Abstract
Background and Objectives
The genus Malasezia currently includes fourteen species that have been isolated from healthy and diseased human and animal skin. However, there were differences with respect to the species most commonly isolated, not only in patients with various skin diseases but also between healthy individuals. The aim of this study was to analyze the prevalence of Malassezia species from clinically normal skin of the scalp and trunk of healthy individuals and to examine if the range of species varies according to body site, gender and age.
Materials and Methods
The study was conducted at the Department of Dermatovenerology, University Clinical Center in Sarajevo, Bosnia and Herzegovina from December 2012 to May 2013. One hundred healthy men and women with no skin diseases and aged from <1 to 82 years were studied. The samples were obtained by scraping the skin surface from the upper and middle part of trunk and from scalps of all subjects and then incubated on modified Dixon agar. The yeasts isolated were identified by their morphological and physiological properties according to Guillot et al. method.
Results
M. sympodialis was the predominant species on trunk skin in older subjects, M. restricta on scalp skin in age groups 21-35 years, while M. globosa was identified as common species in adults (36-50 years), both from scalp skin and trunk skin. From the trunk skin M. furfur was the most frequent in children.
Conclusion
This study confirmed that cutaneous Malassezia microbiota in healthy subjects varies by body part and age but not by gender.
Keywords: Malassezia, species, identification, healthy skin
INTRODUCTION
Malassezia yeasts have been recognized for more than 150 years (1) as members of the normal human cutaneous commensal flora. However, under the influence of predisposing factors these lipolphilic yeasts may become pathogenic and associated with several diseases such as pityriasis versicolor, folliculitis, seborrheic dermatitis, confluent and reticulate papillomatosis, and even systemic infections (2,3).
The taxonomy of Malassezia has been confusing since their early description, because yeasts are dimorphic, existing in both yeast and mycelial phases (4). Today, the genus Malassezia includes 14 lipophilic species which have been identified with a conventional culture method or using molecular methods. Most human isolates belong to the species M. globosa, M. restricta, M. sympodialis, M. furfur, M. slooffiae (5), M. dermatis (6), M. japonica (7) and M. yamotoensis (8).
In humans Malassezia yeasts inhabit sebum-rich areas of the skin, including the trunk and the head region, with population densities peaking between 20-45 years (9). However, there is a great variation regarding presence and density in various skin locations (9-11), in children compared with adults (11-13) and in normal skin compared with diseased skin (2).
Although the role of Malassezia in the pathogenesis of cutaneous diseases is not fully understood, recent studies have shown that decreased density of Malassezia led to improvement of these diseases. There is also some controversy as to whether specific species cause different skin diseases (2,3).
Several studies have been carried out worldwide on the epidemiology of Malassezia species isolated from healthy individuals (11,14-24). The findings vary within the same body sites and same age groups depending on the researcher as different methodologies, isolation media and identification procedures have been employed. The difference in the results may also be attributable to the various internal and external factors such as racial and geographic differences and inevitable errors in each research. Therefore, it is important to recognize the existence of a difference in the distribution of Malassezia species in various ages and different body sites.
The aim of this study was to isolate and identify Malassezia species from the healthy human skin by using morphological, biochemical and physiological criteria and to examine if the range of species varies with different body areas, gender and age groups.
PATIENTS AND METHODS
Patients
The study was conducted from December 2012 till May 2013 at the Department of Dermatovenerology, University Clinical Center, Sarajevo, Bosnia and Herzegovina.
One hundred healthy individuals (50 females and 50 males; age range <1 to 82 years, mean 32) with no skin diseases and without any known underlying disease were included in the study. Subjects were selected among medical students (45), medical staff (20) and their children (17) or relatives (18).
All participants were instructed not to take a shower or use emollients on the day of investigation. Only those subjects who had not used any topical and oral treatment or ultraviolet phototherapy in previous two months were included in the study. All the subjects gave their written informed consent with the requirements of the Institutional Ethics Committee.
Samples
All samples consisted of scales and scrapings from the upper and middle part of trunk and from scalps in all participants. Collected samples were inoculated into Sabouraud dextrose agar and into modified Dixon agar (mDA) consisting of 3.6% malt extract, 0.6% mycological peptone, 2.0% desiccated ox bile (Sigma Chemical Co. Ltd, Dorset, UK), 1% Tween 40, 0.2% glycerol, 0.2% oleic acid, 0.05% chloramphenicol, 0.05% cycloheximide, and 1.2% agar (pH 6.0). The medium was always used within one week of preparation and the cultures were inoculated at 32°C for seven days.
