Abstract
Background and Objectives
Recently, use of herbal medicine and plant extracts as a substitute for commercially available chemical drugs for control of infectious diseases such as dental caries and periodontal disease has become increasingly popular. The present study was aimed to evaluate the effect of Rhus coriaria L. water extract on five common oral bacteria and bacterial biofilm formation on orthodontic wire.
Materials and Methods
For primary assessment of the antibacterial properties of Rhus coriaria L. water extract, the well-plate method in BHIA (Brain Heart Infusion Agar, Merck, Germany) medium was used Using macrodilution method, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the extract against each microorganism were determined. The effect of Rhus coriaria L. on bacterial biofilm formation on orthodontic wire was assessed using viable cell count in biofilm medium (BM) containing 3% sucrose. In the final phase, after fixation of samples in alcohol and glutaraldehyde, samples were prepared for SEM (Scanning Electron Microscopy) analysis.
Results
The diameter of the zone of growth inhibition was proportionate to the tested concentrations of the extract. The lowest MIC (0.390 mg/ml) and MBC (1.5 mg/ml ) of the Rhus coriaria L. were found to be against Streptococcus sobrinus ATCC 27607. Rhus coriaria L. water extract decreased bacterial biofilm formation on orthodontic wire at MIC and 1/8 of MIC by S. sanguinis ATCC 10556, S. sobrinus ATCC 27607, S. salivarius ATCC 9222, S. mutans ATCC 35608 and E. faecalis CIP 55142 by24.2%-43%, 68.5%-91.6%, 10.6%-79.1%, 22.2%-86.1% and 40.6%-76.4%, respectively.
Conclusion
Based on the results, Rhus coriaria L. water extract had significant antibacterial properties against five common oral bacteria and was able to inhibit bacterial biofilm formation on orthodontic wire. Further investigations are recommended for widespread clinical use of this extract.
Keywords: Rhus coriaria L, Water extract, Antibacterial activity, Bacterial plaque, Orthodontic wire
INTRODUCTION
Orthodontic treatment especially with fixed appliances is always associated with a constant concern regarding its side effects in the oral cavity. Orthodontic therapy changes the physico-chemical balance of oral environment and facilitates plaque accumulation on tooth surfaces. Furthermore, orthodontic treatment is usually associated with inadequate oral hygiene and subsequent increase in cariogenic bacteria, Gram-negative flora and consequent gingival and periodontal inflammation (1-4). Dental caries is a multi-factorial infectious disease and despite the great advancements in oral health, tooth caries and periodontal disease are still among the most common oral and dental diseases. Bacterial plaque is a general term that describes accumulation of microorganisms (mainly bacteria) embedded in polymer matrix of salivary and bacterial origin on tooth surfaces which plays a primary role in the pathogenicity of dental caries and periodontal disease (5-9). Oral streptococci are the first plaque forming species; of which, Streptococcus sanguinis is among the first responsible for formation and maturation of bacterial plaque (7,10). Streptococcus salivarius is a common colonizer of oral mucosal surfaces especially in the dorsal aspect of the tongue, buccal mucosa, and saliva (7, 11). Streptococcus mutans and Streptococcus salivarius play an important role in development and maturation of caries The cariogenic properties of these bacteria are attributed to the production of insoluble glucans from sucrose, their ability to adhere to tooth surfaces and their acidogenicity (12).
Numerous efforts have been undertaken to eliminate Streptococcus mutans as the most well recognized cariogenic agent. Solutions and antibiotics like chlorhexidine, penicillin, ampicillin, tetracycline, vancomycin and erythromycin are extremely effective for prevention of dental caries under in-vivo and in-vitro conditions. However, their extensive use changes the microbial oral and intestinal flora and results in development of bacterial resistance, diarrhea, vomiting and tooth discoloration (13). Recognition and use of medicinal plants have a long history. According to the World Health Organization (WHO) reports, 80% of people living in developing countries believe that medicinal plants are beneficial for primary health care. Due to the increased resistance of microorganisms to the commercially available drugs, there has been a growing interest to know more about the medicinal plants and their active ingredients (6, 14).
