Abstract
Background
Data on the prevalence of dementia in India with a large and aging population is scant. We studied prevalence of AD and dementia in Kerala, South India, and effects of age, education and gender on it.
Methods
2-phase survey on 2466 individuals aged ≥55 years living in community. Men constituted 41%, < 75 years age in 76.9% and education ≥4 years in 69.6%. Screening (Phase I) using the instrumental activity of daily living scale for the elderly (IADL-E) and the Addenbrooke’s cognition examination (ACE). Diagnostic-assessment (Phase II) was in 532 screen-positives and 247 (10%) screen-negatives.
Results
93 (3.77%) ≥55 years and 81 (4.86%) ≥65 years of age had dementia. Age adjusted (against US-population in 2000) dementia (and AD) rates were 4.86% (1.91%) in age ≥55 years and 6.44% (3.56%) in ≥65 years. Odds for dementia (and AD) were high with increasing-age 5.89 (15.33) in 75–84, 13.23 (25.92) ≥85 years, and in women 1.62 (2.95); and low 0.27 (0.16) if education was ≥9 years. Age and low education increased dementia. Age and female gender increased AD.
Conclusion
Prevalence of dementia and AD is higher than any reported from the subcontinent suggesting that dementia in Kerala in South India is not uncommon. Increasing age increased dementia and AD. Low-education is associated with dementia and female-gender with AD.
Keywords: dementia, Alzheimer’s disease, vascular dementia, prevalence, India, Addenbrooke’s cognitive examination, instrumental activities of daily living
Introduction
By year 2025, developing countries will account for the majority of the elders in the world, China and India accounting for nearly a fifth (World Health Organisation, 1998) Increasing population and life-expectancy will increase the numbers at risk for dementia, particularly in nations with large population (Ferri et al., 2005; Kalaria et al., 2008). Studies estimating dementia prevalence in India are few (Shaji et al., 1996; Rajkumar et al., 1997; Chandra et al., 1998; Vas et al., 2001; Shaji et al., 2005). Culture and education influences the performance on the neuropsychological tests (Ganguli, 1997; Fillenbaum et al., 1999). Thus, culturally and linguistically fair tests with norms from the local population are required for estimating the true prevalence.
In 2001 we initiated a longitudinal study- ‘cognition in older adults in Trivandrum’ (COAT), in community elders in Kerala, South India. The study, including the informed-consent procedure, was approved by the institute ethics committee. Informed consent was sought verbally from the subject and the head of the family. Verbal consenting was resorted to for the interviews and neuropsychological tests as we anticipated a proportion of the subjects to be unable to read or write and sign. However, in subjects in whom the study team thought it necessary to have additional tests like the blood and/or neuroimaging investigations in Phase II of the study (see below), written consent was obtained. As a token of appreciation for their participation in the study the study team would organise for the participants and their families a free annual general medical-camp in the community centres of the locality. Tools for this study were either developed locally (Mathuranath et al., 2005) or standard-tools were subjected to linguistic and cultural modifications (Mathuranath et al., 2004) for a culture—and language—fair evaluation. We generated population-based norms on them, derived appropriate cut-off scores and validated them for dementia diagnosis (Mathuranath et al., 2007). Data analysed in this study was collected in 2004. Our objective was to estimate the point prevalence of AD and dementia and to study the effect of demographic factors on it.
Methodology
The study was conducted in Trivandrum (population = 524,006), in the state of Kerala (population = 31,838,619). Individuals aged ≥60 years constitute 8.6% of the population, life-expectancy at birth is 73.5 years, and literacy is more than 70% (Director of Census Operations Kerala, 2001). Sampling frame consisted of 41920 subjects from four of the eight wards (administrative districts of the city corporation) of Trivandrum. The four selected wards included a costal district (populated by the economically poorer fisher-folk communities that utilise the federal health services facilities less often and are also a religious minority), a commercial district (populated by progressive, well-educated and economically well-off communities) and an inner-city district populated by moderately-educated families that have migrated decades or a generation ago from rural areas of Kerala or neighbouring states. Residents of these four wards provided a good admixture and faithful representation of the socio-economically and culturally diverse population of Trivandrum. The census information and the Election Commission’s database identified 2932 individuals to be ≥55 years of age. A door-to-door survey in the selected wards revealed that 68 were < 55 years, 112 had died, 62 had relocated outside the study area, and 224 (8.32%) refused consent. Thus, 2466 subjects were alive, residing in the study area, age-confirmed and willing to participate in the study. The sample’s mean age was 68.79 ± 8.0 and education 8.14 ± 5.6 years. Men constituted 40.6%, 23% were ≥75 years of age, and 31.4% had ≤4 years of formal-education. Hindus constituted 69.8%, Christians 24.4%, and Muslims 5.8%. Self-declared monthly income was ≤1000 Indian National Rupees (Rs.) (~ 22 US $) in 35%, and ≥ Rs. 10,001 (≥ 223 US $) in 2%. In work types, unskilled labourers constituted 12.4%, clerks/vocation practitioners 29.2%, professionals 14.8%, and housewives 43.6%.