Identification of Malassezia yeasts
Malassezia species were identified according to their macroscopic and microscopic features and physiological characteristics. The macroscopic features of the predominant colonies included their shape, size, color, consistency, and the characteristics of medium around them. Microscopic features of the yeast cells in culture were described after lactophenol staining and included the predominant morphology, size and budding base of the yeasts.
To assess the physiological properties of the yeasts catalase reaction was determined by using a drop of hydrogen peroxide (30%) onto a culture smear on a glass slide. The production of gas bubbles, indicative of release of oxygen, was considered a positive reaction. Utilization of Tween compounds was done according to the test originally described by Guillot et al. (25) and later modified by Gupta et al. (26). Yeast suspension, obtained by inoculating 5 mL of sterile water with a loopful of actively growing yeasts, was inoculated on Sabouraud glucose agar. The inoculum was then spread evenly. Each plate was divided into four sections and 5 mL of Tween 20, 40, 60 and 80 were added into a hole made in center of each section and incubated for a week at 32°C. Utilization of Tweens was assessed by the degree of growth and/or reaction of the lipophilic yeasts around individual holes.
The ability of the various Malassezia species to growth on mDA at 38°C was studied. A sample of actively growing cultures was transfered to mDA and incubated at 38°C for 7 days after which the ability to grow was investigated.
The β-glucosidase activity (spliting of esculin) of different Malassezia species was assayed using method described by Mayser et al. (27). Briefly, a loop of fresh yeast was inoculated deeply in the esculin agar tube and incubated for 5 days at 32°C. The splitting of esculin is revealed by darkening of the medium.
Statistics
Chi-squared test with Yates’ correction for a small sample size was carried out to determine the statistical significance of differences in proportions. We used a statistical software package Minitab 13.0. Significance level was set at P < 0.05.
RESULTS
Cultures
Malassezia yeasts were found in 65% samples taken from healthy scalp skin. The most frequently isolated species was M. restricta found in 33 patients, followed by M. globosa (16%) M. sympodialis (8%), M. slooffiae (5%), M. furfur (2%) and M. obtusa in one case.
The results of culture obtained from healthy trunk skin were positive for Malassezia yeasts in 69 cases. The predominant species was M. sympodialis found in 30 patients and the prevalence of other species was 20% for M. globosa and 14% for M. furfur. Other species were less frequently isolated: M. restricta 3%, M. slooffiae 2%.
Statistically significant differences were found in the distribution of the species isolated from healthy scalp and trunk skin – M. restricta was more commonly positive in healthy scalp skin cultures (ratio 11.0), while M. furfur and M. sympodialis were more frequent in the healthy trunk skin cultures (ratio 7 and 3.7, respectively) (P < 0.05) (Table 1).
Table 1.
Malassezia species isolated from scalp and trunk of healthy subjects
| No | Age (years) | Gender | Isolated species | |
|---|---|---|---|---|
| Scalp | Trunk | |||
| 1. | 0 (10 months) | F | Negative | M. furfur |
| 2. | 2 | F | M. restricta | Negative |
| 3. | 3 | F | M. restricta | M. globosa |
| 4. | 5 | M | M. restricta | M. sympodialis |
| 5. | 6 | M | M. restricta | M. slooffiae |
| 6. | 6 | F | M. restricta | M. sympodialis |
| 7. | 7 | M | Negative | M. sympodialis |
| 8. | 9 | F | Negative | M. furfur |
| 9. | 9 | F | Negative | M. sympodialis |
| 10. | 9 | F | Negative | M. furfur |
| 11. | 10 | F | M. globosa | M. sympodialis |
| 12. | 10 | F | M. globosa | M. furfur |
| 13. | 10 | F | M. sympodialis | M. furfur |
| 14. | 10 | F | M. sympodialis | M. furfur |
| 15. | 10 | F | M. furfur | M. furfur |
