Abstract
Background and Objectives
Azotobacter is a diazotroph bacterium reported to possess various plant growth-promoting characteristics.The aim of this study was to isolate Azotobacter strains capable of fixing nitrogen and effectively hydrolyzing both organic and inorganic Pi compounds.
Materials and Methods
In this study, soil samples collected from a diverse range of slightly alkaline soil types were screened for Azotobacter isolates. The inorganic and organic phosphate solubilization potentials of twenty competent phosphate solubilizing Azotobacter isolates were assessed.Variations were noted in the solubilization potentials.
Result
Three isolates, identified as Azotobacter vinelandii strains O2, O4 and O6, were able to fix atmospheric N2 effectively. The nitrogenase activity of these isolates ranged between 158.6 and 326.4 C2H4h-1vial-1 (ethylene). Bacterial growth rates and phosphate solubilization activities were measured quantitatively under various environmental conditions. A close association was evident between phosphate solubilizing ability and growth rate as an indicator of active metabolism. All three phosphate solubilizing bacteria (PSB) were able to withstand temperature as high as 45°C, high concentration of NaCl (upto 5%) and a wide range of initial pH from 5 to 10 while hydrolyzing phosphate compounds actively.
Conclusion
Azotobacter vinelandii strains O2, O4 and O6 are superior candidates for biofertilizers that may result in the reduction of chemical nitrogen and phosphate fertilizers leading to increase crop production.
Keywords: Azotobacter, Biofertilizer, Nitrogen fixation, Pisolubilizing
INTRODUCTION
Nitrogen (N) is the most important nutrient and occupies the highest requirement position in plant nutrient compared to the other essential nutrients (1). N is widely distributed throughout the lithosphere, atmosphere, hydrosphere and biosphere. Only a very small part of this element is presents in the soil, as both inorganic and organic forms. The inorganic N in the soil is only a small fraction of the total soil N (2). The total N contents of surface mineral soils normally ranges between 0.05 and 0.2 percent, in most cases less than five percent, is directly available to plants, mainly as nitrate (NO32-) and ammonium (NH4+). Organic N slowly becomes available through mineralization as well (1). Most of the N is to form of dinitrogen (N2) in the earth’s atmosphere. N2 cannot be used directly by plants and animals; therefore, N2 must be first ‘fixed’ or converted into a form such as ammonia before being consumed (2-4). N enters the biosphere through biological nitrogen fixation (BNF) by microorganisms (3,5). N fixation is a process of reducing atmospheric nitrogen to ammonia, catalyzed by the nitrogenase enzyme complex (6-8).
In the fields, phosphorus (P) is the second most essential macro nutrients for plant growth and development that is absorbed only in soluble forms of phosphate ion (Pi), HPO42- or H2PO4-. In fact, total soil phosphorus content is well beyond plant needs (400–1,200 mg/kg); however, it is mostly immobilized in the forms of organic and inorganic compounds such that the only a small fraction of P (1 mg kg−1 or less) is available for plant growth (for reviews see (3, 9)). Even, a large portion of added chemical Pi fertilizer is immobilized rapidly in soil to its insoluble forms (10, 11). Therefore, the availability of Pi is highly dependent on chemical compositions and biological processes occurring in the soil, particularly within the rhizospher micro environment (10). It is generally accepted that the release of insoluble and fixed forms of P carried out by the action of phosphate-solubilizing bacteria (PSB) via the secretion of low molecular weight organic acids mainly gluconic and keto-gluconic acids and phosphatases (9, 12-15). These acids are produced in the periplasm of many Gram-negative bacteria through a direct oxidation pathway of glucose (DOPG, non-phosphorylating oxidation), consequently, the organic acids diffuse freely outside the cells and may release high amounts of soluble P from mineral phosphates, by supplying both protons and metal complex organic acid anions (14, 15).
It has already been shown that the use of nitrogen fixing bacteria with high effective ability in solubilizing organic and inorganic phosphates is one of the most feasible strategies for the development of sustainable agriculture. This can supply sufficient N2 and assist the hydrolysis of a wide range of P compounds leading to increased crop production (3, 9, 16). Among the nitrogen-fixing bacteria those belonging to genus Azotobacter, play a significant role(17, 18). Azotobacter is a gamma-proteobacterium belonging to the family Pseudomonadaceae. It is an obligate aerobic, free-living Gram-negative, broadly dispersed in diverse environments, such as soil, water and sediments (19). In fact, Azotobacter has beneficial effects on plant yields, due to their ability of fixing nitrogen (6, 18, 20, 21), solubilizing phosphates (4, 16, 22) and to the microbial secretion of stimulating phytohormones, like gibberellins, auxins and cytokinins (4, 22). An additional reason which justifies the interest on this microorganism is that the species A. vinelandii and A. chroococcum produces metabolically dormant cysts. The cyst is formed under unfavorable environmental conditions and is the most important basis of application of Azotobacter strains in various environments (20, 23, 24). The use of native soils Azotobacter is an important aspect for biofertilizer applications as it is well adapted to the natural climates of their habitat and particularly with respect to applications under harsh environmental conditions.