Phase I (screening)
Qualified medical social workers and psychologists were provided 3 weeks training in the institute in administering the various survey tools (described below) which included the demographic questionnaire and the neuropsychological batteries and the visual and auditory screening tests. As field-workers they interviewed the participant and families in their homes using a structured-schedule that included symptoms and demographic data. They administered a bed-side auditory and a visual screening (which included a hand-held Snellen’s chart and checking if they could hear a taped music) to check if any auditory—or visual—handicap could interfere with performance on the neuropsychological tests. The data collected by field-workers was reviewed periodically in a consensus meeting by the study team consisting of neurologists, neuropsychiatrists and neuropsychologist in the institute to arrive at the diagnosis.
Cognitive screening battery. We used the Addenbrooke’s cognition examination (ACE) (Mathuranath et al., 2000), a cognition screening battery similar to the CERAD screening battery. Briefly, it consists of the global scale of MMSE, tests for episodic memory (immediate and delayed recall of a seven-item address list), verbal fluency (initial letter P and categories of animals), confrontation naming (10 items), constructional praxis (copying line-drawing of wire-cube), the clock-drawing test, tests for language and remote memory. We adapted it into Malayalam (m-ACE with m-MMSE) and standardised it on the local Malayalam-speaking population (Mathuranath et al., 2004). Education-stratified population based norms were derived on them (Mathuranath et al., 2007).
Activities of daily living. An instrumental activities of daily living scale for the elderly (IADL-E) was specifically developed for the local elders (Mathuranath et al., 2005). Briefly, it consisted of items evaluating the ADL domains of cognitive, social/recreational, community, household and self-care activities. The IADL-E grades the performance of the subject on each task and provides a cognitive disability index (CDI) based on the number of activities applicable to the subject. It has robust psychometric properties and is validated for dementia in the local population (Mathuranath et al., 2005).
As a cut-off for dementia the 5th percentile education-stratified m-ACE composite score, provided a sensitivity of 0.85 and a specificity of 0.95 against a consensus diagnosis of dementia based on clinical, neuropsychological and investigational work-up. When combined with IADL-E the sensitivity increased to 0.95.
Phase II (diagnostic confirmation)
All subjects who in Phase I evaluation scored ≤20th percentile on the education-stratified population norms (Mathuranath et al., 2007) on either the m-ACE (≤33 for 0 years education, ≤45 for 1–4 years, ≤50 for 5–8 years, ≤70 for 9–12 years, ≤79 for >12 years education) or the m-MMSE (≤13 for 0 years education, ≤16 for 1–4 years, ≤19 for 5–8 years, ≤24 for 9–12 years, ≤26 for >12 years education) or ≤16 on the CDI on IADL-E (a score that was validated for dementia detection (Mathuranath et al., 2005)) were considered screen-positives (n = 532, 21.6%). They, along with a randomly selected 10% (n = 247) of the screen-negatives were included in Phase II. Clinical psychologists and neurologists evaluated the selected participants. If subjects failed to complete the evaluations (because of illness/unwillingness/death between the two phases), we conducted a semi-structured intense family interview with available family members to determine their last available neurological status. All these subjects were included in attrition as described below. The evaluations included:
Cognition and behavioural symptoms (over past month) using a standardised structured questionnaire to elicit all cognition and behaviour symptoms (memory, language, attention, visuospatial orientation and neuropsychiatric). When necessary family members were also interviewed.
Clinical evaluation using a brief structured standardised medical history, examination and a bed-side mental status examination.
Neuropsychological evaluation. Qualified psychologists administered a battery of neuropsychological tests. This included the forward and backward digit span, logical memory and memory for designs components of the Weschler’s memory scale (Wechsler, 1987), trail making forms A and B (Reitan, 1958), block design, (Reitan, 1958) verbal fluency (category and initial letter), confrontation naming test (George and Mathuranath, 2005), hospital anxiety and depression scale (Zigmond and Snaith, 1983) and the modified 5-point Barthel index (Hobart and Thompson, 2001).
After evaluating the above examination results of Phase II, in subjects in whom the study team thought it necessary to have further evaluation for reaching a diagnosis, we did a screening blood investigations (including haemogram, thyroid function tests, and vitamin B12 levels) and/or a neuroimaging (a CT scan or an MRI).
