Abstract
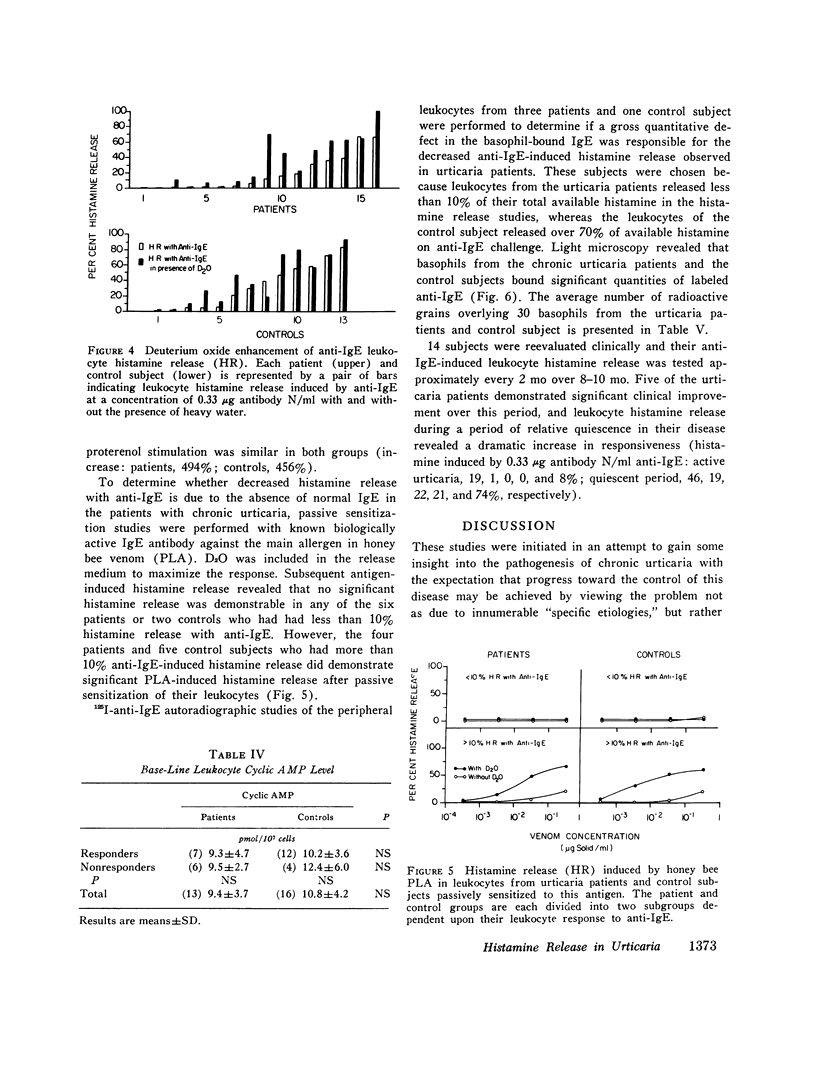
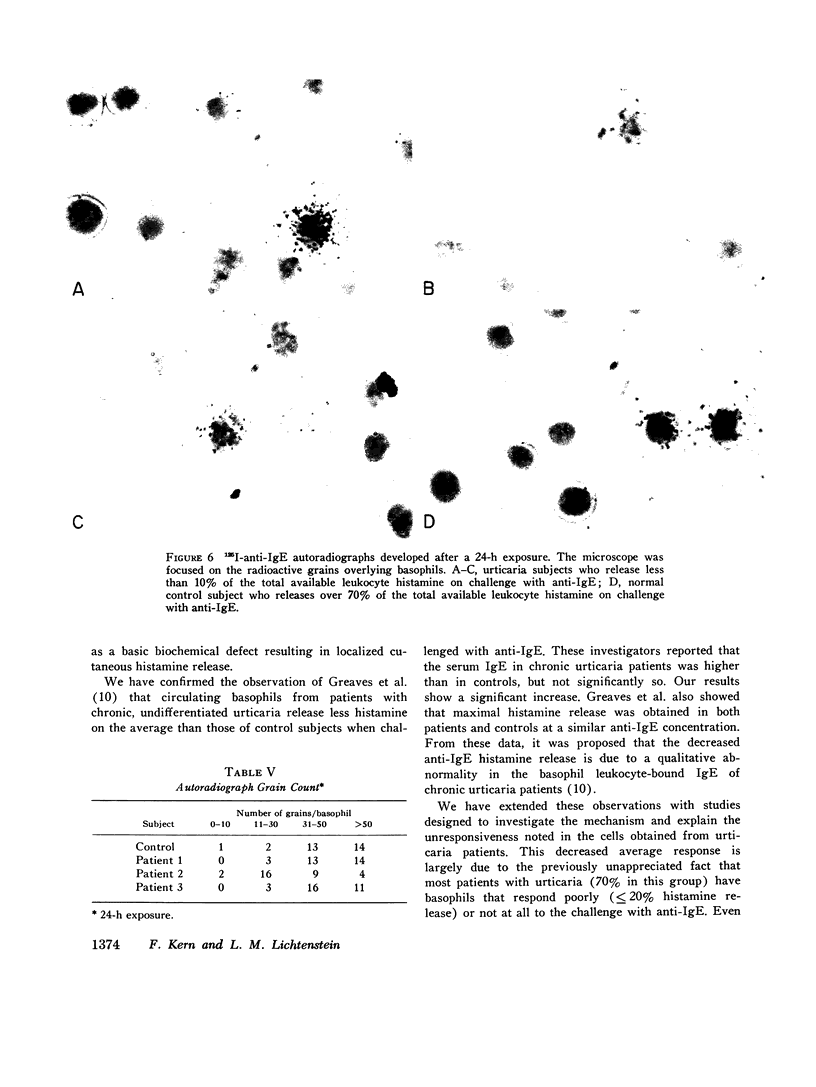
Histamine release from peripheral blood leukocytes challenged with anti-human IgE was studied in patients with chronic urticaria and nonatopic controls. 19 of 23 controls, but only 6 of 20 patients, released over 20% of the total available leukocyte histamine. The response to anti-IgE concentrations of 1.66, 0.33, 0.066, and 0.013 mug antibody N/ml was significantly lower in patients than in controls. Serum IgE levels were significantly higher in the patients but total histamine content of about 10(7) leukocytes was not. Deuterium oxide (D2O) greatly increased histamine release (in both groups), indicating that the anti-IgE interacted with the basophils of urticaria patients. Passive sensitization of leukocytes with biologically active IgE was achieved in both patients and control subjects whose cells responded to anti-IgE, but was not achieved in either patients or control subjects whose cells were nonresponsive to anti-IgE challenge. 125I-anti-IgE autoradiographic studies revealed no obvious quantitative abnormality in the amount of basophil-bound IgE in chronic urticaria patients. Ionophore stimulation of aliquots of the same leukocytes used for anti-IgE challenge demonstrated that the urticaria patients' basophils were capable of releasing normal amounts of histamine. Leukocyte cyclic AMP levels in the two groups were not significantly different either in base-line levels or in responsiveness to stimulation with isoproterenol. These data indicate that chronic urticaria patients have a (acquired?) defect in leukocyte histamine release that occurs after the anti-IgE-IgE interaction, but before the actual (second-stage) release process, and that is comparable to the phenomenon of desensitization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. H., Adamik R. Control of histamine release: effects of various conditions on rate of release and rate of cell desensitization. J Immunol. 1975 Mar;114(3):1034–1041. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie E., Lichtenstein L. M. Histamine release from human leukocytes: studies with deuterium oxide, colchicine, and cytochalasin B. J Clin Invest. 1972 Nov;51(11):2941–2947. doi: 10.1172/JCI107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. W., Plummer V. M., McLaughlan P., Stanworth D. R. Serum and cell bound IgE in chronic urticaria. Clin Allergy. 1974 Sep;4(3):265–271. doi: 10.1111/j.1365-2222.1974.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970 Aug;7(8):687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., De Bernardo R., Tomioka H., Lichtenstein L. M., Ishizaka K. Identification of basophil granulocytes as a site of allergic histamine release. J Immunol. 1972 Apr;108(4):1000–1008. [PubMed] [Google Scholar]
