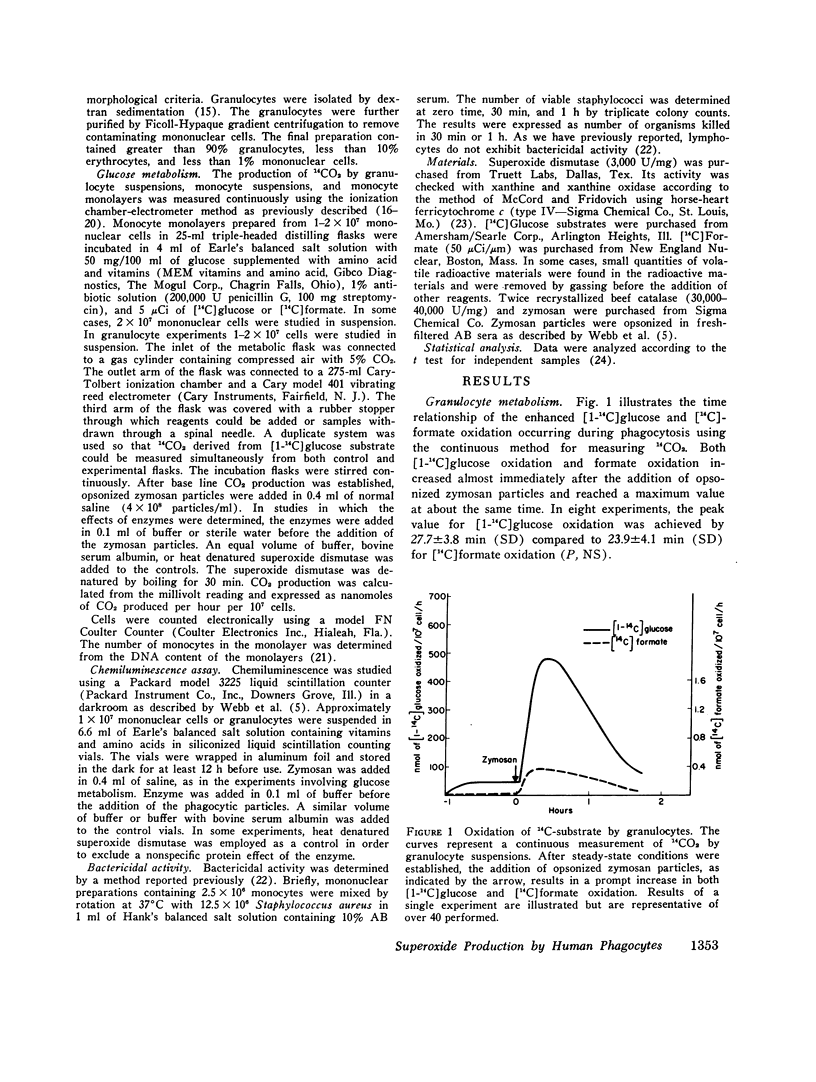
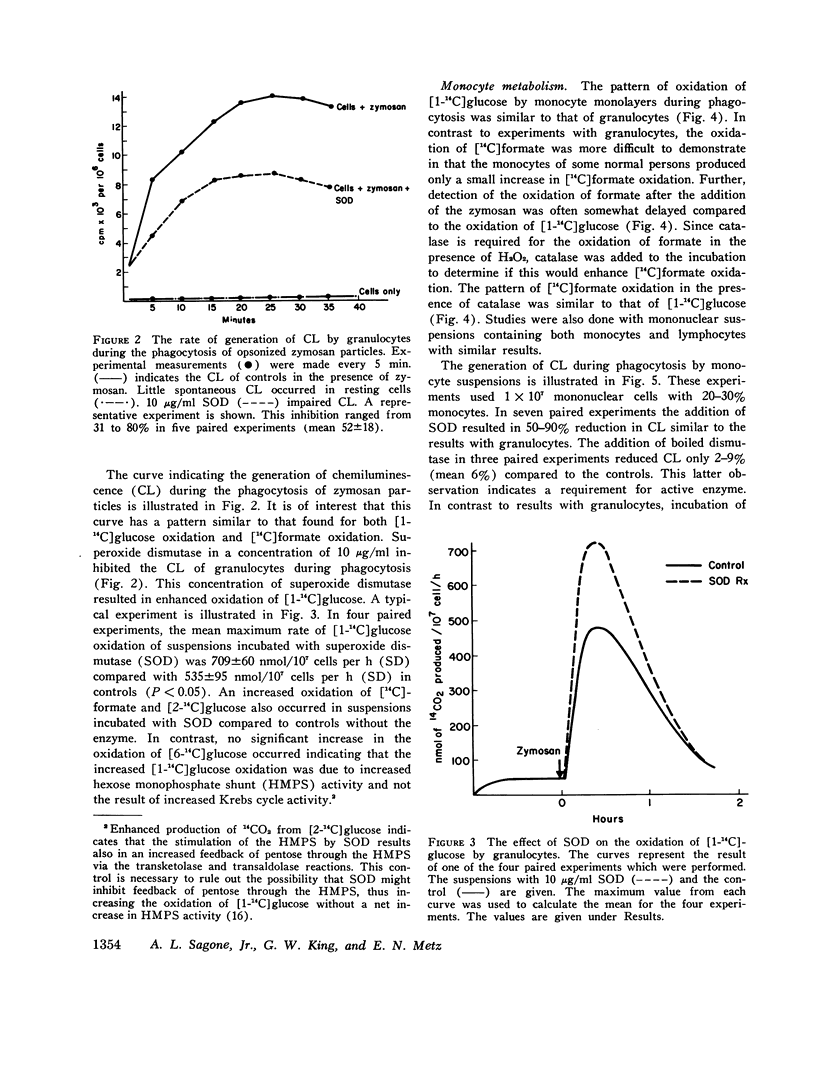
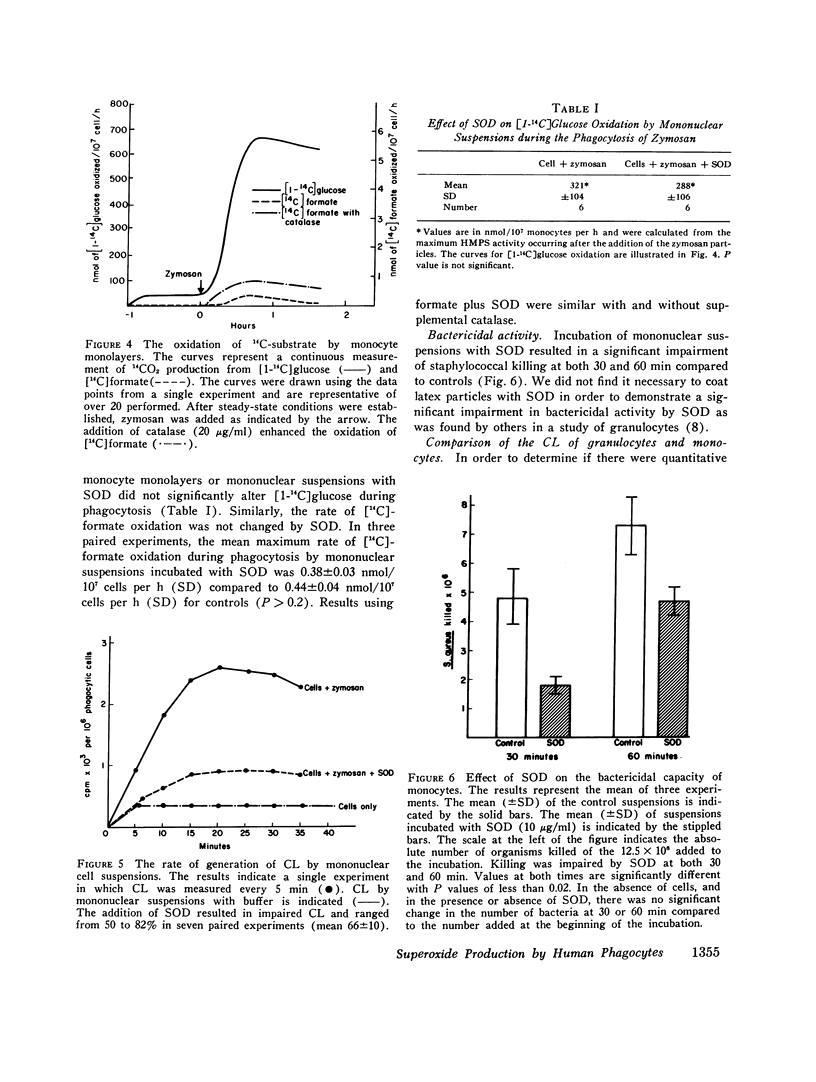
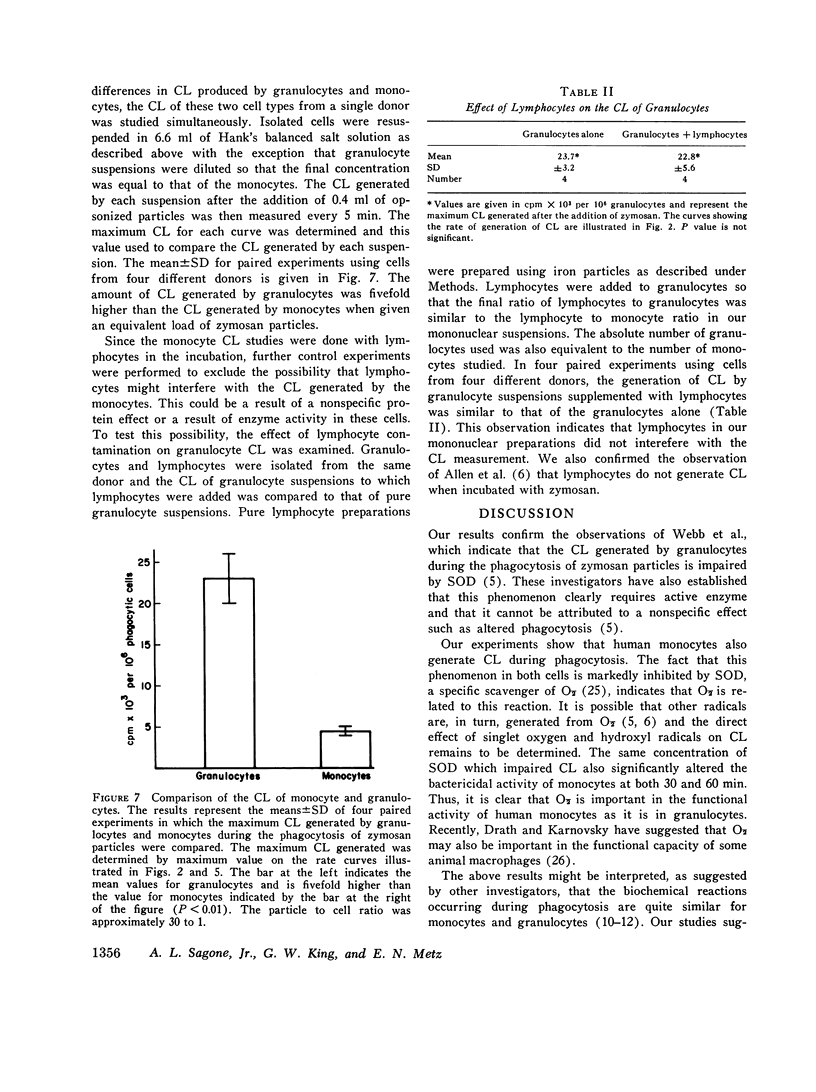
Abstract
Recent studies indicate that oxygen radicals such as superoxide or singlet oxygen may be important in the functional activity of human granulocytes. We have examined the possible importance of these radicals in the functional capacity of human blood monocytes. Monocytes, like granulocytes, generate chemiluminescence during phagocytosis. Chemiluminescence is impaired 50-90% by superoxide dismutase, an enzyme which enhances the dismutation of superoxide to hydrogen peroxide. These results indicate that superoxide is related to the chemiluminescence generated by monocytes. Superoxide dismutase in a concentration which impaired chemiluminescence also impaired the staphylococcal killing by monocytes. Hexose monophosphate shunt activity and hydrogen peroxide production by granulocytes and monocytes were also evaluated. The oxidation of [1-14C]glucose was used as a measure of hexose monophosphate shunt activity and the oxidation of [14C]formate as an estimation of hydrogen peroxide