Commonly called Elm-Leaved Sumac or Tanner’s Sumac with the scientific name Rhus coriaria L. is a deciduous shrub to small tree that grows 1 to 5 meters. Its leaves comprise 9 to 15 hairy leaflets.
The term Sumac in Saami and Arami languages means red and being red in color. Today, the term Sumac is used in general for several species of Rhus plant but the most common commercially available species of Sumac is Rhus coriaria L. Sumac is cultivated in a wide area from the Canary Islands to the Mediterranean coast, Iran and Afghanistan. In traditional medicine, this plant has been used for treatment of anorexia, diarrhea, hemorrhage, and hyperglycemia (15-20).
To date, limited studies have evaluated the antibacterial effects of water extract of Rhus coriaria L. on oral bacteria and its impact on bacterial biofilm formation on orthodontic wire. Thus, the present study was conducted aiming at in-vitro assessment of the effects of Rhus coriaria L. water extract on five oral bacteria and bacterial biofilm formation on orthodontic wire.
MATERIALS AND METHODS
Sample preparation
Rhus coriaria L. was obtained from Nesa village in Chaloos road in October 2012 and was identified at the Pharmacognosy Department of Shahid Beheshti University of Medical Sciences under the voucher number 2 1121.
Extract preparation
In order to prepare the extract, 1000 ml of boiling water was added to 100 g of Rhus coriaria L. shade died peel fruit and the mixture was stored at room temperature for 4 hours to allow the infusion process. The obtained mixture was filtered through Whatman No.1 filter paper. The extract was dried by Bain-marie set to reach dry extract. The extract was stored in a sterile bottle at 4°C. The yield of extract was 15% (15 g of dried extract from 100g of Rhus coriaria. L).
Micoorganisms
The bacterial strains including Streptococcus mutans ATCC 35608, Streptococcus sanguinis ATCC 10556, Streptococcus sobrinus ATCC 27607, Streptococcus salivarius ATCC 9222 and Enterococcus faecalis CIP 55142 were received from Iranian Research Organization for Science and Technology (Tehran, Iran).
Primary evaluation of the antibacterial effect
Microbial suspension with a bacterial count of 1.5 x 108 CFU/ml was prepared with normal saline solution. The prepared microbial suspension for each bacterial strain was cultured on plates containing Brain Heart Infusion Agar (Merck, Germany) and then wells with 6 mm diameter were created. 100 μl of the extract solution prepared with sterile distilled water at 3.125, 6.25, 12.5, 25, 50 and 100 mg/ml concentrations were poured into the wells. All plates were stored at 35° C for 16-24 hours. The test repeated for three times. Afterwards, the diameter of growth inhibition zone was measured in mm and recorded.
Determination of the MIC
MIC is defined as the minimum concentration of the extract that completely inhibits visible bacterial growth compared to the negative control group. The macrodilution method was used to determine the MIC of Rhus coriaria L. Water extract of Rhus coriaria L. was prepared at 0.097, 0.195, 0.390, 0.781, 3.125, 6.25, 12.5, 25 and 50 mg/ml concentrations in Brain Heart Infusion Broth medium (Merck, Germany) and inoculated with the microbial suspension with the bacterial count of 104 CFU/ml. A series of tubes containing the extract were considered as the negative control group while the tube containing culture medium and microorganism was considered as the positive control group. All tubes were stored at 35°C for 16-24 hours. This test was repeated three times for each microorganism.
Determination of the MBC
In order to determine the MBC of extract against each microorganism, 20 μl of each tube with no visible growth of microorganism was inoculated on plates containing Brain Heart Infusion Agar. After storing for 16-24 hours at 35°C, growth of microorganisms was evaluated. Each test was repeated three times. MBC was determined as the minimum concentration of the extract at which no bacterial growth was observed.
