every 10 minutes for a total of 16 samples (3 baseline samples, 2 sample during handling of the rats for the intra-DMH CORT injection and placement of rats into restraint, 4 samples during the restraint period, and 7 samples following the end of restraint; Figure 1C) as described in the automated blood sampling section of the General procedures section.
General procedures
Jugular cannulae assembly
Jugular cannulae were assembled from three different types of tubing (a figure illustrating the construction of the jugular cannulae is available in supplementary material). Briefly, a section of Silastic® tubing (i.d. 0.5 mm, o.d. 1.5 mm, 4 cm long; Cat. No. BSIL-T020, Instech Laboratories, Plymouth Meeting, PA, USA) was expanded in petroleum ether and a section of polythene tubing (i.d. 0.58 mm, o.d. 0.98 mm, 36 cm long; Cat. No. 800/100/200, Portex, Kent, UK) was inserted into the silastic tubing with 1 cm of overlap. Two Silastic® tubing lugs (i.d. 0.5 mm, o.d. 1.5 mm, 2 mm long; Cat. No. BSIL-T020, Instech Laboratories), used to secure the cannula in the jugular vein, were expanded in petroleum ether and slipped onto the opposite end of the polythene tubing. A final section of Silastic® tubing (i.d. 0.5 mm, o.d. 0.93 mm, 3.5 cm long; Cat. No. SFM1-1350, SF Medical, Hudson, MA, USA) was expanded in petroleum ether and placed over the end of the polythene tubing with 1 cm overlap, 1 cm from two Silastic® tubing lugs placed on the polythene tubing.
Jugular venous cannulation surgery
In all experiments, rats were anesthetized with isoflurane (5– 10% induction and 2–5% maintenance in 100% oxygen). Following induction of anesthesia, a small area of skin above the right jugular vein was cleaned with an antibacterial solution (Actril, Cat. No. 176-02-046, Medivators, Minneapolis, MN, USA) then shaved in preparation for the surgery. A 2 cm incision was made superficial to the jugular vein and the tissue was separated via blunt dissection down to the vein. Once the jugular vein was exposed, the surrounding connective tissue was dissected so that the forceps could easily be moved back and forth under the jugular vein. Cotton ligatures were positioned, but not tightened, at both the caudal and rostral ends of the vein and the surgical field was rinsed with sterile heparinized saline. A small longitudinal incision was made along the jugular vein and the Silastic®-tipped polythene jugular cannula was flushed with sterile heparinized saline then inserted into the vein, with the tip of the cannula positioned rostral to the right atrium. The cannula was secured in the vein by tying both ligatures. The cannula was then tunneled subcutaneously out through an incision on the scalp. The ventral incision was closed using silk sutures (Cat. No. MV-683, Med-Vet International, Mettawa, IL, USA) and wound dressing liquid (Nolvasan, Cat. No. NDC 0856-0253-01, Fort Dodge, IA, USA) was applied. The jugular cannula was run through a metal spring (30.5 cm long, 4 mm diameter), which was later attached to a liquid swivel (Cat. No. 375/22, Instech Laboratories) mounted above the cage in order to protect the cannulae while allowing the rat maximal freedom of movement. The spring containing the jugular cannula was secured to the skull using two cheese head screws (Cat. No. 6809, BoltDepot.com, North Weymouth, MA, USA). For Experiments 1 and 2, dental cement (Cat. No. NC9655089, Fisher Scientific, Pittsburgh, PA, USA) was then added to create a head cap that secured the spring to the skull. Following jugular cannulation, rats were either allowed to recover from the anesthesia in a warm cage (Experiment 1) or underwent further surgery (Experiments 2 and 3). Rats were allowed to recover for at least 5 days prior to beginning the automated blood sampling protocol. Jugular cannulae were manually flushed daily by withdrawing 0.2 ml of blood and replacing the volume with 0.3 ml of sterile heparinized saline (0.2 ml to replace blood volume; 0.1 ml to clear blood from cannulae) to maintain patency of the cannulae.
Intracranial guide cannula implantation surgery
Following jugular cannulation in Experiment 3 rats were placed into a stereotaxic frame (SAS-4100, ASI Instruments, Warren, MI, USA) for implantation of bilateral guide cannulae into the DMH. During implantation of guide cannulae, the jugular cannula was run through a metal spring, which was later attached to a liquid swivel mounted above the cage in order to protect the cannulae while allowing the rat maximal freedom of movement. Bilateral guide cannulae (Cat. No. C235G-1.0SPC, Plastics One, Roanoke, VA, USA; cut 8 mm below the pedestal) were placed at the following stereotaxic coordinates relative to bregma: AP: −3.3 mm; ML: +/− 0.5 mm, DV: −6.8 mm; the guide cannulae were placed 2 mm above the intended microinjection site, in order to avoid damage to the microinjection site. The spring containing the jugular cannula was secured to the skull using two cheese head screws and dental cement was added to create a head cap that secured both the spring and guide cannulae. Stylets (Cat. No. C235DC/SPC, Plastics One) were placed into the guide cannulae (extending to, but not extending past, the tip of the guide cannulae) to prevent contamination and ensure patency of the cannulae. Dust caps (Cat. No. 303DC/1, Plastics One) were placed over the stylets to secure them. Following bilateral intra-DMH cannulation surgery, rats underwent either sham-ADX or ADX surgery (Experiment 3). Rats were then allowed to recover following surgery for at least 5 days prior to beginning the experiment. Rats were habituated to i.c. cannulae insertion every day following surgery by handling rats and removing, then replacing, the dummy cannulae.
Adrenalectomy or sham-adrenalectomy surgery
Bilateral adrenalectomies were performed under continued isoflurane anesthesia in Experiments 2 and 3. Adrenalectomies were performed by making transverse, bilateral, 1 cm incisions directly caudal to the rib cage. The border between the liver and kidney was located and separated allowing the surgeon to visualize the adrenal gland, just deep and rostral to the kidney. The adrenal gland was grasped and removed from the body cavity using tuttle forceps (Cat. No. 22-7470, Sklar, Westchester, PA, USA). Fine scissors and a hemostat were used to cut the attached artery and vein. The muscle wall was closed using sutures (Cat No. MV-683, Med-Vet International) and incisions in the skin were then closed using sterile wound clips (Autoclips, 9 mm stainless steel, No. B-2355-100, Clay Adams, Parsipany, NJ, USA). Sham-ADX surgeries were identical to ADX surgeries except that the adrenal glands were not physically contacted. Following ADX or sham-ADX surgery, rats were allowed to recover from the anesthesia in a warm cage. Rats were then allowed to recover for at least 5 days prior to beginning the experiment. Adrenalectomized rats were given CORT-replacement solution (25 µg/ml CORT in 0.9% saline and 0.45% HBC), while sham-ADX rats were given tap water with 0.45% HBC to drink. The CORT-replacement solution was replaced with 0.9% saline with 0.45% HBC 24 h prior to the beginning of experimentation.