The aim of this research was to isolate Azotobacter strains capable of fixing nitrogen and effectively hydrolyzing both organic and inorganic Pi compounds. As a new approach, we have set up a method for screening of Azotobacter with high phosphatase activity. The effects of several environmental conditions on propagations and Pi solubilizing activities of the isolates were assessed too.
MATERIALS AND METHODS
Bacterial Isolation and Identification
Sixty-four different soil samples from the rhizosphere of agricultural crop of Iran were transferred to laboratory (Tehran, Qazvin, Guilan, Mazandaran, Ardabil and Isfahan). Strategies used for isolation were:(i) Enrichment of Azotobacter strains,one gram from each of the soil samples were added into 100 ml Erlenmeyer flasks containing 20 ml of Azotobacter broth of the following composition; mannitol 20g, K2HPO4 0.8 g, KH2PO4 0.2 g, MgSO4·7H2O 0.5 g, FeSO4·6H2O 0.10 g, CaCO3 20 g, NaMoO4·2H2O 0.05 g supplemented with ZnSO4.7H2O 10 mg, MnSO4.4H2O 1.0 mg and cycloheximide (100μg/ml) per liter (Adjust to pH 7.2). Incubation was at 28°C for 2-5days. (ii) Isolation was carried out by preparing serial dilutions from enrichment culture followed by streaking and incubation at 28°Cfor 2-5 days. All the isolates were subcultured on selective nitrogen-free specific medium Azotobacter Agar plates.
Physiological and biochemical characteristics were performed according to Bergey’s Manual of Systematic Bacteriology instructions (19), including colony morphology, the Gram staining, cyst and PHB granules staining as well as production of pigment. Molecular identification was performed with PCR using selective nifH-g1 primers fD1 (5’GGTTGTGACCCGAAAGCTGA-3’) and rP1 (5’-GCGTACATGGCCATCATCTC-3’) (5).
Three selected isolates with the highest nitrogen fixation properties were identified by 16S rDNA amplification and sequencing. Amplifications of the rDNA gene of bacterial strains were carried out using universal bacterial primers (fD1) 27F; (AGAGTTTGATCCTGGCTCAG) and (rP1)1492R; (GGTTACCTTGTTACGACTT)to amplify ∼1.5 kb product from 16S rDNA (25). Sequence similarities were analyzed at NCBI GenBank database and BLAST program at http://www.ncbi.nih.gov/blast.
Phosphate solubilization
To examine Pi solubilization capabilities, 20 μl of the bacterial suspensions (∼104CFU/ml) was spotted on the center of Sperber medium plate containing insoluble Pi. Sperber medium composed of 10 g glucose, 0.5 g yeast extract, 0.1 g CaCl2, 0.25 g MgSO4.7H2O was supplemented with 2.5 g Ca3(PO4)2 (TCP) and 15 g agar (in solid medium) per liter at pH: 7.2 (26). The inoculated plates were incubated at 28°C. The diameter of zone of clearance (halo) surrounding the bacterial colony as well as the diameter of colony were measured after 2, 4 and 7 days in triplicates.Pi solubilizing index (PSI) was calculated as the ratio of diameter of halo(mm)/diameter of colony(mm) (27).
All the isolates were also assayed for phosphatase activity according to the method described by Malboobi et al. (26). The isolates were cultured on the Sperber agar medium containing 50 mg/l BCIP (5-bromo-4-choloro-3-indolyl phosphate; Sigma, St. Louis, Mo) in the presence of soluble phosphate (KH2PO4) or insoluble phosphate (TCP)and incubated at 28°C. Phosphatase activities in isolates were compared based on scoring the intensities of blue stained colonies at 24, 48 and 72 hours.