Bacterial adhesion to orthodontic wire
In order to determine the amount of biofilm formation on orthodontic wire, viable cell count method was employed (17, 18). For this purpose, biofilm medium (BM) containing sterile 3% sucrose was used. Sterile orthodontic wire (Stainless Steel, rectangular, 0.016 3 0.022 inch, M Unitek, St. Paul, Minn.) was cut into 2 cm pieces and stored at 35°C for 40 hours along with 1 ml of the MIC of the extract and three lower concentrations (MIC, 1/2, 1/4 and 1/8 of the MIC) determined for each bacterial strain and prepared using biofilm medium (BM) containing 3% sucrose and also 0.1 ml of microbial suspension with a bacterial count of 104 CFU/ml. The biofilm medium containing extract and a piece of sterile orthodontic wire was considered as the negative control group while biofilm medium containing a piece of sterile orthodontic wire and microorganisms was considered as the positive control group. After completion of this time period, each wire segment along with 1 ml of Phosphate Buffered Saline (8 g NaCl,0.2 g KCl,1.44 g Na2HPO4 and 0.25 g KH2PO4 with a pH of 7.2 was placed in Sonicator (Techna 3, Italy, HZ=50-60, V=230±10%, KW=0.13) for 10 minutes. Afterwards, 4 dilutions were prepared of each PBS sample (1/10-1/10,000) and microbial count was done by pour plate method using Brain Heart Infusion Agar medium (Merck, Germany). Plates were kept at 35°C for three days and after this time period the colonies were counted. The microbial count of each sample was determined separately. All the mentioned steps were repeated three times.
Preparation of samples for SEM analysis
Orthodontic wires were placed in an incubator for 48 hours along with1.562 mg/ml of the prepared extract in biofilm medium containing 3% sucrose and 0.15 ml of Streptococcus mutans suspension. Samples were then washed with 0.1M PBS, stored in glutaraldehyde 2.5% and placed consecutively in 30, 50, 70, 80, 90% and absolute alcohol dilutions. Samples were then placed under the safety hood for 24 hours to be prepared for evaluation with electron microscopy. Fig. 1.
Fig. 1.
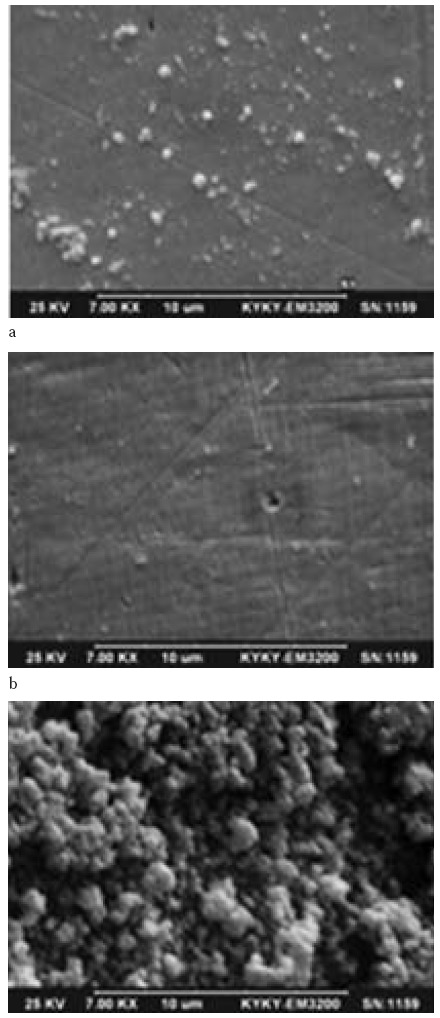
Scanning Electron Microscopy images of S. mutans with an orthodontic wire placed in a. positive control, b. negative control c. Rhus coriaria L. water extract with a concentration of 1.562 mg/ml.