Automated blood sampling
Automated blood sampling was conducted as previously described (Windle et al., 1997). The jugular cannulae were attached to the system using a liquid swivel that allowed for unobstructed 360-degree movement around the cage. Flow of blood or heparinized saline between rat, reservoir, and fraction collector was achieved by cooperative actions of a peristaltic pump and a 3-way valve controlled by a computer outside of the procedure room. Rats were connected to the system at 1700 h the night before the experiment and, using the automated blood sampling system, jugular cannulae were flushed by sampling 50 µl of blood, discarding it, and replacing it through the jugular cannulae with 50 µl of sterile heparinized saline (10 IU/ml) every 60 minutes, until the beginning of the experiment, to maintain cannula patency. Following every sample the blood volume that was removed from the animal was replaced with heparinized saline in order to prevent reductions in blood volume.
Restraint stressor
Custom-made adjustable restraint tubes (Alpha Plastics, Fort Collins, CO, USA; a 3D rendering of the restraint tube, illustrating construction details, is available in supplementary material), constructed using clear acrylic tubing, were used as restraining devices. The restraint tubes were 17.8 cm long, with an insert that served to adjust the level of restraint. The tubes had a 5.2 cm i.d., and had a 1.3 cm wide channel along the top to allow for continuous blood sampling; a removable acrylic door at the front of the tube was used to allow rats to voluntarily exit the tube at the end of the restraint session. The length of the tube was adjusted using the moveable insert to prevent backward and forward movement while not compressing the rats. Rats were placed into restraint tubes for 40 minutes and blood samples were collected at 10 min intervals during and after restraint. Following restraint, the rats were placed back into their home cages and left undisturbed.
Spectrophotometric blood dilution assay
In order to account for potential variations in the amount of blood collected by the ABS system in individual samples, we developed a technique to determine the proportion of blood, relative to heparinized saline, that was collected for each sample. To make this assessment, we inverted sample tubes multiple times before removing a 3 µl volume from each sample and, using a spectrophotometer (Synergy HT, BioTek Instruments, Winooski, VT, USA), compared the samples to a standard curve based on known proportions of blood in sterile heparinized saline (0%, 12.5%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5%, 100%). Samples and standards for the standard curve were diluted 1:90 in sterile heparinized saline and 85 µl of diluted blood in heparinized saline was loaded into 96-well plates in triplicate and read at 630 nm. A linear regression line was fit to the standard curve to produce an equation that was used to solve for the unknown sample dilutions. The dilution of each sample was transformed into a coefficient that was applied to the hormone assay results in order to correct for different blood dilutions in samples (mean: 62.8% blood, range: 0%–100% blood). Blood samples containing less than 15% blood were not reliable among duplicates or triplicates in the CORT enzyme immunoassay and therefore were excluded from the analysis.
CORT enzyme immunoassay
Total plasma CORT concentrations were assayed using CORT Enzyme Immunoassay (EIA) kits (Cat. No. ADI-900-097, Enzo Life Sciences, Plymouth Meeting, PA, USA) according to kit instructions, except for the concentration of steroid displacement reagent, which was increased twenty-fold. These conditions were chosen based on optimizations that were performed with samples from ADX rats that were spiked with known concentrations of exogenous CORT (Cat. No. C2505, Sigma-Aldrich). Samples were run in duplicate or triplicate and plates were read with a spectrophotometer set at 405 nm (Multiskan EX, VWR, West Chester, PA, USA). The dilution coefficient was applied during the analysis of the hormone concentrations to correct for the dilution of the samples. The inter- and intra-assay coefficients of variation were 10.1% and 8.4%, respectively.
Adrenocorticotropic hormone enzyme immunoassay
In Experiment 2 and 3, adrenocorticotropic hormone (ACTH) enzyme immunoassay kits (Phoenix Pharmaceuticals, Cat No. EK-001-21, Burlingame, CA, USA) were used in an attempt to measure plasma ACTH concentrations in sham-ADX and ADX rats from blood samples containing heparinized saline. The manufacturer’s information indicates that the assay functions in the presence of heparin. However, our results, using heparinized samples and the recommended plasma extraction, were inconsistent even among triplicate samples. Heparin has been shown to interfere with a number of assay techniques, both ACTH enzyme immunoassay and ACTH radioimmunoassay (Dupouy et al., 1980). Physiologic baseline ACTH concentrations are considered ~60–110 pg/ml (Park et al., 2013). Studies using the same kits provided by Phoenix Pharmaceuticals under various conditions have reported basal plasma ACTH concentrations in the range of 25–5000 pg/ml (Anseloni et al., 2005;Goebel et al., 2011;Kobayashi et al., 2009;Ochi et al., 2008;Quinn et al., 2012;Samson et al., 2002;Tian et al., 2005). We were not able to reliably measure ACTH in the presence of heparin in samples from the ABS system using the kits in ADX animals. All ADX rats had plasma CORT concentrations below 25 ng/ml.
Drug administration
In Experiment 3, on the day of the experiment, stylets were removed from guide cannulae and injection cannulae (Cat. No. C235l, 1 mm center-to-center, Plastics One) were inserted to a level 2 mm below the bottom of the guide cannulae to allow direct administration of CORT or vehicle into the DMH (from bregma, AP: −3.3 mm; ML: +/− 0.5 mm; DV: −8.8 mm) (Paxinos and Watson, 1998). Rats received bilateral microinjections of CORT (10 ng, 0.5 µL, 0.25 µL/min, per side) or vehicle (aCSF (0.402 g KCL, 17.18 g NaCl, 0.248 g NaH2PO4, 0.398 g Na2HPO4, 0.203 g MgCl2, and 0.176 g CaCl2 in 1 liter of distilled water, pH 7.2–7.4) containing 0.2% EtOH) immediately prior to restraint stress. The dose, volume, and rate of infusion of CORT were based on our previous study using ADX rats demonstrating that CORT infusions into the PVN, 60 min before a 15-min restraint stress or home cage control conditions, prevented stress-induced increases in plasma ACTH, measured 15 min after stress onset (Weiser et al., 2011). The dose of CORT used was also consistent with other studies involving glucocorticoid microinfusion into the PVN (Evanson et al., 2010). Microinjections were administered with a 10 µl syringe (Cat. No. 80365, Hamilton, Reno, NV, USA) using an infusion pump (Model PHD 2000; Harvard Apparatus, Holliston, MA, USA). Injection cannulae were left in place for one min after the microinjection was completed in order to ensure that the drug was not siphoned into the guide cannulae as the injection cannulae were removed. Rats were placed in restrainers next to their home cages immediately following CORT or vehicle microinjection in the DMH.