Nitrogen fixation and Nitrogenase activity
The isolates able to solubilize phosphate on Sperber agar were used for nitrogen fixation capability. The N-fixing efficiency of these isolates was assessed by the acetylene reduction assay (ARA) as described by Hardy et al. (28). Briefly, 5ml of the Azotobacter broth in 12 ml vials was inoculated with ∼104CFU/ml of each isolate and incubated at 28 °C for 48-96 h. Once visible growth was observed, the vials were plugged with rubber. 10% of the head space (7 ml) was injected with pure acetylene gas(C2H2) after removing an equal amount of air from vials by means of a disposable plastic syringe (29-31). The gas samples (0.7 μl) were removed after 24 h incubation, and were assayed for ethylene production with a gas chromatograph (GOW MAC - GM 816 model). The chromatograph was fitted with Poropak N column and a H2-FID detector. The rate of nitrogen fixation was calculated (30, 31) and values were expressed as nanomoles of ethylene produced per hour per vial (nmoles C2H4 h−1 vial−1) (31, 32).
Bacterial growth and phosphate solubilization
Two indices, including growth index (GI) which is the logarithm of CFU/ml of culture and Pi solubilizing index (PSI) that represents the amounts of hydrolyzed Pi from TCP, were monitored. Time-coursed quantitative measurements were carried out for three isolates with the highest nitrogen fixation (O2, O4 and O6) in 100 ml Erlenmeyer flasks containing 25 ml of Sperber broth medium. 100μl of bacterial suspension (with∼104 CFU/ml of each isolate) were inoculated into medium and incubated at 28°C and 120 rpm. The effect of temperature was examined by incubation at 15, 28, 37 and 45°C. For high-salt treatments, the extra amounts of salt (1, 2.5, 5 and 7%; w/v) were added to Sperber medium. Initial pH values of medium ranging from 5 to 9 were adjusted by the addition of either KOH or HCl accordingly. In general, no added NaCl, pH: 7 and temperature of 28°C was used as control conditions. Sampling was carried out within 96 h. For estimation of the growth rate, 100 μl of medium was removed after 3, 6, 12, 18, 24, 36, 48, 72 and 96 h. Serial dilution were prepared and the growth curve was drawn, while simultaneous released Pi in culture supernatant was measured. In brief, 400μl of the medium were removed and centrifuged for 10 min at 5000 rpm. 200μl from supernatant were mixed with an equal amount of vanadate-molybdate reagent and diluted to 2 ml with ddH2O. After 10 min, the optical densities of the samples were measured at 450 nm against a blank. PSI was estimated using standard curve. All the experiments were performed in triplicates.
Data analysis and graph drawing were done using statistics software Graph Pad Prism (v5.0.4) and Microsoft Excel program.
RESULTS
Bacterial Isolation and Identification
A total of 43 isolates, named O1 to O43, were selected after enrichment, isolation and screening from 64 soil samples. According to biochemical characteristics and molecular method, three species of Azotobacter, viz., A. chroococcum, A. virelandiiand A. beijerinckii were identified. Three isolates with the highest nitrogenase activity, O2, O4 and O6 were chosen for further characterization. Several biochemical and biological indicators as well as 16S rDNA sequence were inspected to figure out the taxonomy of these isolates. The compiled data (not shown) revealed that O2, O4 and O6 isolates must be strains of Azotobacter vinelandii. 16S rDNA sequences of these isolates (GenBank accession Nos. EU935082, EF620439 and EF634040) were all in agreement with the biochemical tests.
Phosphate solubilization activity
Phosphate solubilization was measured in all isolates; as shown in Table 1, twenty isolates were able to produce clear zone or blue color in minimal medium containing insoluble Pi or synthetic substrate, respectively, in the course of plating compared to the other isolates. In a comparative experiment set up, the largest clear zone was observed for O4 (21.5 mm) followed by O25 (20.6 mm) within the first 2 days whereas O1, O3 and O32 isolate did not show any clear zone. The PSI values were increased in the time course of culture of each isolate. The highest level of PSI was observed for O4 (3.5±0.1) during 7 days while the lowest rate was for O24 (∼1.2) during the same period of time (Table 1). The strongest blue color was developed by O4, O17 and O25 within the 24 h and 48h while the others stained weakly (Table 1). Phosphatase activity was not detected in O9 and O24.
Table 1.
