Abstract
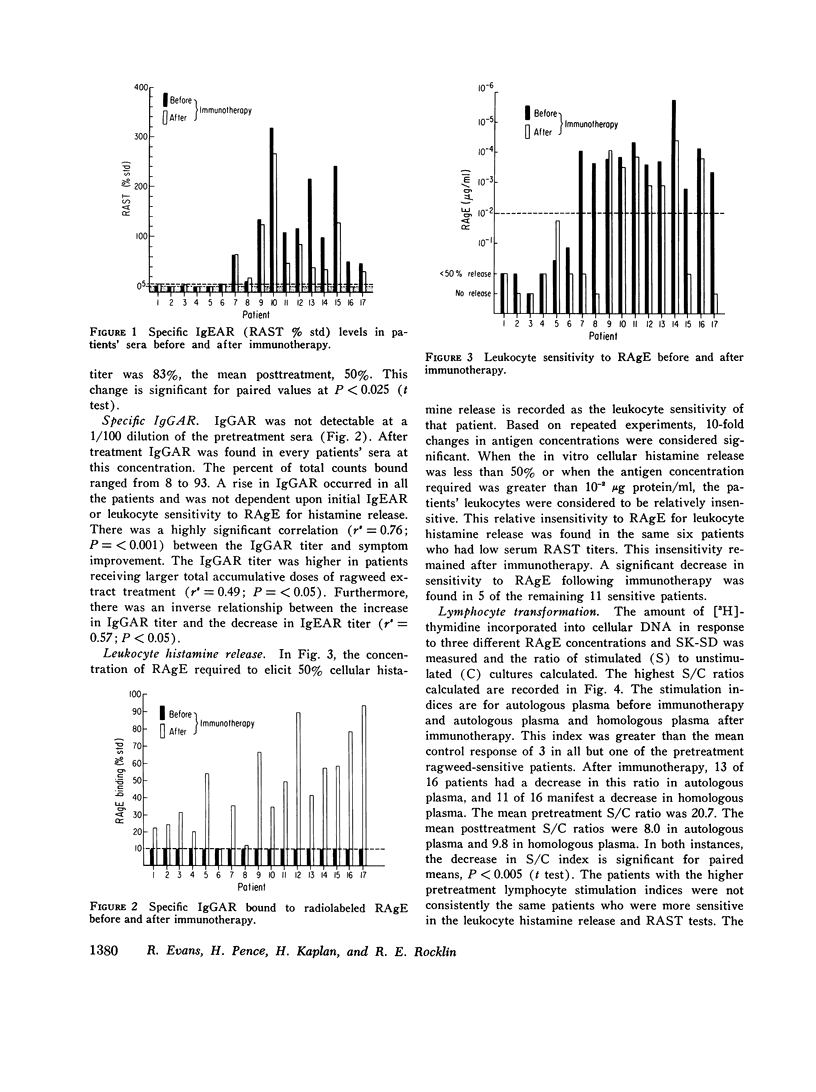
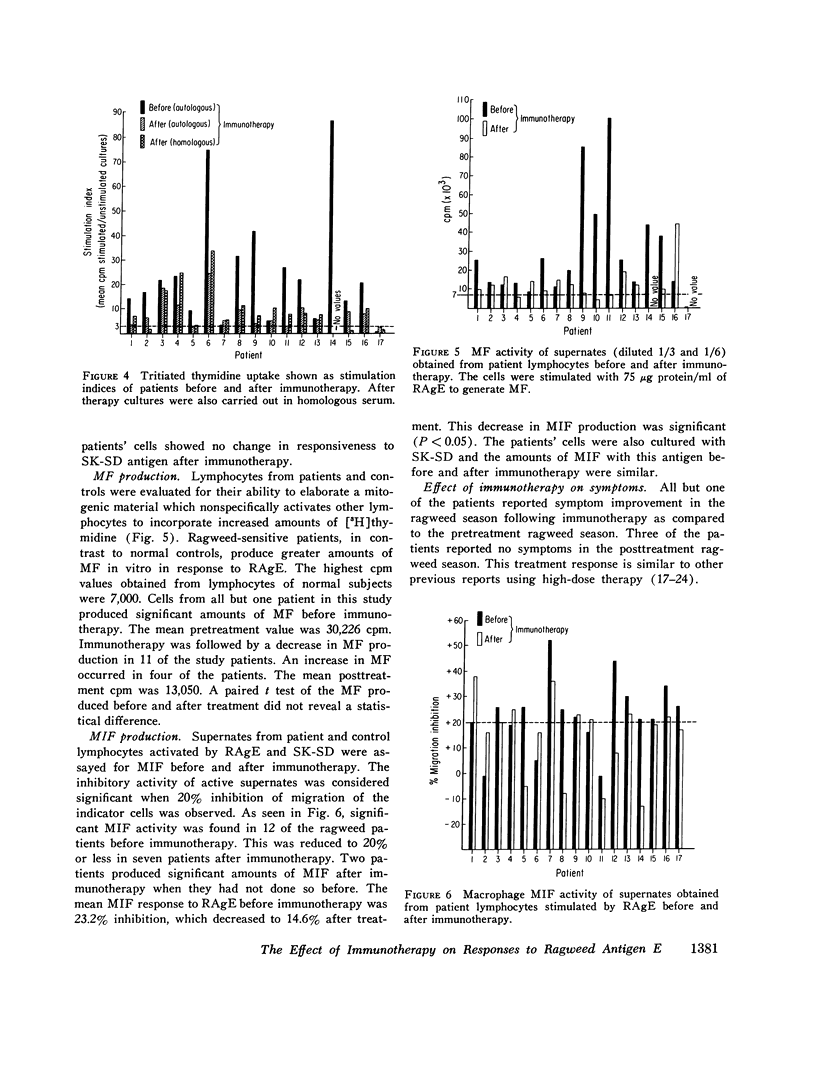
The effect of specific immunotherapy on several in vitro responses to ragweed antigen E has been evaluated in 17 atopic patients with ragweed hayfever. The methods employed were leukocyte histamine release, measurement of specific IgE anti-ragweed antibody and specific IgG anti-ragweed antibody, lymphocyte proliferation, and the production of two lymphocyte mediators (migration inhibitory factor and mitogenic factor). The duration of treatment and symptom improvement were also recorded for comparison. Immunotherapy was associated with a decrease in leukocyte sensitivity for histamine release to ragweed antigen E in a majority of the patients. In addition, there was a significant decrease in IgE anti-ragweed antibody and a significant increase in IgG anti-ragweed antibody. Immunotherapy also resulted in a significant decrease in lymphocyte responsiveness to ragweed antigen E as measured by proliferation and the production of mediators. Symptomatic improvement was best correlated with the presence of IgG anti-ragweed antibody responses. The production of this antibody was also associated with a decrease in lymphocyte responsiveness. The results of this study indicate that specific immunotherapy in ragweed-sensitive patients induces alterations in immunologic reactivity to ragweed antigen in vitro. This response is antigen specific, includes elements of both humoral and cellular immunity, and may account for the clinical improvement that is often observed in patients who undergo this form of therapy.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Lane S. R., Howard W. A., Leiken S., Oppenheim J. J. The effect of hyposensitization on alternaria-induced lymphocyte blastogenesis. Cell Immunol. 1974 Mar 15;10(3):442–449. doi: 10.1016/0008-8749(74)90135-x. [DOI] [PubMed] [Google Scholar]
- Brostoff J., Roitt I. M. Cell-mediated (delayed) hypersensitivity in patients with summer hay-fever. Lancet. 1969 Dec 13;2(7633):1269–1272. doi: 10.1016/s0140-6736(69)90807-1. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatien J. G., Merler E., Colten H. R. Allergy to ragweed antigen E: effect of specific immunotherapy on the reactivity of human T lymphocytes in vitro. Clin Immunol Immunopathol. 1975 May;4(1):32–37. doi: 10.1016/0090-1229(75)90036-7. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Girard J. P., Rose N. R., Kunz M. L., Kobayashi S., Arbesman C. E. In vitro lymphocyte transformation in atopic patients: induced by antigens. J Allergy. 