Confirmation of cannulae placements
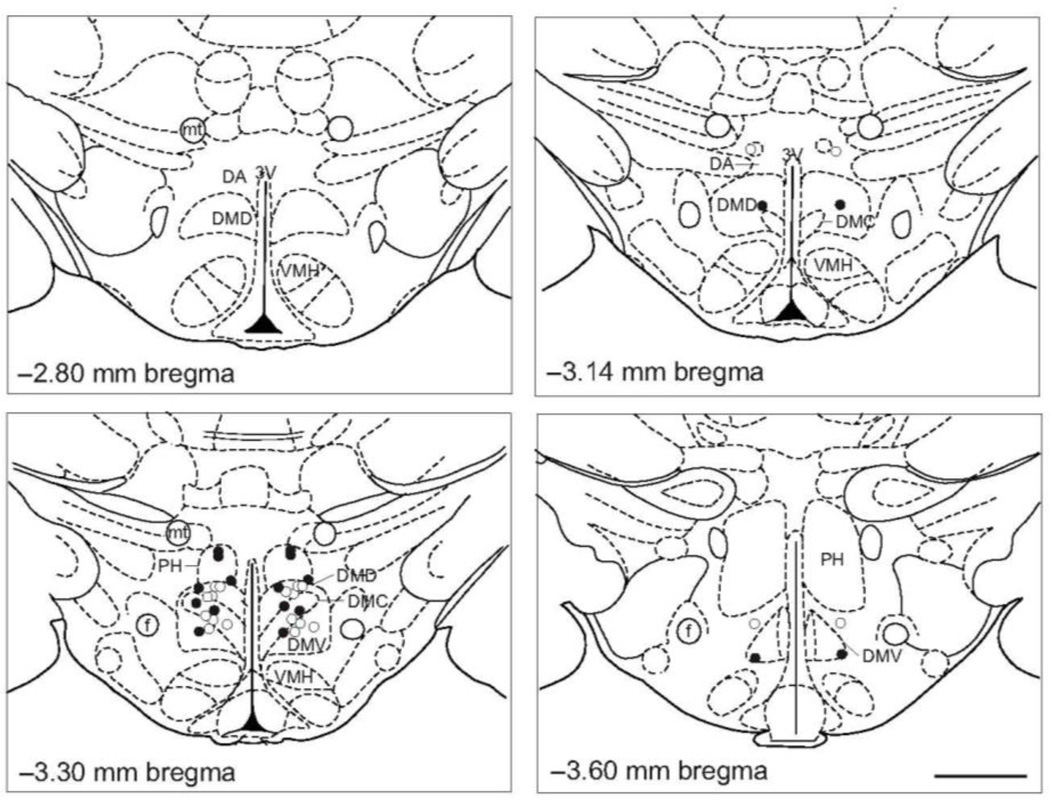
Four hours after the onset of the restraint stress in Experiment 3, rats were euthanized with sodium pentobarbital (Fatal Plus, 0.2 ml i.v., Vortech Pharmaceuticals, Dearborn, MI, USA) and brains were extracted and flash-frozen on dry ice. The brains were serially sectioned at 30 µm for cannulae placement confirmation in the DMH (−2.6 mm to −3.8 mm bregma) using a cryostat (CM 1950, North Central Instruments, Plymouth, MN, USA) at −23 °C. Sections for cannulae placement confirmation were mounted on HistoBond slides (Cat No. 16004-406, VWR) in the cryostat and stained using cresyl violet to assist in the visualization of structures. Rats included in the analysis had confirmed bilateral cannulae placements in the DMH (Figure 2). The limits of the DMH were as defined by Fontes and colleagues (Fontes et al., 2011). Among sham-ADX rats, after confirming cannulae placements, eighteen of twenty-four rats were determined to be bilateral hits (sham-ADX/vehicle, n = 10; sham-ADX/CORT, n = 8).
Figure 2.
Bilateral injection cannulae placements for Experiment 3. The four panels were adapted from a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998). The dorsomedial hypothalamus (DMH) was defined based on Fontes et al. (2011). Open and closed circles represent cannulae placements considered inside the DMH for sham-adrenalectomized (ADX)/vehicle-treated rats and sham-ADX/corticosterone (CORT)-treated rats, respectively. Abbreviations: 3V, 3rd ventricle; ADX, adrenalectomized; CORT, corticosterone; DA, dorsal hypothalamic area; DMC, dorsomedial hypothalamic nucleus, compact part; DMD, dorsomedial hypothalamic nucleus, dorsal part; DMV, dorsomedial hypothalamic nucleus, ventral part; f, fornix; mt, mammillothalamic tract; PH, posterior hypothalamic area; VMH, ventromedial hypothalamic nucleus. Scale bar, 1 mm.
Statistical analysis
Prior to statistical analysis, outliers were removed from the dataset using the Grubbs’ method (Grubbs, 1969). In addition, samples with a blood concentration of less than 15% were not included in the analysis. In Experiment 1, the final sample size used for statistical analysis was n = 7. No animals were removed from Experiment 2 (n = 8 per group). In Experiment 3, it was not possible to conduct all intra-DMH microinjections and place all rats in restrainers simultaneously as the ABS system collects samples from all 8 rats simultaneously. Therefore, the onset of handling, microinjection, and restraint, relative to sample collection, was not uniform for every rat, introducing the possibility of variability between plasma CORT concentrations at a single critical time point (i.e. start of restraint). For this reason the data for 5 rats (sham-ADX/Vehicle: n = 3; sham-ADX/CORT: n = 2) were normalized so that the first sample with elevated plasma CORT concentration (>50 ng/ml) was set to the 10-min time point. In addition, because of the variability in the onset of handling, microinjection, and restraint, between-group comparisons were not made at either time point 0 or 10. Rats that had baseline plasma CORT concentrations (prior to the onset of handling, intra-DMH microinjections, and placement in restrainers) exceeding 50 ng/ml were removed from the dataset (n = 1; sham-ADX/CORT). Rats with one or both cannulae placements outside of the DMH were removed from the dataset (sham-ADX/Vehicle (n = 1); sham-ADX/CORT (n = 2)). In addition, rats with blood in the brain section that obscured the confirmation of the cannula placement were not included in the dataset (sham-ADX/Vehicle (n = 1); sham-ADX/CORT (n = 1)). The final sample sizes used for statistical analyses were: sham-ADX/Vehicle (n = 10), sham-ADX/CORT (n = 8).