Diagnosis was made in a consensus conference involving the neurologist, neuropsychiatrist and psychologists. DSM IV criteria (American Psychiatric Association, 1994) was used for diagnosing dementia, NINCDS–ADRDA criteria (McKhann et al., 1984) for possible or probable AD and Hachinski’s ischemic scale (Hachinski and Bowler, 1993) for vascular dementia (VaD).
Statistical considerations
Attrition was classified as ‘attrition-with-information’ if the intense family interview in Phase II was considered contributory to reach a consensus on the presence or absence of dementia. All other instances were classified as ‘attrition-without-information’.
Age was stratified into 55–64, 65–74, 75–84 and ≥85 years. Education was stratified as 0 (no formal education), 1–4 (primary), 5–8 (high school), 9–12 (higher secondary), ≥12 (university) years. Based on our earlier studies, an education ≤4 year was illiteracy (Mathuranath et al., 2007). Age-specific prevalence was estimated by excluding—(a) all attritions or (b) only attrition-without-information. Age-adjusted rates against US-population in 2000. Groups were compared by t-test. For gender, education and age bivariate-relations explored by Mantel-Haenszel χ2 test and odds ratio estimated. Bivariate logistic-regression models was used for exploring effects of age, education and gender on prevalence.
Results
Phase I
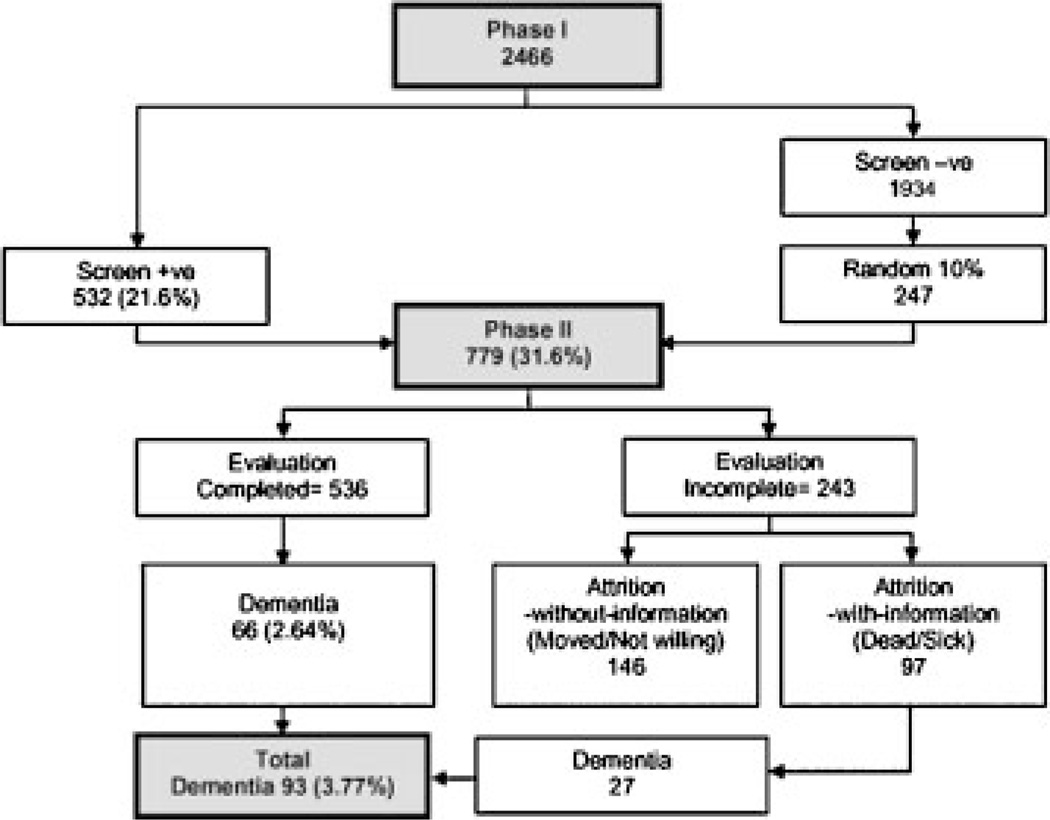
Figure 1 shows the outcome of each phase. Table 1 shows the age-, gender- and literacy-wise breakdown of the entire cohort. The illiterates were significantly older (69.8 ± 8.4 vs. 68.3 ± 7.7; p < .001), and had lower average monthly income (Rs. 1303.4 ± 1843.9 vs. Rs. 4081.8 ± 3627.1; p < .001). Association of illiteracy with older age was significant in women as well as men.