1967 Feb;39(2):65–81. doi: 10.1016/0021-8707(67)90113-x. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Biological function of gamma E antibodies and mechanisms of reaginic hypersensitivity. Clin Exp Immunol. 1970 Jan;6(1):25–42. [PMC free article] [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S., CONNELL J. T. ISOLATION AND CHARACTERIZATION OF ALLERGENS FROM RAGWEED POLLEN. II. Biochemistry. 1964 Mar;3:458–468. doi: 10.1021/bi00891a026. [DOI] [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S. Isolation studies of allergens from regweed pollen. Biochemistry. 1962 Jul;1:709–720. doi: 10.1021/bi00910a027. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The function and interrelationships of T-cell receptors, Ir genes and other histocompatibility gene products. Transplant Rev. 1975;22:175–195. doi: 10.1111/j.1600-065x.1975.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Ishizaka K. Regulation of antibody response in vitro. V. Effect of carrier-specific Helper cells on generation of hapten-specific memory cells of different immunoglobulin classes. J Immunol. 1973 Jul;111(1):1–9. [PubMed] [Google Scholar]
- Levy D. A., Lichtenstein L. M., Goldstein E. O., Ishizaka K. Immunologic and cellular changes accompanying the therapy of pollen allergy. J Clin Invest. 1971 Feb;50(2):360–369. doi: 10.1172/JCI106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Ishizaka K., Norman P. S., Sobotka A. K., Hill B. M. IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest. 1973 Feb;52(2):472–482. doi: 10.1172/JCI107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L. Clinical and in vitro studies on the role of immunotherapy in ragweed hay fever. Am J Med. 1968 Apr;44(4):514–524. doi: 10.1016/0002-9343(68)90052-1. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L., Osler A. G. In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization. J Clin Invest. 1966 Jul;45(7):1126–1136. doi: 10.1172/JCI105419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell F. C., Franklin W. A double-blind study of the effectiveness and specificity of injecton therapy in ragweed hay fever. N Engl J Med. 1965 Sep 23;273(13):675–679. doi: 10.1056/NEJM196509232731302. [DOI] [PubMed] [Google Scholar]
- Maini R. N., Dumonde D. C., Faux J. A., Hargreave F. E., Pepys J. The production of lymphocyte mitogenic factor and migration-inhibition factor by antigen-stimulated lymphocytes of subjects with grass pollen allergy. Clin Exp Immunol. 1971 Oct;9(4):449–465. [PMC free article] [PubMed] [Google Scholar]