RESULTS
In this study, primary assessment of the antibacterial effect of Rhus coriaria L. water extract was done using the well-plate method. The mean diameter (mm) of growth inhibition zone for each bacterial strain exposed to different concentrations of the extract is demonstrated in Table 1. Rhus coriaria L. water extract showed antibacterial effects on the studied bacteria since the growth of all five bacterial strains were inhibited by various concentrations of the extract. Diameter of the growth inhibition zone was proportional to the extract concentration. The size (diameter) of growth inhibition zone ranged from 11±1.0 to 31±3.6 mm. The largest growth inhibition zone as the result of exposure to extract was observed for S. sabrinius ATCC 27607. No growth inhibition zone was observed for any studied bacteria in extract concentrations in the range of 3.1 to 12.5 mg/ml.
Table 1.
Mean size (diameter) of growth inhibition zone of understudy bacteria due to exposure to different concentrations of Rhus coriaria L. water extract
| Microorganisms | Concentration (mg/ ml) | |||||
|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | 6.25 | 3.1 | |
| S. mutans | 21±0.00 | 15±1 | nd | nd | nd | nd |
| S. sanguinis | 29.33±3.05 | 20±0.00 | 13.66±0.577 | nd | nd | nd |
| S. salivarius | 19.33±2.08 | 13.0±1 | 11±1 | nd | nd | nd |
| S. sobrinus | 31±3.6 | 23±2 | 13.33±1.52 | nd | nd | nd |
| E. faecalis | 17.66±2.51 | 11±1 | nd | nd | nd | nd |
Mean diameter of growth inhibition zone± SD in mm nd: Growth inhibition zone not detected
MIC and MBC of Rhus coriaria L. water extract against each microorganism are demonstrated in Table 2. MIC and MBC were in the range of 0.390 to 1.562 mg/ml and 1.562 to 25 mg/ml, respectively. The lowest MIC and MBC values (0.390 and 1.562 mg/ml ) were observed against S. sobrinus ATCC 27607. The highest MIC of this extract was 1.5 mg/ml against S. salivarius ATCC 9222, E. faecalis CIP 55142 and S. mutans ATCC 35608. The highest MBC was 25 mg/ml against E. faecalis CIP 55142. The results of the inhibitory effects of 4 different concentrations of the extract on bacterial biofilm formation on orthodontic wire are demonstrated in Table 3. Rhus coriaria L. water extract at MIC and 1/8 of MIC reduced bacterial biofilm formation on orthodontic wire by S. sanguinis ATCC 10556, S. sobrinus ATCC 27607, S. salivariusATCC 9222, S. mutans ATCC 35608 and E. faecalis CIP 55142 by 24.2-43%, 68.5-91.6%, 10.6-79.1%, 22.2-86.1% and 40.6-76.4%, respectively. Reduction of bacterial biofilm formation by S. sobrinus ATCC 27607 on orthodontic (68.5-91.6%) wire was achieved by a lower concentration of the extract.
Table 2.
MIC and MBC of Rhus coriaria L. water extract against five studied bacteria.
| Microorganisms | MIC (mg/ml) | MBC (mg/ml) |
|---|---|---|
| S. mutans | 1.562 | 6.25 |
| S. sanguinis | 1.562 | 3.125 |
| S. salivarius | 1.562 | 12.5 |
| S. sobrinus | 0.390 | 1.562 |
| E. faecalis | 0.781 | 25 |
Table 3.