Data were analyzed using a linear mixed model with treatment (CORT or vehicle) as the fixed factor and time as the repeated measure. A survey of covariate structures was performed by examining the −2 Restricted Log Likelihood value (i.e., an information criteria function used to measure goodness-of-fit) and the number of parameters (i.e., a measure of parsimony) to determine the covariate structure that best fit the model. A linear mixed model analysis was used because it has several advantages over the repeated measures ANOVA when analyzing repeated measures data, including 1) the accommodation of multiple missing data values (in this experiment resulting from outliers and complications during the experiment), 2) the ability to more effectively estimate model parameters in unbalanced experimental designs, 3) more flexibility in model fitting through the objective selection of covariance structures that better fit the correlations between data points, and 4) the ability to model nonlinear changes in a dependent variable across time and treatment (Cnaan et al., 1997;Krueger and Tian, 2004). When appropriate, Student’s t-test was used to compare plasma CORT concentrations between the two treatment groups at specific time points. Area under the curve (AUC) was calculated using the trapezoidal method. Missing values were replaced using the Peterson method (Petersen, 1985). Significance for the linear mixed model and Student’s t-tests was accepted when p < 0.05.
Results
Experiment 1
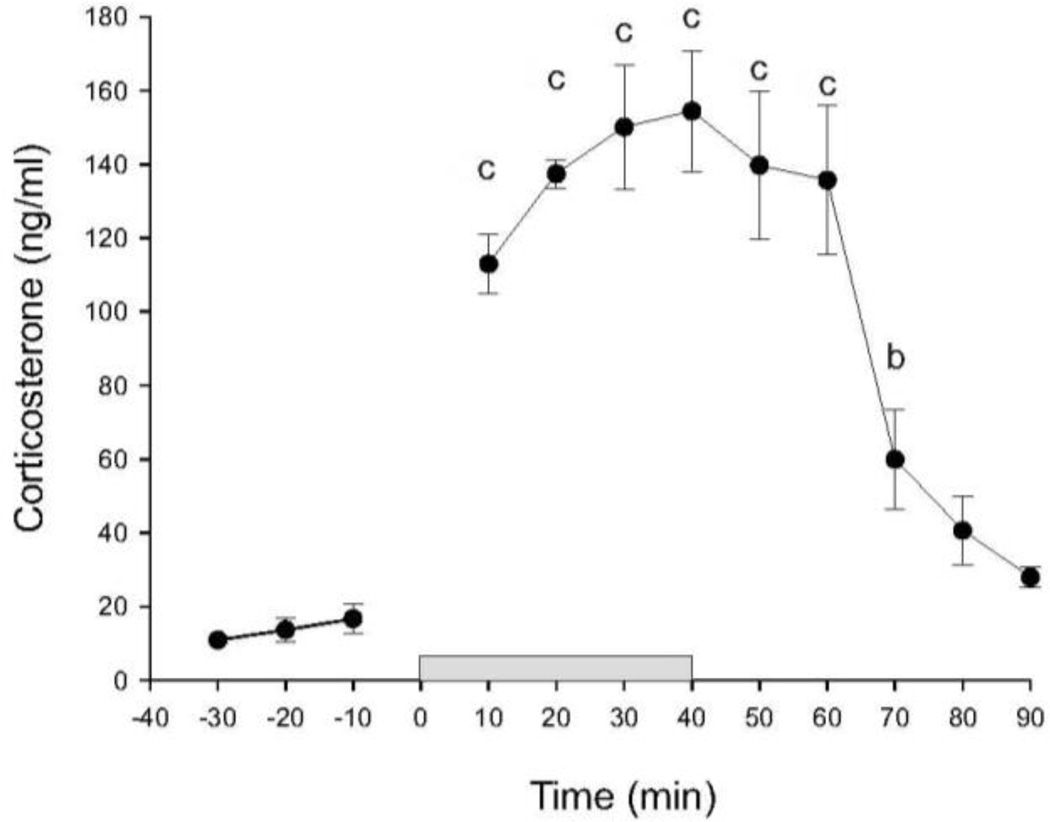
In order to validate the use of restraint stress in combination with automated blood sampling for measurement of stress-induced increases in HPA axis activity, we collected blood samples at 10 minute intervals starting 30 minutes prior to a 40 minute period of restraint, during restraint, and during the 50 minute period following restraint in adrenal-intact adult male Sprague Dawley rats (n = 7, Figure 3). Baseline CORT concentrations were approximately 15 ng/ml. Plasma CORT concentrations were maximal within 20 minutes after the onset of restraint and were approximately 140 ng/ml. Linear mixed model analysis revealed an effect of time (F(11, 39.9) = 21.01, p < 0.001). Linear mixed model analysis revealed significant effects of restraint on plasma CORT concentrations, relative to baseline concentrations 10 min prior to restraint, throughout restraint and for 30 min after termination of restraint. Plasma CORT concentrations returned to baseline within 40 min following the termination of restraint.
Figure 3.
Baseline and restraint-induced increases in plasma corticosterone (CORT) concentrations (ng/ml) in rats in Experiment 1 (n = 7). The break between the −10 and 10 min time points is due to intentional omission of automated sampling at that time point. The gray bar between time points 0 and 40 represents the time period during which rats were restrained. Data are presented as means ± SEM (n = 7). Within group comparisons: b, p < 0.01; c, p < 0.001, generated by a linear mixed model statistical analysis.