- Massie F. S., Wutanasupta R. Detection of delayed hypersensitivity in ragweed pollinosis by an in vitro method. Ann Allergy. 1972 Dec;30(12):676–684. [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. A proposal that one in vivo function of t cells is to regulate the availability of antigen for b cells. Transplant Rev. 1975;23:119–125. doi: 10.1111/j.1600-065x.1975.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Norman P. S. A rational approach to desensitization. J Allergy. 1969 Sep;44(3):129–145. doi: 10.1016/0021-8707(69)90137-3. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Lichtenstein L. M. Capacity of purified antigens and whole pollen extracts to release histamine from leukocytes of hay fever patients. J Allergy Clin Immunol. 1973 Aug;52(2):94–104. doi: 10.1016/0091-6749(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Winkenwerder W. L., Lichtenstein L. M. Immunotherapy of hay fever with ragweed antigen E: comparisons with whole pollen extract and placebos. J Allergy. 1968 Aug;42(2):93–108. doi: 10.1016/0021-8707(68)90139-1. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. 3. Effect of thymectomy and splenectomy. J Immunol. 1971 Apr;106(4):1019–1025. [PubMed] [Google Scholar]
- Richter M., Naspitz C. K. The in vitro blastogenic response of lymphocytes of ragweed-sensitive individuals. J Allergy. 1968 Mar;41(3):140–151. doi: 10.1016/0021-8707(68)90054-3. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Meyers O. L., David J. R. An in vitro assay for cellular hypersensitivity in man. J Immunol. 1970 Jan;104(1):95–102. [PubMed] [Google Scholar]
- Rocklin R. E., Pence H., Kaplan H., Evans R. Cell-mediated immune response of ragweed-sensitive patients to ragweed antigen E. In vitro lymphocyte transformation and elaboration of lymphocyte mediators. J Clin Invest. 1974 Mar;53(3):735–744. doi: 10.1172/JCI107612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Biliotti G., Ricci M. Depression of grass pollen-induced lymphocyte transformation by serum from hyposensitized patients. Clin Exp Immunol. 1975 Jan;19(1):83–91. [PMC free article] [PubMed] [Google Scholar]
- Sadan N., Rhyne M. B., Mellits E. D., Goldstein E. O., Levy D. A., Lichtenstein L. M. Immunotherapy of pollinosis in children: investigation of the immunologic basis of clinical improvement. N Engl J Med. 1969 Mar 20;280(12):623–627. doi: 10.1056/NEJM196903202801201. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Wide L., Bennich H., Johansson S. G. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967 Nov 25;2(7526):1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]