production. The oxidation of both substrates by monocytes was increased during phagocytosis but, in contrast to results in granulocytes, was not further increased by the addition of superoxide dismutase. These data indicate that superoxide may be important in bactericidal activity of human monocytes. Our results also suggest that the metabolism of oxygen radicals in monocytes and granulocytes may be different.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Baehner R. L., Murrmann S. K., Davis J., Johnston R. B., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A. A., Sagone A. L., Jr, Metz E. N., Balcerzak S. P. Relationship of glucose oxidation to aggregation of human platelets. Blood. 1973 Feb;41(2):249–258. [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The production of superoxide radical during the decomposition of potassium peroxochromate(V). Biochemistry. 1974 Aug 27;13(18):3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller C. A., King G. W., Hurtubise P. E., Sagone A. L., LoBuglio A. F. Characterization of glass adherent human mononuclear cells. J Immunol. 1973 Nov;111(5):1610–1612. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Metz E. N., Sagone A. L., Jr The effect of copper on the erythrocyte hexose monophosphate shunt pathway. J Lab Clin Med. 1972 Sep;80(3):405–413. [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- Prasad A. S., DuMouchelle E., Koniuch D., Oberleas D. A simple fluorometric method for the determination of RNA and DNA in tissues. J Lab Clin Med. 1972 Oct;80(4):598–602. [PubMed] [Google Scholar]
- Rinehart J. J., Balcerzak S. P., Sagone A. L., LoBuglio A. F. Effects of corticosteroids on human monocyte function. J Clin Invest. 1974 Dec;54(6):1337–1343. doi: 10.1172/JCI107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. J., Sagone A. L., Balcerzak S. P., Ackerman G. A., LoBuglio A. F. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975 Jan 30;292(5):236–241. doi: 10.1056/NEJM197501302920504. [DOI] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Balcerzak S. P., Metz E. N. The response of red cell hexose monophosphate shunt after sulfhydryl inhibition. Blood. 1975 Jan;45(1):49–54. [PubMed] [Google Scholar]