| 16. | 11 | M | M. restricta | M. sympodialis |
| 17. | 13 | F | Negative | Negative |
| 18. | 15 | F | M. restricta | Negative |
| 19. | 16 | F | M. restricta | Negative |
| 20. | 16 | F | M. globosa | Negative |
| 21. | 17 | M | M. restricta | M. restricta |
| 22. | 18 | F | M. restricta | M. resticta |
| 23. | 18 | M | M. sympodialis | M. furfur |
| 24. | 19 | M | M. restricta | Negative |
| 25. | 19 | M | M. sympodialis | Negative |
| 26. | 19 | M | M. restricta | M. sympodialis |
| 27. | 19 | F | M. globosa | M. globosa |
| 28. | 19 | F | M. restricta | Negative |
| 29. | 20 | F | M. slooffiae | Negative |
| 30. | 20 | M | M. restricta | Negative |
| 31. | 20 | M | M. furfur | M. furfur |
| 32. | 20 | M | M. restricta | M. furfur |
| 33. | 20 | F | M. obtusa | Negative |
| 34. | 20 | M | M. restricta | M. sympodialis |
| 35. | 21 | F | M. restricta | Negative |
| 36. | 21 | F | M. restricta | Negative |
| 37. | 21 | M | M. globosa | Negative |
| 38. | 23 | M | M. restricta | Negative |
| 39. | 23 | F | Negative | M. sympodialis |
| 40. | 23 | M | M. restricta | Negative |
| 41. | 24 | M | Negative | M. sympodialis |
| 42. | 24 | F | M. restricta | Negative |
| 43. | 24 | F | Negative | M. slooffiae |
| 44. | 24 | M | Negative | Negative |
| 45. | 24 | F | M. restricta | Negative |
| 46. | 25 | M | Negative | M. furfur |
| 47. | 25 | F | M. restricta | Negative |
| 48. | 25 | M | M. restricta | M. sympodialis |
| 49. | 25 | M | M. restricta | M. furfur |
| 50. | 26 | F | M. globosa | M. globosa |
| 51. | 26 | M | M. restricta | M. restricta |
| 52. | 26 | M | M. slooffiae | Negative |
| 53. | 27 | M | Negative | Negative |
| 54. | 28 | M | M. restricta | M. globosa |
| 55. | 28 | M | Negative | Negative |
| 56. | 28 | M | M. slooffiae | Negative |
| 57. | 29 | M | Negative | M. globosa |
| 58. | 29 | M | M. restricta | Negative |
| 59. | 30 | F | Negative | M. restricta |
| 60. | 31 | M | M. sympodialis | Negative |
| 61. | 32 | M | Negative | M. furfur |
| 62. | 33 | M | M. sympodialis | Negative |
| 63. | 35 | F | M. restricta | M. sympodialis |
| 64. | 35 | M | Negative | Negative |
| 65. | 36 | M | M. restricta | M. globosa |
| 66. | 37 | M | M. globosa | M. globosa |
| 67. | 37 | F | M. globosa | M. sympodialis |
| 68. | 38 | F | M. slooffiae | M. sympodialis |
| 69. | 39 | M | M. globosa | M. globosa |
| 70. | 39 | F | Negative | M. sympodialis |
| 71. | 40 | M | M. globosa | M. globosa |
| 72. | 40 | F | M. sympodialis | M. globosa |
| 73. | 41 | F | Negative | M. sympodialis |
| 74. | 42 | F | Negative | M. globosa |
| 75. | 43 | F | M. sympodialis | M. sympodialis |
| 76. | 44 | F | M. globosa | M. sympodialis |
| 77. | 45 | M | M. globosa | M. globosa |
| 78. | 47 | F | Negative | M. globosa |
| 79. | 48 | M | M. globosa | M. globosa |
| 80. | 49 | F | Negative | Negative |
| 81. | 49 | M | Negative | M. sympodialis |
| 82. | 50 | F | M. globosa | M. sympodialis |
| 83. | 50 | F | M. globosa | M. sympodialis |
| 84. | 50 | M | M. restricta | M. globosa |
| 85. | 51 | M | M. restricta | M. sympodialis |
| 86. | 54 | F | Negative | M. globosa |
| 87. | 56 | M | Negative | M. globosa |
| 88. | 59 | F | Negative | M. sympodialis |
| 89. | 59 | F | Negative | Negative |
| 90. | 61 | M | Negative | M. sympodialis |
| 91. | 66 | M | Negative | M. furfur |
| 92. | 66 | M | M. restricta | M. sympodialis |
| 93. | 67 | M | Negative | M. globosa |
| 94. | 67 | M | Negative | M. sympodialis |
| 95. | 68 | M | Negative | M. sympodialis |
| 96. | 70 | F | M. slooffiae | M. sympodialis |
| 97. | 74 | F | Negative | M. globosa |
| 98. | 78 | F | Negative | M. globosa |
| 99. | 80 | M | M. globosa | M. sympodialis |
| 100. | 82 | F | Negative | M. sympodialis |
Distribution of Malassezia species isolated according to demographic parameters
Gender
The same numbers were woman and man (50%). No statistically significant differences were found between the genders in the species isolated from scalp and trunk skin (P > 0.05) (Fig. 1).