Experiment 2
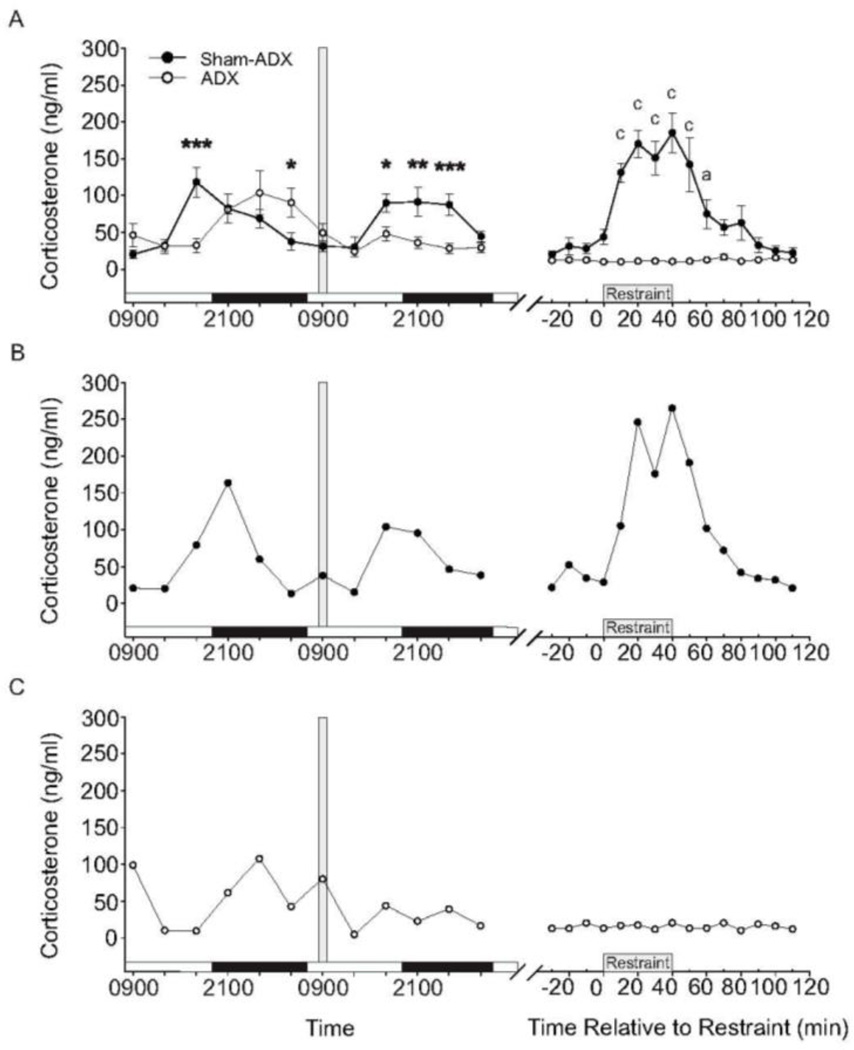
In order to characterize the diurnal rhythm of plasma CORT concentrations in ADX rats with CORT-replacement solution (25 µg/ml CORT in 0.9% saline containing 0.45% HBC), we compared plasma CORT concentrations in sham-ADX rats drinking tap water containing 0.45% HBC and ADX rats with CORT replacement at 4-h intervals for 48 h prior to a restraint stress challenge (Figure 4). Sham-ADX rats displayed a diurnal rhythm with peak CORT concentrations of approximately 125 ng/ml at 1700 h (2 h before the onset of the dark phase) and nadir in CORT concentrations of approximately 20 ng/ml at 0900 h (2 h after the onset of the light phase). ADX rats given CORT re-established a rhythm similar in amplitude and period to the diurnal rhythm of sham-ADX rats, but with a phase delay of approximately 4 h as measured by plasma CORT concentrations. The phase delay in ADX rats receiving CORT-replacement solution is manifested in the greater plasma CORT concentration of sham-ADX rats, relative to ADX rats with CORT replacement, 2 h prior to the dark phase (p = 0.002, Figure 4A), while ADX rats do not reach similar plasma CORT concentrations until the early part of the dark phase (4 h later). Thus, the diurnal rise in CORT concentrations in ADX rats with CORT-replacement was delayed until after dark onset, presumably due to the onset of drinking behavior at dark onset. In addition, the phase delay persisted throughout the dark phase, presumably due to continued drinking while the rats were active, leading to the higher plasma CORT concentrations in ADX rats at the end of the dark phase (p = 0.03). After 48 h of sampling, CORT-replacement solution was removed at 0900 h and replaced with 0.9% saline containing 0.45% HBC in tap water; this eliminated the rise in plasma CORT observed during the subsequent dark phase in ADX rats, with differences between the sham-ADX and ADX rats seen at 1700 h, 2100 h, and 0100 h (1700 h, p = 0.02; 2100 h, p = 0.01; 0100 h, p = 0.003). Plasma CORT concentrations were low in ADX rats (between 10.7 ng/ml and 17.2 ng/ml with a mean of 12.8 ± 0.5 ng/ml) with saline replacement for several hours before the beginning of restraint. Linear mixed model analysis of the plasma CORT concentrations for the sham-ADX rats revealed an effect of time (F(14, 44.8) = 10.61, p < 0.001). Linear mixed model analysis revealed significant effects of time on plasma CORT concentrations, relative to baseline concentrations 10 min prior to restraint. Time-dependent increases in plasma CORT concentrations were observed starting 20 min following the onset of restraint, and persisted for 20 minutes after the termination of restraint. Plasma CORT concentrations returned to baseline within 30 min following termination of restraint. These data indicate that the method of replacing CORT in ADX rats established an amplitude and period of rhythmic plasma CORT concentrations similar to that observed in sham-ADX rats, but with a significant phase delay. In addition, these results indicate that removing CORT 24 h prior to the onset of restraint stress resulted in clearance of exogenous CORT.
Figure 4.
Plasma corticosterone (CORT) concentrations (ng/ml) in adrenalectomized (ADX) (n = 8) and sham-ADX (n = 8) rats over the course of Experiment 2. (A) The data illustrated before the break in the x-axis illustrate the diurnal rhythm of plasma CORT concentrations, measured at 4 h intervals, during a 48 h period. The white and black horizontal bars along the x-axis represent the light cycle phase (white indicates the light phase, black indicates the dark phase). The vertical gray bar represents the time at which CORT-replacement solution (25 µg/ml CORT in 0.9% saline containing 0.45% hydroxypropyl-beta-cyclodextrin (HBC)) was replaced with 0.9% saline containing 0.45% HBC for ADX rats 24 h prior to the onset of restraint. The data after the break in the x-axis illustrate baseline, restraint, and post-restraint samples taken at 10 min intervals. (B) Graph illustrating plasma CORT concentrations in a representative sham-ADX rat. (C) Graph illustrating plasma CORT concentrations in a representative ADX rat. Data in A represent means ± SEM of plasma CORT concentrations for each treatment group. Between-group comparisons: *p < 0.05, **p < 0.01, ***p < 0.001. Within-group comparisons: a = p < 0.05, c = p < 0.001. Abbreviations: ADX, adrenalectomy; CORT, corticosterone; HBC, hydroxypropyl-beta-cyclodextrin.
Experiment 3
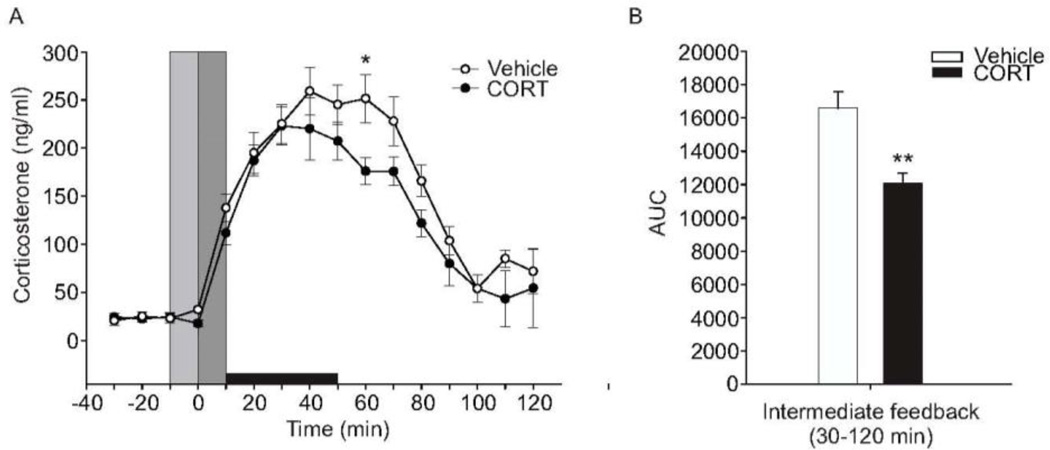
To test if the DMH plays a role in CORT-mediated negative feedback inhibition of the HPA axis, we microinjected CORT or vehicle into the DMH of sham-ADX and ADX rats. Intra-DMH cannulae placements are shown in Figure 2. Only cannulae placements within the DMH as defined by Fontes and colleagues (2011) were used in the analysis. Both CORT- and vehicle-treated sham-ADX rats showed increases in plasma CORT concentrations starting at the 10-min time point (Figure 5). Sham-ADX rats treated with intra-DMH vehicle microinjection showed peak plasma CORT concentrations of approximately 250 ng/ml at the 40 min time point. Sham-ADX rats treated with intra-DMH CORT microinjection showed peak plasma CORT concentrations of approximately 220 ng/ml within 30 min. Linear mixed model analysis revealed a treatment × time interaction (F(15,64.9) = 2.03, p = 0.026). CORT-treated sham-ADX rats showed lower peak plasma CORT concentrations at the 60 min time point (p = 0.042; Figure 5A). Analysis of the area under the curve of the intermediate (0.5 –2 h) negative feedback time frame (Dallman et al., 1987;Keller-Wood and Dallman, 1984) showed a significant treatment effect (p < 0.01; Figure 5B), with a reduction in plasma CORT concentrations in rats that received intra-DMH CORT treatment.