Figure 1.
Flow chart of the two phases and its outcomes.
Table 1.
Age, gender and education distribution of the cohort
| Age (years) | Male | Female | Total | ||||
|---|---|---|---|---|---|---|---|
| Education | Total | Education | Total | ||||
| ≤ 4 years | > 4 years | ≤ 4 years | > 4 years | ||||
| Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | Count (%) | |
| 55–59 | 0 | 9 (100) | 9 (0.9) | 3 (8.6) | 32 (91.4) | 35 (2.4) | 44 (1.8) |
| 60–64 | 51 (17.8) | 235 (82.2) | 286 (28.5) | 159 (34.3) | 305 (65.7) | 464 (31.7) | 750 (30.4) |
| 65–69 | 43 (15.1) | 242 (84.9) | 285 (28.4) | 148 (41.2) | 211 (58.8) | 359 (24.5) | 644 (26.1) |
| 70–74 | 46 (24.1) | 145 (75.9) | 191 (19.0) | 111 (41.3) | 158 (58.7) | 269 (18.4) | 460 (18.7) |
| 75–79 | 34 (28.3) | 86 (71.7) | 120 (11.9) | 88 (46.1) | 103 (53.9) | 191 (13.1) | 311 (12.6) |
| 80–85 | 14 (24.1) | 44 (75.9) | 58 (5.8) | 41 (47.1) | 46 (52.9) | 87 (5.9) | 145 (5.9) |
| 85+ | 18 (33.3) | 36 (66.7) | 54 (5.4) | 37 (63.8) | 21 (36.2) | 58 (3.9) | 112 (4.5) |
| 55+ | 206 (20.5) | 797 (79.5) | 1003 (100) | 587 (40.1) | 876 (59.9) | 1463 (100) | 2466 (100) |
| 65+ | 155 (21.9) | 553 (78.1) | 708 (70.6) | 425 (44.1) | 539 (55.9) | 964 (65.9) | 1672 (67.8) |
Phase II
Excluding all attrition
Phase II completed in 536 and 66 had dementia, including five (2%, 5/247) Phase I screen negative.
Including attrition-with-information
Phase II could not be completed in 243 (78 dead, 9 sick or aphasic, 120 relocated, 26 unwilling). Intense family interview was completed in 171, data was contributory (attrition with- information) in 97 and dementia diagnosed in 27 (in nine of whom scrutiny of copies of hospital records available with family showed them as having been medically diagnosed as having dementia before their death). Data from Phase I showed that these 27 were comparable on education (6.3 ± 4.6 vs. 5.5 ± 5.2), but were younger (73.1 ± 10.1 vs. 77.6 ± 8.8), had lower (p < .01) m-ACE (21.7 ± 18.9 vs. 39.2 ± 16.9), and m- MMSE (7.0 ± 6.7 vs. 14.4 ± 6.3) scores than the 66 who underwent complete Phase II evaluation before receiving a diagnosis of dementia. Thus, in all, 93 (66 + 27) had dementia.
Subtypes of dementia
The subtyping of dementia completed in 67 (including 66 completing Phase II) of 93. Probable or possible AD was diagnosed in 47 (71.3%), VaD in 11 (16.6%) and other dementias in eight (12.1%). Six (54.5%) VaD and 38 (81%) AD were women. In women and men AD (and VaD) accounted for 79.2% (12.5%) and 50% (27.8%) of all dementias, respectively. Comparison of AD (n = 47) with VaD (n = 11) showed that the AD were older (78.1 ± 8.3 vs. 69.3 ± 10.9) than VaD. The extent of dementia was a shade greater in VaD than in AD as indicated by CDR Box scores (8.3 ± 4.0 vs. 6.5 ± 4.6), IADL-CDI (48.8 ± 28.1 vs. 45.6 ± 33.8) and m-ACE (34.8 ± 26.8 vs. 38.2 ± 16.4) scores. Expectedly the depression score on HADS was also more for VaD (34.8 ± 26.8) than for AD (6.5 ± 4.9).
Prevalence
After excluding all attrition, the crude prevalence rate is (66/2466) 2.64%, (95% CI: 2.01–3.27) and the age-adjusted rate in age ≥55 years 2.95% (95% CI 2.28–3.62). Including attrition-with-information the crude (and the age-adjusted) rate is 3.77 (4.1) for dementia and 1.91 (1.91) for AD in age ≥55 years and 4.86 (6.44) for dementia and 2.64 (3.56) for AD in age ≥65 years (Table 2).
Table 2.