Reduction percentage of bacterial biofilm formation compared to the positive control group caused by different concentrations of Rhus coriaria L. water extract for each microorganism
| 1.5621 | 0.781 | 0.390 | 0.195 | 0.097 | 0.048 | |
|---|---|---|---|---|---|---|
| S. mutans | 86.1 | 85.9 | 66.4 | 22.2 | - | - |
| S. sanguinis | 43.6 | 42.4 | 41.3 | 24.2 | - | - |
| S. salivarius | 79.1 | 75.3 | 20.5 | 10.6 | - | - |
| S. sobrinus | - | - | 91.6 | 83.9 | 73.5 | 68.5 |
| E. faecalis | - | 76.4 | 74.5 | 54.9 | 40.6 | - |
Not using the mentioned concentrations for the respective bacteria
DISCUSSION
Plaque accumulation and subsequent dental caries and periodontal disease in orthodontic patients are common due to the hardship of maintaining adequate oral hygiene in presence of brackets, bands, wires and elastomeric ligatures. Poor oral hygiene can eventually lead to the development of white spots, caries and hyperplastic gingival tissue requiring dental interventions other than orthodontic treatment (21). Tooth brushing and dental flossing are the gold standard for oral hygiene control by patients. Despite the high emphasis on mechanical plaque control, prevalence of oral and dental diseases is still high (22). Therefore, at present, special attention is paid to additional means for maintaining oral health like the mouthwashes since they can clinically enhance the effects of mechanical plaque removal methods.
For the first time, in 1969 the effects of chlorhexidine gluconate as an antiseptic with low toxicity and a wide spectrum antimicrobial activity were evaluated in the field of dentistry (23). Several investigators reported positive effects of chlorhexidine mouthwash on reducing microbial plaque, gingivitis and bleeding in orthodontic as well as other patients (24-29). However, its most common side effect is tooth discoloration following its consumption which is not pleasant for the patients (1). Thus, there is an increasing tendency to find medicinal plants with antibacterial properties and minimum side effects to control infectious diseases like dental caries and periodontal disease. The present study aimed at in-vitro evaluation of the effect of Rhus coriaria L. water extract on five common oral bacteria and bacterial biofilm formation on orthodontic wire. Very limited studies have evaluated the antimicrobial effects of this extract on oral bacteria. Therefore, comparison of the results is not feasible. Due to the extensive use of Sumac as a spice in food industry, most studies have focused on its effects on food bacteria (30-33). In general, these studies show that this extract has a more significant effect on Gram positive than Gram negative bacteria with no antiviral or antifungal effect (34, 35).
In a study conducted on standard and clinical strains of Staphylococcus epidermidis and Corynebacterium xerosis it was revealed that Sumac has a significant effect on cutaneous bacteria (36). Number of studies on the antifungal effects of Sumac is very limited and most of them have mentioned no significant effect on Listeria monocytogenes, M. luteus, S. aureus, P. aeruginosa, and E. coli (38). Motaharinia et al., (2012) reported strong antibacterial effects of ethanolic extract of Rhus coriaria L. on Helicobacter pylori with a MIC of 214.28(mm) μg/ml. Diameter of growth inhibition zone was ≥ 42/19 mm for 20 mg/ml and ≥ 57/28mm for 40 mg/ml concentration of the extract (40). Babpour et al., in 2009 demonstrated that the alcoholic extract of Rhus coriaria L. had MIC and MBC of 250 μg/ml against S. mutans and S. sanguinis (5). In the present study, the antibacterial effect of Rhus coriaria L. water extract was determined on five common oral bacteria using the macrodilution and well-plate methods (Tables 1 and 2).
Based on the results of previous studies, pure extract of Sumac fruit peel had a stronger antibacterial activity compared to the extract derived from the fruit seeds; thus, in the present study we used the fruit peel (41). The highest MIC, MBC and the largest size of growth inhibition zone were observed against S. sobrinus ATCC 27607 in an amount of 0.390, 1.562 mg/ml and range of 13.33± 1.52 and 31± 3.6 mm.