Fig. 1.
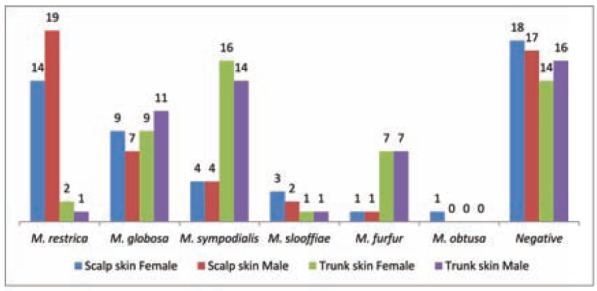
Malassezia species distribution from scalp and trunk skin of healthy subjects according to gender
Age
Subjects were grouped according to age as follows: 0-10 (15%), 11-20 (19%), 21-35 (30%), 36-50 (20%), and ≥51 years of age (16%).
Site of infection
Among Malassezia species cultured from scalp skin, M. restricta predominated in age groups 21-35 (39%) and 11-20 (33%). M. globosa was identified as common species in adults (36-50 years), both from scalp skin and trunk skin, in a frequency of 56% and 50%, respectively. From the trunk skin, M. sympodialis was most frequently found in older subjects (>51 years) – 30%, while M. furfur was the most predominant in the group of less than 10 years – 50 (Fig. 2).
Fig. 2.
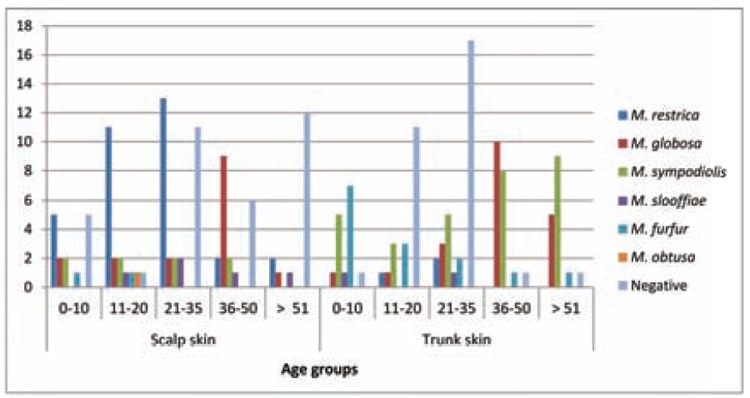
Malassezia species distribution from scalp and trunk skin of healthy subjects according to age
Statistically significant differences were observed in the distribution of the species isolated from scalp and trunk skin according to the age groups (P < 0.05).
DISCUSSION
The Malassezia genus contains a group of lipophilic yeasts that form part of the normal human skin. The colony-formation begins immediately after the birth and increases significantly with age of the neonate. It has been reported that skin colonization by Malassezia yeasts was 5% at the first week and 30% at 2-4 weeks of life (28). Using a molecular analysis, Japanese investigators were able to detect Malassezia from 89% and 100% of neonate samples on days 0 and 1 after birth, respectively. Subsequently, the level of Malassezia colonization of the neonates increased with time, whereas that of the mothers did not change (29). Generally, the carriage of Malassezia is the highest during puberty, which is related to the increase in sebaceous gland activity seen at this time.
In one study from Venezuela, 65% cultures yielded positive specimens for Malassezia (30), while earlier studies have identified even greater carriage rates of Malassezia of healthy pubertal children: 74% on the scalp (31), 93% on the back (32), and 87% on the forehead (33).