Prevalence rates of all dementia and AD (figures in parentheses). Break-up across gender and literacy
| Age (years) | Count | Crude rates | Males | Females | ≤ 4 years edu. | > 4 years edu. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | Count | % | Count | % | Count | % | Count | % | ||
| 55–59 | 0/44 | 0/9 | 0/35 | 0/3 | 0/41 | ||||||
| 60–64 | 12/750 (3) |
1.60 (0.4) |
0.70–2.50 (−0.05–0.85) |
5/286 (1) |
1.7 (0.4) |
7/464 (2) |
1.5 (0.4) |
8/210 (2) |
3.8 (0.9) |
4/540 (1) |
0.7 (0.2) |
| 65–69 | 11/644 (4) |
1.56 (0.62) |
0.60–2.52 (0.01–1.23) |
5/285 (2) |
1.8 (0.7) |
6/359 (2) |
1.7 (0.6) |
5/191 (2) |
2.6 (1.1) |
6/453 (2) |
1.3 (0.4) |
| 70–74 | 13/460 (5) |
2.62 (1.09) |
1.16–4.08 (0.14–2.04) |
4/191 (0) |
2.1 | 9/269 (5) |
3.3 (1.9) |
4/157 (2) |
2.5 (1.3) |
9/303 (3) |
3.0 (0.9) |
| 75–79 | 20/311 (14) |
6.77 (4.52) |
3.98–9.57 (2.20–6.83) |
6/120 (2) |
5 (1.67) |
14/191 (12) |
7.3 (6.3) |
9/122 (7) |
7.4 (5.74) |
11/189 (7) |
5.8 (3.70) |
| 80–84 | 18/145 (11) |
12.33 (7.53) |
7.0–17.66 (3.25–11.82) |
2/58 (1) |
3.4 (1.72) |
16/87 (10) |
18.4 (11.5) |
6/55 (5) |
10.9 (9.09) |
12/90 (6) |
13.3 (6.67) |
| 85+ | 19/112 (10) |
17.70 (8.85) |
10.66–24.74 (3.61–14.09) |
6/54 (3) |
11.1 (5.56) |
13/58 (7) |
22.4 (12.1) |
11/55 (5) |
20 (9.09) |
8/57 (5) |
14 (8.87) |
| 55+ | 93/2466 (47) |
3.77 (1.91) |
3.02–4.52 (1.37–2.45) |
28/1003 (9) |
2.79 (0.9) |
65/1463 (38) |
4.44 (2.59) |
43/793 (23) |
5.4 (2.9) |
50/1673 (24) |
3.0 (1.43) |
| 65+ | 81/1672 (44) |
4.86 (2.64) |
3.83–5.89 (1.87–3.41) |
23/708 (8) |
3.25 (1.13) |
58/964 (36) |
5.02 (3.73) |
35/580 (21) |
6.0 (3.62) |
46/1092 (23) |
4.2 (2.11) |
Dementia and age, education and gender
Table 3 shows the odds-ratio for prevalent dementia and AD. Greater age (≥75 years), lower education (≤8 years) and female gender were associated with higher odds for dementia and AD. In logistic regression, age (beta = 0.1, df = 1, p < .001) and education (beta = −0.05, df = 1, p = .028) with dementia and age (beta = 0.12, df = 1, p < .001) and gender (beta = 1.12, df = 1, p = .005) with AD showed significant association. Results were unchanged even after excluding dementias other than AD. Age, gender and education, interactions were insignificant and hence excluded from the model.
Table 3.