Difference between our results and those of Babpour et al., (2009) regarding S. mutans and S. sanguinis can be due to the different method of extract preparation, type of solvent used and method of determining the MIC of the extract. All parts of Sumac plant especially its fruit contain tannins reach in phenolic compounds (16, 17, 39).Tannins are among the most potent antibacterial agents showed high antimicrobial properties. They are absorbed by the bacterial cell membrane and interfere with the metallic ions and function of bacterial enzymes (5, 42). As observed in Table 2 and SEM images of S. mutans ATCC 35608, Rhus coriaria L. water extract could reduce bacterial biofilm formation on orthodontic wire in proportion to the concentration of the Rhus coriaria L. water extract, shows the possible relationship between active compounds present in the extract interfere with bacterial adhesion mechanisms.
Acknowledgments
This study was approved by the Ethics Committee of School of Dentistry, Shahid Beheshti University of Medical Sciences. The authors would like to express their gratitude to the Research Deputy of School of Dentistry and Department of Pharmaceutics School of Pharmacy, Shahid Beheshti University of Medical Sciences for their sincere cooperation in the conduction of this study.
References
- 1.Poosti M, Radvar M, Yaghoobi S, Ahmadi R. Comparing the effect of Chlorhexidine and Persica mouth rinse on periodontal status of fixed orthodontic patients. J Mashhad Dent Sch. 2006;30:183–190. [Google Scholar]
- 2.Salehi P, MomeniDanaie Sh. Comparision of the antibacterial effect of persica mouth wash with chlorhexidine on streptococcus mutans in orthodontic patients. DARU. 2006;14:178–182. [Google Scholar]
- 3.Ousehal L, Lazrak L, Es-Said R, Hamdoune H, Elquars F, Khadija A. Evaluation of dental plaque control in patients wearing fixed orthodontic appliances: a clinical study. Int Orthod. 2011;9:140–55. doi: 10.1016/j.ortho.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Park HS, Kim KH, Kwon TY, Hong SH. Effect of garlic on bacterial biofilm formation on orthodontic wire. Angle Orthod. 2011;3:1–6. doi: 10.2319/121010-713.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babpour E, Angaji Ah, Angaji M. Antimicrobial effects of four medicinal plants on dental Plaque. J MED PLANTS RES. 2009;3:132–137. [Google Scholar]
- 6.Palombo EA. Traditional medicinal plants extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011;2011:680354. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semyari H, Owlia P, Farhadi S, Moghadami Tabrizi S. Evaluation of antimicrobial effect of Ammi visnaga against oral streptococci. J Microbiol Antimicrob. 2011;3:126–129. [Google Scholar]
- 8.Chu-hong Hu, Jian He, Randal Eckert, Xiao-yang Wu, Li-na Li, Yan Tian. Development and evaluation of a safe and effective sugar-free herbal lollipop that kills cavity-causing bacteria. Int J Oral Sci. 2011;3:13–20. doi: 10.4248/IJOS11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.JafarzadehKashi TS, Kermanshahic RK, Erfand M, VahidDastjerdie E, Rezaeia Y, Tabatabaei FS. Evaluating the in-vitro antibacterial effect of Iranian propolis on oral microorganisms. Iran J Pharm Res. 2011;10:363–368. [PMC free article] [PubMed] [Google Scholar]
- 10.Hillman JD, Cransky SSS, Shivers M. The relationsips between streptoccocal Species and periodontopathic Bacteria IN Human Dental Plaque. Arch Oral Biol. 1985;30:791–795. doi: 10.1016/0003-9969(85)90133-5. [DOI] [PubMed] [Google Scholar]