In adults, the frequency and density of colonization is related to the age and to the activity of the sebaceous glands in the area studied. The highest population densities were noted from chest, upper back and forehead and men yielded more yeasts in lower back and thigh than women (9). Bergbrant and Faergemann found that the density of Malassezia species on the skin decreased with increasing age, which was probably due to a reduction in the level of lipid on the skin (34).
Several studies have been carried out world-wide on the distribution of the newly defined species of Malassezia on healthy adult human skin in which variable results have been reported from different geographical regions (11,14-24) (Table 2).
Table 2.
Results from epidemiological studies of Malassezia species isolated from scalp and trunk skin of healthy normal subjects
| Country | Sampling method | Culture medium/ Identification method | Site sampled | Number of samples/ pos cultures | Species recovered (% of samples) | Ref. |
|---|---|---|---|---|---|---|
| Spain | Scraping | mDixons/ By Guillot et al. (1996) | Trunk (shoulders) | 75/52 | M. sympodialis (96) M. slooffiae (4) | 14 |
| Japan | Swabing | mDixons/ By Guillot et al. (1996) | Scalp | 35/5 | M. globosa (6) M. sympodialis, M. furfur, M. slooffiae (3 each) | 15 |
| Trunk | 35/24 | M. globosa (51) M. sympodialis (26) | ||||
| Japan | Tape strip | None (direct DNA extraction)/Molecular-Nested PCR | Scalp | 18/14 | M. restricta (61) M. sympodialis (50) M. globosa (44) | 16 |
| Iran | Tape strip | mDixons/ By Guillot et al. (1996) | Not stated | 100/60 | M. globosa (42) M. sympodialis (25) M. furfur (23) | 17 |
| Canada | Contact plates | LNA/ By Guillot et al. (1996) | MS | 245/172 | M. sympodialis (57) M. globosa (32) | 18 |
| Tunis | Swabing | mDixons/ By Guillot et al. (1996) | Trunk (chest) | 30/9 | M. globosa + M. sympodialis (10) M. furfur, M. sympodialis (7 each) | 19 |
| Sweden | Contact plates | LNA/ By Guillot et al. (1996) | Trunk (upper back) | 31/26 | M. sympodialis (69) M. obtusa (15) M. globosa (12) | 20 |
| Korea | Scrub-wash | LNA/ By Guillot et al. (1996) | Scalp | 120/56 | M. restricta (39) M. globosa (22.5) M. slooffiae (1.5) | 11 |
| Chest | 120/101 | M. globosa (49) M. restricta (21) M. sympodialis (10) | ||||
| Iran | Scraping | LNA/ Molecular – PCR-FLPM | Trunk | 95/94 | M. globosa (70) M. furfur (15) M. sympodialis (6) | 21 |
| Japan | Tape strip | None (direct DNA extraction)/Molecular-Nested PCR | Face | 30/30 | M. globosa (87) M. restricta (83) M. sympodialis (37) | 22 |
| Korea | Scrub-wash | LNA/ Molecular-Nested PCR | MS | 110/70 | M. globosa (32) M. restricta (30) M. sympodialis (15) | 23 |
| India | Scraping | mDixons/ By Guillot et al. (1996) | Trunk (upper back) | 45/21 | M. sympodialis (48) M. obtusa (19) M. globosa (14) | 24 |
mDixons, modified Dixon agar; LNA, Leeming and Notman agar; FLPA, fragment length polymorphism analysis; PCR, polymerase chain reaction; MS, multiple sites
In general, it seems that the most common Malassezia species cultured from healthy scalp skin is M. restricta (11,16), while M. globosa and M. sympodilais are two most frequently isolated species from the trunk (11,14,15,17-24). Other species are less common, but not completely absent.
Similar to the majority of other investigations, we found M. sympodialis as the most frequent species on normal trunk skin isolated in 30% of cases. This species emerges as the predominant species on healthy skin, especially on the trunk, where it can be recovered in great numbers in more than 50% of individuals (18,20).
By contrast, M. globosa has been reported as the most frequently isolated species in some other investigations (15,17,19,21-23). In our study, M. globosa is less common species found in 20% of healthy individuals which goes well with the studies of Gupta et al. (18) and Kaur et al. (24). This species is reported to be a causative agent of pityriasis versicolor, found in filamentous form in the scales from this skin disorder (14,15,17,19,21,35).