- Ishizaka T., Soto C. S., Ishizaka K. Mechanisms of passive sensitization. 3. Number of IgE molecules and their receptor sites on human basophil granulocytes. J Immunol. 1973 Aug;111(2):500–511. [PubMed] [Google Scholar]
- Ishizaka T., Tomioka H., Ishizaka K. Degranulation of human basophil leukocytes by anti-gamma E antibody. J Immunol. 1971 Mar;106(3):705–710. [PubMed] [Google Scholar]
- Juhlin L., Johansson G. O., Bennich H., Högman C., Thyresson N. Immunoglobulin E in dermatoses. Levels in atopic dermatitis and urticaria. Arch Dermatol. 1969 Jul;100(1):12–16. doi: 10.1001/archderm.100.1.12. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XIV. Passive sensitization in vitro of human leukocytes to ragweed pollen antigen. J Immunol. 1966 Aug;97(2):203–212. [PubMed] [Google Scholar]
- Lichtenstein L. M., Levy D. A. Is desensitization' for ragweed hay fever immunologically specific? Int Arch Allergy Appl Immunol. 1972;42(4):615–626. doi: 10.1159/000230642. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Levy D. A., Ishizaka K. In vitro reversed anaphylaxis: characteristics of anti-IgE mediated histamine release. Immunology. 1970 Nov;19(5):831–842. [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M. The immediate allergic response: in vitro separation of antigen activation, decay and histamine release. J Immunol. 1971 Oct;107(4):1122–1130. [PubMed] [Google Scholar]
- Lichtenstein L. M. The mechanism of basophil histamine release induced by antigen and by the calcium ionophore A23187. J Immunol. 1975 Jun;114(6):1692–1699. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J. C., KRELL R. A STUDY OF THE CUTANEOUS EFFECTS OF BRADYKININ. J Invest Dermatol. 1964 Sep;43:177–180. [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Smith J. W. Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to -adrenergic agents. J Clin Invest. 1973 Jan;52(1):48–59. doi: 10.1172/JCI107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RORSMAN H. Basophilic leucopenia in different forms of urticaria. Acta Allergol. 1962;17:168–184. doi: 10.1111/j.1398-9995.1962.tb02937.x. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- SPARROW E. M., WILHELM D. L. Species differences in susceptibility to capillary permeability factors: histamine, 5-hydroxytrytamine and compound 48/80. J Physiol. 1957 Jun 18;137(1):51–65. doi: 10.1113/jphysiol.1957.sp005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S. Urticaria and angioedema. Ann Intern Med. 1968 Aug;69(2):361–380. doi: 10.7326/0003-4819-69-2-361. [DOI] [PubMed] [Google Scholar]