- 11.Townsend-Lawman P, Bleiweis AS. Multilevel control of extracellular sucrose metabolism in Streptococcus salivarius by sucrose. Microbiology. 1991;137:15–13. doi: 10.1099/00221287-137-1-5. [DOI] [PubMed] [Google Scholar]
- 12.Kuspradini H, Mitsunaga T, Ohashi H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonolrhamnosides from kempas (Koompassiamalaccensis) extracts. J Wood Sci. 2009;55:308–313. [Google Scholar]
- 13.Saraya S, Kanta J, Sarisuta N, Temsiririrkkul R, Suvathi Y, Samranri K. development of guava extract chewable tablets for anticariogenic activity against Streptococcus mutans. Mah Univer Jou of Pharma Sci. 2008;35(1-4):18–23. [Google Scholar]
- 14.Mohapatra DP, Thakur V, Brar SK. Antibacterial efficacy of raw and processed honey. Biotechnol Res Int. 2011 doi: 10.4061/2011/917505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasar-Abbasa SM, KadirHalkman A. Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int J Food Microbiol. 2004;97:63–69. doi: 10.1016/j.ijfoodmicro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Shabbir A. Rhus coriaria linn, a plant of medicinal, nutritional and industrial importance: a review. J Anim Plant Sci. 2012;22:505–512. [Google Scholar]
- 17.Ahmadian Atari MM, Amin GhR, Fazeli MR, Jamalifar H. A review on antimicrobial activities of sumac fruit (Rhus coriaria L.) J Medi Plan. 2008;7:1–9. [Google Scholar]
- 18.Safarnejad A, Alamdari SBL. Tissue culture in medicinal plant of sumac (Rhus coriaria) Int J Sci Nat. 2011;2:760–763. [Google Scholar]
- 19.Rayne S, Mazza G. Biological activities of extracts from sumac (Rhus spp.): a review. Plant Foods Hum Nutr. 2007;62:165–175. doi: 10.1007/s11130-007-0058-4. [DOI] [PubMed] [Google Scholar]
- 20.Khayatnouri MH, Babazadeh D, Safarmashaei S. Orally administration effect of sumac on blood sugar in rat. Adv Environ Biol. 2011;5:2077–2079. [Google Scholar]
- 21.Tufekci E, Casagrande ZA, Lindauer SJ, Fowler CE, Williams KT. Effectiveness of an essential oil mouth rinse in improving oral health in orthodontic patients. Angle Orthod. 2008;78:294–298. doi: 10.2319/040607-174.1. [DOI] [PubMed] [Google Scholar]
- 22.Gunsolley JC. Clinical efficacy of antimicrobial mouth rinses. J Dent. 2010;38:6–10. doi: 10.1016/S0300-5712(10)70004-X. [DOI] [PubMed] [Google Scholar]
- 23.Schiott C, LOE h, Jensen SB, Kilian M, Davies RM, Glavind K. The effect of chlorhexidin emouth rineses on the human oral flora. J Priodontal Res. 1970;5:84–89. doi: 10.1111/j.1600-0765.1970.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 24.Storhaug K. Hibitan in oral disease in handicap patients. J Clin Periodontal. 1997;4:102–107. doi: 10.1111/j.1600-051x.1977.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 25.Lang NP, Brecx MC. Chlorhexdine digluconate-An agent for chemical plaque control and prevention of gingival inflammation. J Priodontal Res. 1986;21:74–89. [Google Scholar]
- 26.Grossman E. Six month study of the effect of a chlorhexidin mouth rinse on gingivitis in adults. J Priodontal Res. 1986;21:33–34. [Google Scholar]
- 27.Wennstorm JL, Heijl L, Dahlen G, Grondahl K. Periodic subgingival antimicrobial irrigation of periodontal pockets: Clinical observation. J Clin Priodontal. 1987;14:573–580. doi: 10.1111/j.1600-051x.1987.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds MA, Lavigne CK, Mina GE, Suzuki JB. Clinical effects of simultaneous ultrasonic scalling and subgingival irrigation with chlorhexidine. J Clin Priodontal. 1992;19:595–600. doi: 10.1111/j.1600-051x.1992.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 29.Stirrups DR, Laws EA, Honigman JL. The effects of chlorhexidinegluconat emouth rinse on oral health during fixed appliance orthodontic treatment. Br Dent J. 1981;151:84–86. doi: 10.1038/sj.bdj.4804641. [DOI] [PubMed] [Google Scholar]