M. furfur was isolated in 14% and this species is found to be less common inhabitant of healthy skin (11,17,19,21). However, M. furfur is confirmed to also be responsible for pityriasis versicolor, particularly under tropical climate (36).
In contrast to healthy trunk skin, M. sympodialis was recovered less frequently from the scalp skin of same subjects (8%), whereas M. restricta was the commonest species (33%). This species is isolated regularly from the scalp and face of patients with seborrheic dermatitis (5,14,22). Oh et al. in Korea also found M. restricta to be particularly associated with scalp and M. globosa and M. sympodialis with the chest, whereas other species (M. furfur, M. obtusa and M. slooffiae) were less frequently recovered (23).
In our study, M. slooffiae and M. obtusa were isolated only from a few samples taken from our subjects. These species are considered to be very rare on healthy skin (20,24) and only infrequently isolated from the cases of pityriasis versicolor (17,35), atopic dermatitis (15,20,26) and seborrheic dermatitis (14,20).
M. pachydermatis was not recovered from any of our samples either from trunk or from healthy scalp skin. This species is confirmed to be clearly adapted to animals, although it has been involved in some systemic human infection. The presence of this species on human skin is rare and transient, occurring possibly by transmission from animal pets and environmental sources (37).
Several studies demonstrated that the distribution of Malassezia species on head and trunk region is parallel with the density and activity of pilosebaceous glands in these areas. The difference in species according to different body areas is presumably to be attributed to lipid content and different lipid components in each body area (11).
The higher detection rate of Malassezia species was observed using molecular determination method than by conventional culture methods. These variations may be attributed to the different sampling technique and inadequate determination of the relative proportion of species on the skin, or the consent ability of the fungus to grow in each specified medium that have impact on the range of species recovered (20).
A correlation between the prevalence of Malassezia species and gender of the subjects has been observed without differences among the woman and man. However, statistical difference was noted among age groups and isolated species. M. globosa was the predominant species in adult subjects regardless of body part, M. restricta was recovered more frequently from the scalp skin in the teens and young adults, while on the trunk sin M. sympodialis was most frequently found in older subjects. Our results are concordant with the majority of studies worldwide which clarified that the cutaneous Malassezia microbiota of healthy subjects differed by age (11,13,38). This was contrary to observation of Gupta et al. who cultured more frequently M. globosa on younger subjects and M. sympodialis on the skin of adolescents and adults (18).
In conclusion, researches recovered not only different species distributed on the same anatomic area, but also the same species in even the same frequency from different body areas. These facts suggest that besides different sampling methods and culture media used, there are probably some internal and external factors as well as geographical and ethnic variations reflected in the predominance of different species on the skin of individuals in different parts of the world.
New molecular approaches to the identification of different species will certainly answer many outstanding questions associated with Malassezia species and their role in human pathophysiology.
References
- 1.Eishstedt E. Piltzbildung in der Pityriasis versicolor. Froriep Neue Notiz Natur Helk. 1846;39:270. [Google Scholar]
- 2.Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004;51:789–798. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Gaitanis G, Velegraki A, Mayser P, Bassukas ID. Skin diseases associated with Malassezia yeasts: facts and controversies. Clin Dermatol. 2013;31(4):455–463. doi: 10.1016/j.clindermatol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Midgley G. The diversity of Pityrosporum (Malassezia) yeasts in vivo and in vitro. Mycopathologia. 1989;106:143–153. doi: 10.1007/BF00443055. [DOI] [PubMed] [Google Scholar]