Figure 5.
Effects of bilateral intra-dorsomedial hypothalamus (DMH) microinjections of corticosterone (CORT) or vehicle in sham-adrenalectomized (sham-ADX) rats exposed to a 40 min period of restraint stress on plasma CORT concentrations (ng/ml). All rats included in the data analysis had bilateral cannulae placements within the DMH. A) Open and closed circles represent sham-ADX/vehicle-treated (n = 10) and sham-ADX/CORT-treated (n = 8) rats, respectively. Three baseline samples were taken at −10, −20, and −30 min, immediately prior to intra-DMH CORT microinjection and restraint. The vertical light gray bar indicates the window of time from −10 to 0 min during which rats were handled for insertion of injection cannulae and initiation of the intra-DMH microinjections. The vertical dark gray bar represents the time from 0 to 10 min taken to complete the intra-DMH microinjections, remove the injection cannulae, and put the rats into the restraint tube. Data were normalized so that the first sample with elevated plasma CORT concentration (>50 ng/ml) was at the 10 min time point. The horizontal black bar indicates the 40-min restraint period, from 10 min to 50 min. B) Graph illustrating the area under the curve for the intermediate (30–120 min) feedback timeframe. CORT-treated rats showed a significantly attenuated HPA axis response during the intermediate feedback timeframe (p < 0.01). Abbreviations: ADX, adrenalectomy; AUC, area under the curve; CORT, corticosterone. Between-group comparisons: *p < 0.05, **p < 0.01, Fisher’s protected least significant difference (LSD) test.
Discussion
In studies of adrenal intact rats, bilateral intra-DMH CORT injections, immediately prior to exposure to restraint, suppressed stress-induced increases in plasma CORT concentrations. This effect was apparent 60 min following the onset of stress exposure, coinciding with the time frame that has been described as the intermediate (e.g. ~0.5–2 h) negative feedback time frame (Dallman et al., 1987;Keller-Wood and Dallman, 1984). Analysis of area under the curve for CORT concentrations between 0.5–2 h revealed decreased plasma CORT concentrations in rats pretreated with intra-DMH CORT microinjections, consistent with a role for the DMH in negative feedback control of the HPA axis during the intermediate time frame. These studies also confirmed that CORT replacement in the drinking water, at a concentration of 25 µg/ml, results in a rhythmic pattern of CORT concentrations that resembles the endogenous diurnal rhythm, albeit with a phase delay of approximately 4 h.
Here we demonstrate use of an automated blood sampling system to investigate site-specific glucocorticoid-mediated suppression of HPA axis activity, using a novel restraining device to accommodate the hardware associated with the automated blood sampling system. In this model, restraint stress induced a reliable activation of the HPA axis, with roughly an order of magnitude increase in plasma CORT concentration following a 40 min period of restraint. The magnitude of the increase in plasma CORT concentration in Experiments 1 and 2 (approximately 150–175 ng/ml) was notably less than that observed in Experiment 3 (approximately 250 ng/ml). This is likely due to the additional manual restraint and handling required for insertion of bilateral injection cannulae in Experiment 3. Nevertheless, this procedure results in a reliable increase in plasma CORT concentrations that peaks approximately 40 min following the onset of restraint, and returns to basal concentrations approximately 2 h following the onset of restraint.
The DMH has previously been implicated in control of HPA axis responses to aversive stimuli. For example, intra-DMH microinjections of the GABAA receptor agonist muscimol suppress air-jet stress-induced increases in plasma ACTH and CORT concentrations (Morin et al., 2001;Stotz-Potter et al., 1996). Conversely, microinjections of the GABAA receptor antagonist bicuculline into the DMH potentiate HPA axis activity (Bailey and DiMicco, 2001). Interestingly, the DMH appears to play a role in control of HPA axis responses to psychological or exteroceptive stressors, such as restraint, but not to physical stressors, such as hemorrhage (Morin et al., 2001;Thrivikraman et al., 2000). Thrivikraman and colleagues proposed a role for the DMH in fast negative feedback control of the HPA axis based on the observation that air-puff startle, but not moderate hemorrhage, increases c-Fos expression in cortico-limbic structures that have no monosynaptic connection to the PVN, but project to the DMH, which has extensive monosynaptic connections to the PVN (Thrivikraman et al., 2000). As was observed for the cortico-limbic structures, air-puff startle, but not moderate hemorrhage, increased c-Fos expression in the DMH and it was proposed that the cortico-limbic structures modulate HPA axis function through their projections to the DMH (Thrivikraman et al., 2000). Consistent with a role for the DMH in negative feedback control of HPA axis function, the DMH has abundant projections to the PVN, including glutamatergic and GABAergic projections (Herman et al., 2002). Consequently, CORT could act by inhibiting excitatory input to the PVN, or facilitating inhibitory input to the PVN, or both.
The overall effect of intra-DMH CORT on stress-induced CORT secretion was modest and certainly not a full blockade. Similar findings have been reported for the PVN (Evanson et al., 2010) and hippocampus (Kovacs and Makara, 1988). It is clear that the DMH constitutes only one of multiple sites for negative feedback control of the HPA axis. Principal sites of glucocorticoid-mediated feedback inhibition of HPA axis activity include the pituitary (Cole et al., 2000;Keller-Wood and Dallman, 1984;Russell et al., 2010) and PVN (Evanson et al., 2010;Keller-Wood and Dallman, 1984), but also extrahypothalamic sites (Furay et al., 2008), including the paraventricular nucleus of the thalamus (Jaferi et al., 2003), hippocampus (Kovacs and Makara, 1988) and prefrontal cortex (Diorio et al., 1993). It is likely that CORT actions at multiple sites are required for a more substantial blockade.