- Sagone A. L., Jr, LoBuglio A. F., Balcerzak S. P. Alterations in hexose monophosphate shunt during lymphoblastic transformation. Cell Immunol. 1974 Dec;14(3):443–452. doi: 10.1016/0008-8749(74)90195-6. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Metz E. N., Balcerzak S. P. Effect of inorganic phosphate on erythrocyte pentose phosphate pathway activity. Biochim Biophys Acta. 1972 Jan 28;261(1):1–8. doi: 10.1016/0304-4165(72)90306-6. [DOI] [PubMed] [Google Scholar]
- Sbarra A. J., Paul B. B., Jacobs A. A., Strauss R. R., Mitchell G. W., Jr Biochemical aspects of phagocytic cells as related to bactericidal function. J Reticuloendothel Soc. 1972 May;11(5):492–502. [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Smith A. L. Lipid peroxidation by human blood phagocytes. J Clin Invest. 1974 Sep;54(3):638–645. doi: 10.1172/JCI107801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D. Role of superoxide radicals in the lipid peroxidation of intracellular membranes. FEBS Lett. 1975 Mar 1;51(1):180–183. doi: 10.1016/0014-5793(75)80882-9. [DOI] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcester J. The statistical method. N Engl J Med. 1966 Jan 6;274(1):27–36. doi: 10.1056/NEJM196601062740106. [DOI] [PubMed] [Google Scholar]