Odds ratio for the various demographic factors for prevalent dementia and prevalent AD
| All dementias | AD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | p | Odds ratio (95% CI) | χ2 | p | Odds ratio (95% CI) | ||||
| Education (in years) | 0 | 1 | 1 | ||||||
| 1–4 | 1.81 | 0.18 | 0.65 | (0.35–1.22) | 1.53 | 0.216 | 0.59 | (0.25–1.36) | |
| 5–8 | .04 | 0.84 | 0.95 | (0.54–1.65) | 0.71 | 0.79 | 0.90 | (0.43–1.89) | |
| 9–12 | 15.52 | <0.001 | 0.27 | (0.13–0.54) | 13.17 | <0.001 | 0.16 | (0.53–0.49) | |
| >12 | 14.71 | <0.001 | 0.26 | (0.12–0.54) | 9.03 | 0.003 | 0.23 | (0.83–0.65) | |
| Age (in years) | 55–64 | 1 | 1 | ||||||
| 65–74 | 1.07 | 0.3 | 1.44 | (0.72–2.90) | 1.43 | 0.232 | 2.18 | (0.59–8.03) | |
| 75–84 | 34.76 | <0.001 | 5.89 | (3.04–11.39) | 34.54 | <0.001 | 15.33 | (4.60–51.07) | |
| >=85 | 70.33 | <0.001 | 13.23 | (6.22–28.12) | 50.88 | <0.001 | 25.92 | (7.02–95.72) | |
| Gender | Male | 1 | 1 | ||||||
| Female | 4.47 | 0.04 | 1.62 | (1.03–2.54) | 9.19 | 0.002 | 2.95 | (1.42–6.12) | |
Discussion
Strength of this study was the use of sensitive screening tools specifically adapted or developed for the local population and well-defined demographic profile of a representative population of Kerala (Director of Census Operations Kerala, 2001). Our major findings are- (i) The prevalence of dementia (and AD) in our population is 3.77% (1.91%) in age ≥55 and 4.86% (2.64%) in ≥65 years, higher than any reported from the Indian subcontinent (Shaji et al., 1996; Rajkumar et al., 1997; Chandra et al., 1998; Vas et al., 2001; de Silva et al., 2003; Shaji et al., 2005), (ii) AD is the major subtype and VaD constitutes a smaller proportion than in other studies(Shaji et al., 1996; Rajkumar et al., 1997; Vas et al., 2001; Shaji et al., 2005), (iii) AD is a decade older and less depressed than the VaD, (iv) Odds for dementia/AD increase with increasing age, lower education and female gender, (v) On prevalent dementia, age and education, and on prevalent AD, age and gender, have independent effect.
Published reports from India show dementia (and AD) prevalence ranging from 0.84 (.63) in the 55-plus in rural North India (Chandra et al., 1998) to 2.44 (1.5) in the 65-plus in urban west (Vas et al., 2001) to 3.5 (1.13) in the 60-plus age-groups in rural South India (Rajkumar et al., 1997). Two earlier reports from Kerala estimated the dementia (and AD) crude prevalence to be 3.39 in age ≥ 60 in rural (Shaji et al., 1996) and 3.36 (1.55) in ≥65 in urban regions (Shaji et al., 2005). The differences in prevalence rates between our and the above reports from west (Vas et al., 2001) and South India(Shaji et al., 1996; Rajkumar et al., 1997; Shaji et al., 2005) are possibly attributable to methodological differences. First, unlike other studies, we adapted/developed cognitive and ADL tools to suit our population culturally and linguistically. Performances on the neuropsychological test, particularly verbal, and relevance of activities of daily living, particularly instrumental, are known to be influenced by cultural and/or linguistic differences. Second, rather than adapt standard western cut-off scores, we derived norms on healthy adults (Mathuranath et al., 2007) and used cut-off scores from it. Third, instead of a single cut-off score for the entire population, we chose education-stratified cut-off scores. The rationale being that educational attainment of our sample was diverse and that education influences performance on the neuropsychological tests, particularly verbal, as shown by our earlier studies (Mathuranath et al., 2003; Mathuranath et al., 2007). Similar findings prompted investigators in China to use education-stratified cut-off scores on cognitive tests (Zhang et al., 1990). Fourth, we explored the causes of attrition, classified it and used the information, wherever available, for estimating prevalence. Attrition is often left unaccounted in many studies and in multi-phased studies this could lead to under-estimation.
Our methodology is akin to that of the Indo-US study (Chandra, 1998) from north India. In contrast to our study (as well as other Indian studies), however, the Indo-US study reported one of the lowest prevalence rates in literature. As opposed to the largely illiterate (73%) rural north Indian population of Ballabhgarh in the Indo-US study, ours is a largely literate (69%), urban and semi-urban population. The earning potential, standards of living, health indices, access to health care, male-female ratio and life-expectancy are all distinctly different in South India in general, and in Kerala in particular, where these indices are far above the national average (Director of Census Operations Kerala, 2001) and often comparable to that in the west. As explained above, the higher dementia prevalence in our study, when compared to other studies reported from India, could be contributed to by methodological differences in the studies. Nevertheless, it could also suggest a truly higher prevalence of dementia in this part of India, contributed to by its better living standards and health indices. It is also possible that the m-ACE and the IADL-E have greater sensitivity in detecting dementia in this population when compared to some of the scales used by earlier studies.
We also compared our results with that from neighbouring nations. In age ≥65 our rate of 4.86% is comparable to the 4.6% reported from Shanghai in China (Zhang et al., 1990), and marginally higher than the 3.92% reported from Sri Lanka (de Silva et al., 2003). The fraction of dementia subjects with AD (71%), however, was comparable to that reported from Sri Lanka (71%) and China (65%). Both these studies report a systematic 2-phase survey using education-stratified cut-off scores derived on the local population and use the same dementia classification criteria as in our study.