The solubilization efficiency of Azotobacter strains in plate assay for which Pi solubilizing index (PSI) and presence or absences of phosphatase activity are given
| Bacterial strain | Pi solubilizing index (PSI) a | Phosphatase activityb,c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2days | 4days | 7days | With soluble Pi | With insoluble Pi | |||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||
| O1 | 0 | 1.7±0.1 | 2.1±0.1 | + | + | + | + | + | + |
| O2 | 1.4 | 1.5±0.1 | 2±0.2 | + | + | + | + | + | + |
| O3 | 0 | 1.7±0.3 | 2.1±0.1 | + | + | + | - | + | + |
| O4 | 2.7±0.2 | 3.3±0.1 | 3.5±0.1 | ++ | ++ | ++ | ++ | ++ | ++ |
| O5 | 1.6±0.1 | 1.7±0.1 | 1.8±0.3 | + | + | + | + | + | + |
| O6 | 1.6±0.2 | 1.7±0.1 | 1.9±0.1 | + | + | + | + | + | + |
| O8 | 1.7±0.3 | 2±0.1 | 2.4±0.1 | + | + | + | - | + | + |
| O9 | 1 | 1.1±0.1 | 1.3±0.1 | - | - | - | - | - | - |
| O11 | 1.1 | 1.4±0.2 | 1.6±0.3 | + | + | + | - | + | + |
| O14 | 2.6±0.4 | 2.8±0.2 | 3.2±0.1 | - | - | + | - | - | + |
| O15 | 1.2±0.1 | 1.3 | 1.4±0.1 | + | + | + | + | + | + |
| O16 | 1.4 | 1.7±0.1 | 1.8±0.2 | + | + | + | + | + | + |
| O17 | 2.4 | 3±0.3 | 3.4±0.3 | ++ | ++ | ++ | + | + | ++ |
| O21 | 1.5 | 1.6±0.2 | 1.7±0.2 | + | + | + | + | + | + |
| O24 | 1 | 1±0.1 | 1.2±0.1 | - | - | - | - | - | - |
| O25 | 2.2±0.2 | 2.6±0.2 | 3 | ++ | ++ | ++ | ++ | ++ | ++ |
| O32 | 0 | 1.3±0.2 | 1.4±0.2 | + | + | + | + | + | + |
| O33 | 1.1 | 1.4±0.2 | 1.5±0.2 | - | + | + | - | - | - |
| O34 | 1.4 | 1.4 | 1.6±0.1 | + | + | + | + | + | + |
| O39 | 1.7 | 1.8±0.1 | 1.8±0.1 | + | + | + | + | + | + |
Results presented are means of three replicates ± standard errors
Soluble phosphate is KH2PO4 and insoluble phosphate is TCP
+ Light blue stain and ++ dark blue stain
Nitrogen fixation and nitrogenase activity
Nitrogenase activities were determined by checking the acetylene reduction activity for phosphate solubilizing isolates. Rates obtained in samples were in the range of 12.1 to 326.4 nmol C2H4/h/vial while cell numbers were adjusted to 107CFU/ml. Five isolates showed the highest N2-fixing activity. Three isolates (O2, O4 and O6) were identified as A. vinelandii and the other two strains (O1 and O3) were A. chroococcum. The acetylene reduction activity (ARA) result of pure cultures of these strains is shown in Table 2. A. vinelandi O6 showed the highest activity (326.4nmol C2H4/h/vial) followed by strain O2 with an activity of 265.5nmol C2H4/h/vial. Strain O5 had the lowest nitrogenase activity (12.1nmolC2H4/h/vial).
Table 2.
Acetylene reduction activities in the selected isolates with maximum nitrogenase activity.Values are means of triplicates followed by the standard deviation
| Isolates | Nitrogenase activity (nmol C2H4 h−1 vial−1) | Identified strain |
|---|---|---|
| O1 | 87.2 ± 4.6 | A.chroococcum |
| O2 | 265.5 ± 7.2 | A. vinelandii |
| O3 | 135.7 ± 3.6 | A.chroococcum |
| O4 | 158.6 ± 4.7 | A. vinelandii |
| O6 | 326.4 ± 8.7 | A. vinelandii |
Correlation between bacterial growth and phosphate solubilization
Three isolates with the highest nitrogen fixation (A. vinelandii O2, A. vinelandii O4 and A. vinelandii O6) were chosen for further investigations. In a series of preliminary experiments, the effects of various growth conditions on the survival of these isolates were assessed. A. vinelandii O2 was capable of growing and Pi solubilizationon solid medium with the added salt of up to 2.5% between 25 to 45°C and initial pH 6-8. A. vinelandii O4 was capable of Pi solubilization at 15 to 40°C, pH 5-9 and salt as high as 7% when grown on solid medium. In contrast, A. vinelandii O6 was able to form colonies at a range of 15-45°C, pH 5-9 and 5% salt. Pi solubilization were observed only at 20-40°C, pH 5-8 and salt concentration of 2.5% for this strain.