Studies involving intra-PVN administration of the synthetic glucocorticoid receptor agonist dexamethasone found glucocorticoid-mediated suppression of HPA axis activity following exposure of rats to a 30 min period of restraint, with suppressed stress-induced ACTH concentrations 15 min (fast feedback) following the onset of restraint, and suppressed stress-induced CORT concentrations 60 min (intermediate feedback) following the onset of restraint (Evanson et al., 2010), as observed here. These effects of intra-PVN dexamethasone to exert fast feedback inhibition of HPA axis activity may be mediated by rapid, non-genomic effects of glucocorticoids such as altering endocannabinoid or nitric oxide signaling (Evanson et al., 2010;Tasker and Herman, 2011). Similarly, Weiser and colleagues (2011) found that intra-PVN microinfusions of CORT 60 min before a 15-min restraint stress prevented stress-induced increases in plasma ACTH measured 15 min after stress onset, an effect that coincides with the intermediate timeframe of negative feedback. Intra-PVN CORT also prevented stress-induced increases in corticotropin-releasing hormone (Crh) heteronuclear RNA within the PVN and stress-induced increases in pro-opiomelanocortin mRNA expression in the anterior pituitary (Weiser et al., 2011). It is not clear if the same mechanisms underlying negative feedback inhibition of HPA axis activity operate within the DMH and PVN.
The following evidence supports the conclusion that the intra-DMH-induced suppression of stress-induced increases in plasma CORT concentrations is not due to diffusion of the microinjected CORT from the DMH to the PVN. First, a validation study of microinjections in the brain determined that the injection volume used in our study (0.5 µl) should not diffuse to the PVN (Lohman et al., 2005). Diffusion of 0.2–0.5 µl microinjections of dye (0.04 µl/min) into the rat brain 90 min after injection had an average radial diffusion of 520 ± 120 µm (Lohman et al., 2005). Our most rostral injection was at −3.14 mm bregma and the most caudal aspect of the PVN is at −2.12 mm bregma, which is ~1.0 mm of separation (Paxinos and Watson, 1998). Consequently, it is unlikely that intra-DMH CORT spread to the PVN, or, if it did so, it did so at greatly reduced concentrations. Secondly, in a previous study involving intra-PVN injections of CORT, using the same dose of CORT, injection volume, and rate of infusion as used in our study, CORT injections that were 0.5 mm beyond the rostral, caudal and/or dorsal borders of the PVN had no effect on restraint stress-induced HPA axis activation (Weiser et al., 2011). The majority of the “misses” were located at −2.28 mm bregma, a location that lies caudal to the PVN, but rostral to the DMH. Thus, there appears to be a zone located between the PVN and DMH where intra-hypothalamic microinfusions of CORT have no effect on stress-induced HPA axis activity. Together, these findings support the hypothesis that both the PVN and DMH participate in glucocorticoid-mediated negative feedback control of HPA axis function.
Intermediate feedback (~2 h) appears to depend on serotonergic signaling (Dallman et al., 1987;Kaneko and Hiroshige, 1978;Keller-Wood and Dallman, 1984). A potential role of serotonergic signaling in CORT-mediated feedback control of HPA axis function during the intermediate timeframe is consistent with previous studies demonstrating a delayed increase in serotonin release in some limbic forebrain structures, from approximately 0.5–2 h following infusion of the stress-related neuropeptide, Crh, into the dorsal raphe nucleus, a time interval that corresponds with the intermediate feedback timeframe. This delayed release of serotonin has been proposed to mediate stress recovery, as it coincides with the cessation of fear following Crh infusions into the dorsal raphe nucleus. Furthermore, infusion of CORT into the DMH potentiates d-fenfluramine-induced increases in extracellular serotonin concentrations (Feng et al., 2009), while activation of 5-HT1A receptors in the medial hypothalamus inhibits HPA axis activity (Hanley and Van de Kar, 2003). In addition, activation of 5-HT1A receptors in the DMH with microinjections of (±)-8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide (8-OH-DPAT), a 5-HT1A receptor agonist, has anti-panic-like effects on escape reactions in male rats, suggesting that serotonergic signaling in the DMH may play a role in suppression of both HPA axis and behavioral responses to threatening stimuli (de Bortoli et al., 2013). A potential molecular mechanism of CORT action in the DMH may involve blockade of CORT-sensitive organic cation transporter 3 (OCT3). Organic cation transporter 3 is a low affinity, high capacity transporter of organic cations, including serotonin, that is highly expressed in the DMH and is rapidly inhibited by physiological concentrations of CORT (Gasser et al., 2009). Corticosterone blockade of OCT3 would be predicted to enhance serotonergic signaling in the presence of high extracellular serotonin concentrations, as might occur under stress conditions (Feng et al., 2009;Feng et al., 2010;Gasser et al., 2009). These potential mechanisms of action should be evaluated in future studies.
These studies also confirmed that CORT replacement, at a concentration of 25 µg/ml in the drinking water, results in a rhythmic pattern of plasma CORT concentrations in ADX rats that resembles the endogenous diurnal rhythm in intact rats, albeit with a phase delay of approximately 4 h. The 4 h delay in the diurnal CORT rhythm is likely due to the timing of drinking behavior. Most drinking behavior in rats is restricted to the dark phase of the photoperiod, which coincides with their highest level of physical activity (Johnson and Johnson, 1990). Therefore, the rhythmic fluctuations in plasma CORT concentrations in ADX rats with CORT replacement in the drinking water does not reflect either the circadian or the feedback regulation of the HPA axis, but rather the circadian rhythm of drinking behavior. Nevertheless, it is likely that glucocorticoid responsive targets (both peripherally and in the central nervous system) in ADX rats with CORT replacement in the drinking water were exposed to levels of CORT that approximated those found in sham-ADX controls, although with a phase delay. In addition, removal of CORT-replacement solution 24 h before the start of restraint in ADX rats was sufficient for clearance of exogenous CORT. This would be expected as the half-life for CORT in adult male rats is ~20 min (Kitay, 1961).
Conclusions
These studies suggest a potential role for the DMH in negative feedback control of HPA axis activity, with inhibition in the intermediate timeframe. However, these data are consistent with the hypothesis that a highly distributed system of brain sites, including the DMH, functions to exert negative feedback inhibition of HPA axis activity. Further studies will be required to elucidate the cellular and molecular mechanisms involved.