Although the differences were not large, the odds for dementia (and AD) were significantly lower with more than 8 years of education. Interestingly, the EURO-DEM pooled analysis (Launer et al., 1999) and the Canadian study of health and aging also reported that 8 or more years of education reduced the risk of incident dementia and AD, respectively (Lindsay, 1994). We also found that women were one and half times more at risk for developing dementia than men, a finding similar to that in other reports (Shaji et al., 1996). Age significantly influenced the prevalence rates of both, dementia and AD. In contrast, education only influenced prevalent dementia and gender only influenced prevalent AD.
VaD in our study accounted for a much lower fraction of dementia than 22–58% reported in other studies from Kerala (Shaji et al., 1996; Shaji et al., 2005) and south and west of India (Rajkumar et al., 1997; Vas et al., 2001). This is possibly attributable to two reasons. First, the other studies have used ICD-10 criteria to diagnose VaD while we have used the Hachinski’s modified ischemic scale, which is more specific. Second, the majority of those with dementia in our study who had died between the two phases of ascertainment (n = 27/29) were left unclassified on subtype for want of adequate data. It is possible, therefore, that many patients with VaD remained unclassified in our study as they have a shorter life-span following diagnosis than those with AD. AD (71%) constituted the commonest dementia subtype. This figure is comparable to the 74% (32/43) reported in the Indo-US study but higher than the 41–65% reported from western and southern India (Rajkumar et al., 1997; Vas et al., 2001). This may be because of the differences in criteria used—the latter studies used ICD-10 criteria for AD.
A limitation of our study was that it represented a largely urban sample. Previous reports from Kerala have suggested that the urban and rural prevalence rates of dementia are comparable, although the urban rates are a little higher. Women constituted a larger proportion (59%) in our sample, possibly reflecting the sex distribution in Kerala, which favours them. Although our results do not show the effect of education on prevalent AD to be significant, we would exercise caution in interpreting this result. Most AD patients in our sample (81%) were females and the vast majority of them had education of 8 years or less. It is possible, therefore, that the effect of education on AD, if present, could not show up in this sample. Another limitation is that 27 subjects with dementia were from the ‘attrition with information’ group and were not available to the study team for detailed Phase II evaluation for confirmation of dementia. This admittedly raises the possibility of over diagnosis of dementia that could contribute to higher prevalence rates (we also estimated the prevalence rates after excluding all attrition). This possibility, however, is likely to be low since in nine of the 27, copies of hospital records available with the family members revealed a medical diagnosis of dementia. Furthermore, when compared to 66 subjects diagnosed dementia after completing Phase II evaluation, 27 diagnosed after intense family interviews had significantly lower cognitive scores on m-ACE and m-MMSE in Phase I, suggesting that cognitive impairment in them had possibly commenced early.
The fact that the prevalence rates in this study are higher than earlier thought emphasises the fact that cognitive dysfunction, particularly in some sections of urban India (Banerjee et al., 2008), is present and supports the need for community dementia case-finding methods (Shaji et al., 2002) to reveal the hidden problem. Despite being the highest so far from the Indian subcontinent, our rates are still lower than that reported from the west (Jorm, 1990). This raises the need to explore if the lower rates are related to lower prevalence of some of the risk factors, e.g. the cerebrovascular risk factors, Apo E4 etc., or alternatively, higher prevalence of certain protective factors related to the diet, life-style etc. The COAT study provides us an opportunity to explore some of these hypotheses.
Key Points.
In 2004, in those aged 65 years or more, point prevalence of dementia in Kerala, South India, was 4.86%.
On adjusting for age (against US 2000 population) the prevalence rates of dementia (and AD) were higher 6.44 (and 3.56).
Odds for dementia and AD were high with higher age, low education and in females.
Acknowledgements
The authors wish to thank Mrs Meera Pattabhi and Mr Radhamoni of the Trivandrum Chapter of the Alzheimer’s & Related Disorders Society of India (ARDSI), the office bearers of the various Resident’s Associations in the survey area for facilitating the survey, and the participants of the COAT study. This study was supported in parts by research grants to Dr Mathuranath from Sir Ratan Tata Trust, Mumbai, Kerala State Council for Science Technology & Environment (grant no.5462/B5/2002/STED),Trivandrum, and National Institute on Aging (grant no. R21AG029799), USA. Dr Ranjith is supported by a fellowship from Lady Tata Trust, Mumbai.