- 30.Fazeli MR, Amin Gh, Ahmadian-Attari MM, Ashtiani H, Jamalifar H, Samadi N. Antimicrobial activities of Iranian sumac, avishan-e shirazi (Zataria multiflora) against some food-borne bacteria. Food Contr. 2007;18:646–649. [Google Scholar]
- 31.Nsar-Abbas SM, KadirHalkman A. Anti microbial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food born bacteria including pathogens. Int J Food Microb. 2004;97:63–69. doi: 10.1016/j.ijfoodmicro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Nasar-Abbas SM, KadirHalkman A. Inhibition of some foodborne bacteria by alcohol extract of sumac (Rhus coriaria L.) J Food Safety. 2004;24:257–67. [Google Scholar]
- 33.Gulmez M, Oral N, Vatansever L. The effect of water extract of Sumac (Rhus coriaria L.) and lactic acid on decontamination and shelf life of raw broiler wings. Poult Sci. 2006;85:1466–1471. doi: 10.1093/ps/85.8.1466. [DOI] [PubMed] [Google Scholar]
- 34.Abu Shanab B, Adman C, Abu Safiya D, Adwan K, Abu Shanab M. Antibacterial activity of Rhus coriaria L. extracts growing in Palestine. J Islam Univ Gaza. 2005;13:147–153. [Google Scholar]
- 35.Shidfar F, Rahideh ST, Rajab A, Khandozi N, Hosseini S, Shidfar Sh, Mojab F. The effect of Sumac Rhus coriaria L. powder on serum glycemic status, ApoB, ApoA-I and total antioxidant capacity in type 2 diabetic patients. 2014;13:1249–1255. [PMC free article] [PubMed] [Google Scholar]
- 36.Fazeli MR, Ashtiani H, Ahmadian-Attari MM, Jamalifar H, Zaheri A. Antimicrobial effect of Rhus coriaria L. (Sumac) total extract on skin isolates Staphylococcus epidermidis and Corynebacterium xerosis. J Med Plant. 2006;5:27–31. [Google Scholar]
- 37.Sokman A, Jones BM, Erturk M. The in vitro antibacterial activity of Turkish medicinal plants. J Ethnopharmacol. 1999;67:79–86. doi: 10.1016/s0378-8741(98)00189-5. [DOI] [PubMed] [Google Scholar]
- 38.Digrak M, Alma MH, Ilçim A. Antibacterial and antifungal activities of Turkish medicinal plants. Pharm Biol. 2001;39:346–350. [Google Scholar]
- 39.Mahboubi A, Kamalinejad M, Ayatollahi AM, Babaeian M. Total phenolic content and antibacterial activity of five plants of Labiatae against four foodborne and some other bacteria. Iran J Pharm Res. 2014;13:559–566. [PMC free article] [PubMed] [Google Scholar]
- 40.Motaharinia Y, Hazhir MS, Rezaee MA, Vahedi S, Rashidi A, Hosseini W, Hakhamaneshi MS, Rahmani MR. Comparison of in vitro antimicrobial effect of ethanol extracts of Satureja khuzestanica, Rhus coriaria, and Ocimum basilicum L. on Helicobacter pylori. J Med Plants Res. 2012;6:3749–3753. [Google Scholar]
- 41.Shahidibonjar GH, Kariminik A, Heydari MR, Ghasemzadeh MH, Rashid Farrokhi P, Moin MR, et al. Anti-pseudoma and anti-bacilli activity of some medicinal plants of Iran. DARU. 2003;11:157–163. [Google Scholar]
- 42.Duman AD, Ozgen M, Dayisoylu KS, Erbil N, Durgac C. Antimicrobial activity of six Pomegranate (Punica granatum L.) varieties and Their relation to some of their Pomological and Phytonutrient Characteristics. Molecules. 2009;14:1808–1817. doi: 10.3390/molecules14051808. [DOI] [PMC free article] [PubMed] [Google Scholar]