- 5.Gueho E, Midgley G, Guillot J. The Genus Malassezia with description of four new species. Antonie van Leeuwenhoek. 1996;69:337–355. doi: 10.1007/BF00399623. [DOI] [PubMed] [Google Scholar]
- 6.Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, Ogawa H, Nishikawa A. New yeast species, Malassezia dermatis, isolated from a patient with atopic dermatitis. J Clin Microbiol. 2002;40:1363–1367. doi: 10.1128/JCM.40.4.1363-1367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugita T, Takashima M, Kodama M, Ryoji T, Nishikawa A. Description of a new species Malassezia japonica and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003;41:4695–4699. doi: 10.1128/JCM.41.10.4695-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, Nishikawa A. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Imunol. 2004;48:579–483. doi: 10.1111/j.1348-0421.2004.tb03554.x. [DOI] [PubMed] [Google Scholar]
- 9.Leeming JP, Notman FH, Holland KT. The distribution and ecology of Malassezia furfur and cutaneous bacteria on human skin. J Appl Bacteriol. 1989;67:47–52. doi: 10.1111/j.1365-2672.1989.tb04953.x. [DOI] [PubMed] [Google Scholar]
- 10.Faergemann J, Aly R, Maibach HI. Quantitative variations in distribution of Pityrosporum orbiculare on clinically normal skin. Acta Derm Venereol. 1983;63:346–348. [PubMed] [Google Scholar]
- 11.Lee YW, Yim SM, Lim SH, Choe YB, Ahn KJ. Quantitative investigation on the distribution of Malassezia species on healthy human skin in Korea. Mycoses. 2006;49(5):405–410. doi: 10.1111/j.1439-0507.2006.01239.x. [DOI] [PubMed] [Google Scholar]
- 12.Faergemann J, Fredriksson T. Age incidence of Pityrosporum orbiculare on human skin. Acta Derm Venereol. 1980;60:531–535. [PubMed] [Google Scholar]
- 13.Sugita T, Suzuki M, Goto S, Nishikawa A, Hiruma M, Yamazaki T, Makimura K. Quantitative analysis of the cutaneous Malassezia microbiota in 770 healthy Japanese by age and gender using a real-time PCR assay. Med Mycol. 2010;48:229–233. doi: 10.1080/13693780902977976. [DOI] [PubMed] [Google Scholar]
- 14.Crespo Erchiga V, Ojeda Martos AA, Vera Casaño A, Crespo Erchiga A, Sánchez Fajardo F. Isolation and identification of Malassezia spp. in pytiriasis versicolor, seborrheic dermatitis and healthy skin. Rev Iberoam Micol. 1999;16:16–21. [PubMed] [Google Scholar]
- 15.Nakabayashi A, Sei Y, Guillot J. Identification of Malasseziaspecies isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000;38:337–341. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- 16.Sugita T, Suto H, Unno T, Tsuboi R, Ogawa H, Shinoda T, Nishikawa A. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J Clin Microbiol. 2001;39(10):3486–3490. doi: 10.1128/JCM.39.10.3486-3490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarazooie B, Kordbacheh P, Zaini F, Zomorodian K, Saadat F, Zeraati H, Hallaji Z, Rezaie S. Study of the distribution of Malassezia species in patients with pityriasis versicolor and healthy individuals in Tehran, Iran. BMC Dermatol. 2004;4:5. doi: 10.1186/1471-5945-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta AK, Kohli Y. Prevalence of Malassezia species on various body sites in clinically healthy subjects representing different age groups. Med Mycol. 2004;42(1):35–42. doi: 10.1080/13693780310001610056. [DOI] [PubMed] [Google Scholar]
- 19.Ben Salah S, Makni F, Marrakchi S, Sellami H, Cheikhrouhou F, Bouassida S, Zahaf A, Ayadi A. Identification of Malassezia species from Tunisian patients with pityriasis versicolor and normal subjects. Mycoses. 2005;48(4):242–245. doi: 10.1111/j.1439-0507.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- 20.Sandström Falk MH, Tengvall Linder M, Johansson C, Bartosik J, Bäck O, Särnhult T, Wahlgren CF, Scheynius A, Faergemann J. The prevalence of Malassezia yeasts in patients with atopic dermatitis, seborrhoeic dermatitis and healthy controls. Acta Derm Venereol. 2005;85:17–23. doi: 10.1080/00015550410022276. [DOI] [PubMed] [Google Scholar]