Footnotes
Conflict of interest
None known.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Banerjee TK, Mukherjee CS, Dutt A, Shekhar A, Hazra A. Cognitive dysfunction in an urban Indian population—some observations. Neuroepidemiology. 2008;31(2):109–114. doi: 10.1159/000146252. [DOI] [PubMed] [Google Scholar]
- Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky ST. Prevalence of Alzheimer’s disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51(4):1000–1008. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- de Silva HA, Gunatilake SB, Smith AD. Prevalence of dementia in a semi-urban population in Sri Lanka: report from a regional survey. Int J Geriatr Psychiatry. 2003;18(8):711–715. doi: 10.1002/gps.909. [DOI] [PubMed] [Google Scholar]
- Director of Census Operations Kerala. Provisional Population Totals. Kerala: Director of Census Operations; 2001. [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum GG, Chandra V, Ganguli M, Pandav R, Gilby JE, Seaberg EC, et al. Development of an activities of daily living scale to screen for dementia in an illiterate rural older population in India. Age Ageing. 1999;28(2):161–168. doi: 10.1093/ageing/28.2.161. [DOI] [PubMed] [Google Scholar]
- Ganguli M. The use of screening instruments for the detection of dementia. Neuroepidemiology. 1997;16(6):271–280. doi: 10.1159/000109697. [DOI] [PubMed] [Google Scholar]
- George A, Mathuranath PS. Primary progressive aphasia: a comparative study of progressive nonfluent aphasia and semantic dementia. Neurol India. 2005;53(2):162–166. doi: 10.4103/0028-3886.16398. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Bowler JV. Vascular dementia. Neurology. 1993;43:2159–2160. doi: 10.1212/wnl.43.10.2159-a. [DOI] [PubMed] [Google Scholar]
- Hobart JC, Thompson AJ. The five item Barthel index. J Neurol Neurosurg Psychiatr. 2001;71(2):225–230. doi: 10.1136/jnnp.71.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF. The Epidemiology of Alzheimer’s Disease and Related Disorders. London: Chapman and Hall; 1990. [Google Scholar]
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. Neurology. 1999;52(1):78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- Lindsay J. The Canadian study of health and aging: risk factors for Alzheimer’s disease in Canada. Neurology. 1994;44(11):2073–2080. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, Cherian PJ, Mathew R, George A, Alexander A, Sarma P. Mini mental state examination and the Addenbrooke’s cognitive examination: effect of education and norms for a multicultural population. Neurol India. 2007;55(2):106–110. doi: 10.4103/0028-3886.32779. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, George A, Cherian PJ, Alexander A, Sarma SG, Sarma PS. Effects of age, education and gender on verbal fluency. J Clin Exp Neuropsychol. 2003;25(8):1057–1064. doi: 10.1076/jcen.25.8.1057.16736. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, George A, Cherian PJ, Mathew R, Sarma PS. Instrumental activities of daily living scale for dementia screening in elderly people. Int Psychogeriatr. 2005;17(3):461–474. doi: 10.1017/s1041610205001547. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, Hodges JR, Mathew R, Cherian PJ, George A, Bak TH. Adaptation of the ACE for a Malayalam speaking population in southern India. Int J Geriatr Psychiatry. 2004;19(12):1188–1194. doi: 10.1002/gps.1239. [DOI] [PubMed] [Google Scholar]
- Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000;55(11):1613–1620. doi: 10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Rajkumar S, Kumar S, Thara R. Prevalence of dementia in a rural setting: a report from India. Int J Geriatr Psychiatry. 1997;12(7):702–707. doi: 10.1002/(sici)1099-1166(199707)12:7<702::aid-gps489>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Shaji KS, Arun Kishore NR, Lal KP, Prince M. Revealing a hidden problem. an evaluation of a community dementia case-finding program from the Indian 10/66 dementia research network. Int J Geriatr Psychiatry. 2002;17(3):222–225. doi: 10.1002/gps.553. [DOI] [PubMed] [Google Scholar]
- Shaji S, Bose S, Verghese A. Prevalence of dementia in an urban population in Kerala, India. Br J Psychiatry. 2005;186:136–140. doi: 10.1192/bjp.186.2.136. [DOI] [PubMed] [Google Scholar]
- Shaji S, Promodu K, Abraham T, Roy KJ, Verghese A. An epidemiological study of dementia in a rural community in Kerala, India. Br J Psychiatry. 1996;168(6):745–749. doi: 10.1192/bjp.168.6.745. [DOI] [PubMed] [Google Scholar]
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13(4):439–450. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- World Health, Organisation W. Fact Sheet No. 135. Geneva: World Health Organisation; 1998. Population ageing—a public health challenge. [Google Scholar]
- Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27(4):428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. Hospital anxiety and depression (HAD) scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]