Supplementary Material
Acknowledgments
This work was supported by awards from the National Science Foundation to CAL (NSF- IOS 0921969), KJR (NSF-IOS 0921874), and MO (NSF-IOS 0922085) and an award from the National Institute of Mental Health to RLS(R01MH075968). CAL also receives grant support from the National Institute of Mental Health (R01MH065702, R01DA019921), the Depressive and Bipolar Disorder Alternative Treatment Foundation, and is the recipient of a NARSAD, Brain & Behavior Research Foundation 2010 Young Investigator Award. We are grateful to Peter Hibl, Kathleen Dady, Adam Kapitz, and Laura Shultz for technical support. Finally, we are grateful to Alpha Plastics and Design (Fort Collins, CO, USA) for manufacturing the custom restrainers and providing the 3D rendered image.
Reference List
- Anseloni VC, He F, Novikova SI, Turnbach RM, Lidow IA, Ennis M, Lidow MS. Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience. 2005;131:635–645. doi: 10.1016/j.neuroscience.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Bailey TW, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc Soc Exp Biol Med. 1998;218:284–306. doi: 10.3181/00379727-218-44296. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–167. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SK, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: Variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- de Bortoli VC, Yamashita PS, Zangrossi H., Jr 5-HT1A and 5-HT2A receptor control of a panic-like defensive response in the rat dorsomedial hypothalamic nucleus. J Psychopharmacol. 2013;27:1116–1123. doi: 10.1177/0269881113492900. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupouy JP, Chatelain A, Godaut M. Influences of heparin on ACTH distribution and immunoreactivity in plasma of the rat. In vivo and in vitro studies. J Physiol (Paris) 1980;76:631–635. [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Lowry CA, Lukkes JL, Orchinik M, Forster GL, Renner KJ. Organic cation transporter inhibition increases medial hypothalamic serotonin under basal conditions and during mild restraint. Brain Res. 2010;1326:105–113. doi: 10.1016/j.brainres.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Telefont M, Kelly K, Orchinik M, Forster GL, Renner KJ, Lowry CA. Local perfusion of corticosterone in the rat medial hypothalamus potentiates D-fenfluramine-induced elevations of extracellular 5-HT concentrations. Horm Behav. 2009;56:149–157. doi: 10.1016/j.yhbeh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Xavier CH, de Menezes RC, DiMicco JA. The dorsomedial hypothalamus and the central pathways involved in the cardiovascular response to emotional stress. Neuroscience. 2011;184:64–74. doi: 10.1016/j.neuroscience.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Reeve J, Jr, Tache Y. Lipopolysaccharide increases plasma levels of corticotropin-releasing hormone in rats. Neuroendocrinology. 2011;93:165–173. doi: 10.1159/000322590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic--pituitary-adrenal axis in health and disease. Vitam Horm. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Nowak N, Bhatnagar S. Negative feedback functions in chronically stressed rats: role of the posterior paraventricular thalamus. Physiol Behav. 2003;78:365–373. doi: 10.1016/s0031-9384(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Johnson AK. Light/dark cycle modulates food to water intake ratios in rats. Physiol Behav. 1990;48:707–711. doi: 10.1016/0031-9384(90)90215-p. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hiroshige T. Site of fast, rate-sensitive feedback inhibition of adrenocorticotropin secretion during stress. Am J Physiol. 1978;234:R46–R51. doi: 10.1152/ajpregu.1978.234.1.R46. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Machida T, Takahashi T, Takatsu H, Shinkai T, Abe K, Urano S. Elevation by oxidative stress and aging of hypothalamic-pituitary-adrenal activity in rats and its prevention by vitamin e. J Clin Biochem Nutr. 2009;45:207–213. doi: 10.3164/jcbn.09-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474:205–210. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- Krueger C, Tian L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs. 2004;6:151–157. doi: 10.1177/1099800404267682. [DOI] [PubMed] [Google Scholar]
- Lohman RJ, Liu L, Morris M, O'Brien TJ. Validation of a method for localised microinjection of drugs into thalamic subregions in rats for epilepsy pharmacological studies. J Neurosci Methods. 2005;146:191–197. doi: 10.1016/j.jneumeth.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Burke KA, Renner KJ, Moore FL, Orchinik M. Rapid changes in monoamine levels following administration of corticotropin-releasing factor or corticosterone are localized in the dorsomedial hypothalamus. Horm Behav. 2001;39:195–205. doi: 10.1006/hbeh.2001.1646. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Morin SM, Stotz-Potter EH, DiMicco JA. Injection of muscimol in dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1276–R1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [DOI] [PubMed] [Google Scholar]
- Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, Higuchi K, Arakawa T. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci. 2008;82:862–868. doi: 10.1016/j.lfs.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Park SY, Walker JJ, Johnson NW, Zhao Z, Lightman SL, Spiga F. Constant light disrupts the circadian rhythm of steroidogenic proteins in the rat adrenal gland. Mol Cell Endocrinol. 2013;371:114–123. doi: 10.1016/j.mce.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition. San Diego: Academic Press; c1998. [Google Scholar]
- Petersen RG. Design and Analysis of Experiments. New York: Marcel Dekker, Inc; c1985. [Google Scholar]
- Quinn M, Ueno Y, Pae HY, Huang L, Frampton G, Galindo C, Francis H, Horvat D, McMillin M, Demorrow S. Suppression of the HPA axis during extrahepatic biliary obstruction induces cholangiocyte proliferation in the rat. Am J Physiol Gastrointest Liver Physiol. 2012;302:G182–G193. doi: 10.1152/ajpgi.00205.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell GM, Henley DE, Leendertz J, Douthwaite JA, Wood SA, Stevens A, Woltersdorf WW, Peeters BW, Ruigt GS, White A, Veldhuis JD, Lightman SL. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J Neurosci. 2010;30:6106–6115. doi: 10.1523/JNEUROSCI.5332-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol Biochem Behav. 1995;50:253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Katner JS, Sajdyk TJ, Kohl RR. Role of norepinephrine in the dorsomedial hypothalamic panic response: an in vivo microdialysis study. Pharmacol Biochem Behav. 2002;71:493–500. doi: 10.1016/s0091-3057(01)00688-8. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrivikraman KV, Nemeroff CB, Plotsky PM. Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000;870:87–101. doi: 10.1016/s0006-8993(00)02405-7. [DOI] [PubMed] [Google Scholar]
- Tian DR, Li XD, Wang F, Niu DB, He QH, Li YS, Chang JK, Yang J, Han JS. Up-regulation of the expression of cocaine and amphetamine-regulated transcript peptide by electroacupuncture in the arcuate nucleus of diet-induced obese rats. Neurosci Lett. 2005;383:17–21. doi: 10.1016/j.neulet.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Osterlund C, Spencer RL. Inhibitory effects of corticosterone in the hypothalamic paraventricular nucleus (PVN) on stress-induced adrenocorticotrophic hormone secretion and gene expression in the PVN and anterior pituitary. J Neuroendocrinol. 2011;23:1231–1240. doi: 10.1111/j.1365-2826.2011.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa AP, Ingram CD, Lightman SL. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol. 1997;9:407–414. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.