- 21.Zomorodian K, Mirhendi H, Tarazooie B, Zeraati H, Hallaji Z, Balighi K. Distribution of Malassezia species in patients with psoriasis and healthy individuals in Tehran, Iran. J Cutan Pathol. 2008;35(11):1027–1031. doi: 10.1111/j.1600-0560.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 22.Tajima M, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: comparison with other diseases and healthy subjects. J Invest Dermatol. 2008;128(2):345–51. doi: 10.1038/sj.jid.5701017. [DOI] [PubMed] [Google Scholar]
- 23.Oh BH, Song YC, Lee YW, Choe YB, Ahn KJ. Comparison of Nested PCR and RFLP for Identification and Classification of malassezia yeasts from Healthy human skin. Ann Dermatol. 2009;21(4):352–357. doi: 10.5021/ad.2009.21.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur M, Narang T, Bala M, Gupte S, Aggarwal P, Manhas A. Study of the distribution of Malassezia species in patients with pityriasis versicolor and healthy individuals in Tertiary Care Hospital, Punjab. Indian J Med Microbiol. 2013;31(3):270–274. doi: 10.4103/0255-0857.115636. [DOI] [PubMed] [Google Scholar]
- 25.Guillot J, Gueho E, Lesourd M, Midgley G, Chevrier G, Dupont B. Identification of Malassezia species. A ppractical approach. J Mycol Med. 1996;6:103–110. [Google Scholar]
- 26.Gupta AK, Kohli Y, Summerbell RC, Faergemann J. Quantitative culture of Malassezia species from different body site of individuals with and without dermatoses. Med Mycol. 2001;38:243–251. doi: 10.1080/mmy.39.3.243.251. [DOI] [PubMed] [Google Scholar]
- 27.Mayser P, Haze P, Papavassilis C, Pickel M, Gruender K, Gueho E. Differentiation of Malassezia species: selectivity of Cremophor EL, castor oil and ricinoleic acid for M. furfur. Br J Dermatol. 1997;137:208–213. doi: 10.1046/j.1365-2133.1997.18071890.x. [DOI] [PubMed] [Google Scholar]
- 28.Ayhan M, Sancak B, Karaduman A, Arikan S, Sahin S. Colonization of neonate skin by Malassezia species: relationship with neonatal cephalic pustulosis. J Am Acad Dermatol. 2007;57(6):1012–1018. doi: 10.1016/j.jaad.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Nagata R, Nagano H, Ogishima D, Nakamura Y, Hiruma M, Sugita T. Transmission of the major skin microbiota, Malassezia, from mother to neonate. Pediatr Int. 2012;54(3):350–355. doi: 10.1111/j.1442-200X.2012.03563.x. [DOI] [PubMed] [Google Scholar]
- 30.González-Morán E, Rodríguez-Valero S, Del Monte ML, Briceño M, Sintjago S, Mesa LM, García D, Villalobos R, Pereira N. Isolation and identification of Malassezia species isolated from healthy skin of malnourished and eutrophic children cared for in daycare centers in Venezuela. Invest Clin. 2009;50(2):145–152. [PubMed] [Google Scholar]
- 31.Noble WC, Midgley G. Scalp carriage of Pityrosporum species: the effect of physiological maturity, sex and race. Sabouraudia. 1978;16:229–232. doi: 10.1080/00362177885380311. [DOI] [PubMed] [Google Scholar]
- 32.Garcia RL. Skin disorders in air force recruits. J Assoc Mil Dermatol. 1976;2:61. [Google Scholar]
- 33.Bergbrant IM, Broberg A. Pityrosporum ovale culture from the forehead of healthy children. Acta Dermato-Venereol. 1994;74:260–261. doi: 10.2340/0001555574260261. [DOI] [PubMed] [Google Scholar]
- 34.Bergbrant IM, Faergemann J. Variations of Pityrosporum orbiculare in middle-aged and elderly individuals. Acta Dermato-Venereol. 1988;68:537–540. [PubMed] [Google Scholar]
- 35.Prohic A, Ozegovic L. Malassezia species isolated from lesional and non-lesional skin in patients with pityriasis versicolor. Mycoses. 2007;50:58–63. doi: 10.1111/j.1439-0507.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 36.Midgley G. The lipophilic yeasts: state of the art and prospects. Med Mycol. 2000;38:9–16. [PubMed] [Google Scholar]
- 37.Prohic A, Kasumagic-Halilovic E. Identification of Malassezia pachydermatis from healthy and diseased human skin. Med Arh. 2009;63:317–319. [PubMed] [Google Scholar]
- 38.Akaza N, Akamatsu H, Sasaki Y, Takeoka S, Kishi M, Mizutani H, Sano A, Hirokawa K, Nakata S, Matsunaga K. Cutaneous Malassezia microbiota of healthy subjects differ by sex, body part and season. J Dermatol. 2010;37:786–792. doi: 10.1111/j.1346-8138.2010.00913.x. [DOI] [PubMed] [Google Scholar]