This study was further carried out in liquid minimal medium for GI and PSI. In all cases, a close association between GI and PSI was noticeable (Figs. 1-3). Under such conditions, the maximum levels of both GI and PSI for A. vinelandii O2 reached after 48 h of incubation at 28°C while exponential growth phase of A. vinelandii O6 delayed for 72 h and arrived at stationary phase and maximum PSI (∼ 230mg/l) after 72 h. Generally, bacterial growth and consequently PSI of both bacteria were reduced at both high and low temperatures (Fig. 1). In comparison; the most PSI was obtained for A. vinelandii O4 after 24 h at 37°C (225 mg/l) (Fig. 1). This was gradually decreased when incubation was extended beyond 24 h. Bacterial growth and consequently PSI of this strain were reduced at 15°C and 45°C (Fig. 1). This was more pronounced for O4 strain such that it never entered exponential growth phase when incubated at 45°C, although they were alive.
Fig. 1.
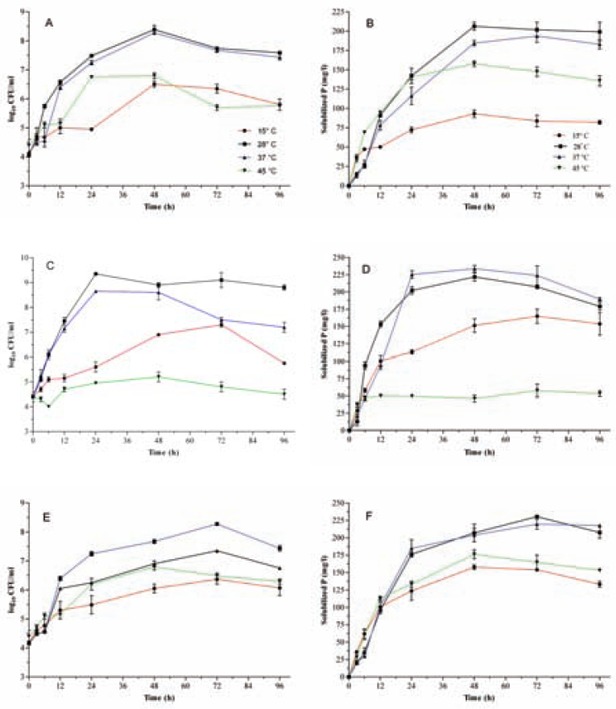
The effect of incubation temperature on the bacterial growth rates (panels A, C and E) and the Pi solubilization abilities (panels B, D and F). Bacterial strains O2 (panels A and B), O4 (panels C and D) and O6 (panels E and F) were grown in liquid medium containing tricalcium phosphate. All data points are the means of three replicates. Standard errors are shown by vertical bars.
Fig. 3.
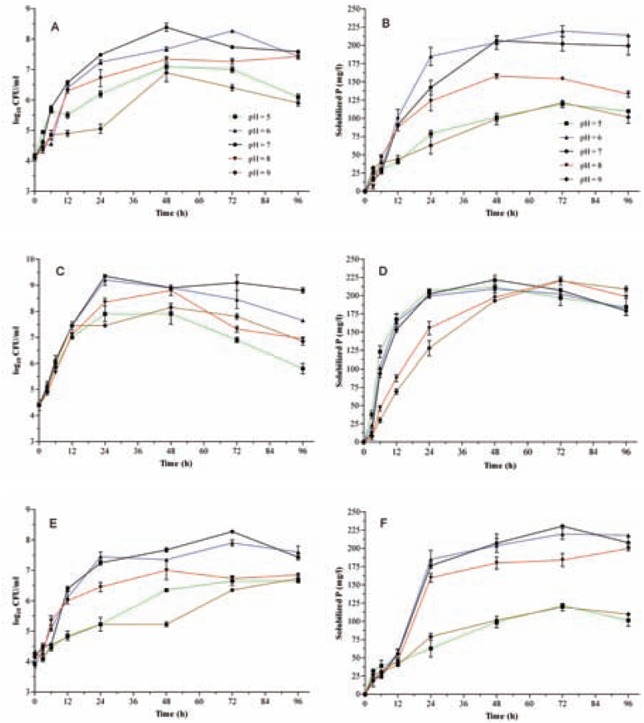
The effect of different initial pH of medium on bacterial growth rates and Pi solubilizationindicies. Panels are arranged as Fig. 1. All data points are the means of three replicates. Standard errors are shown by vertical bars.
As shown in Fig. 2, increased NaCl concentration in medium was associated with reduced bacterial growth and PSI values. In other words, the least amount of both GI and PSI values were detected at 7% NaCl. A different pattern was observed for O4 strain; Pi solubilization was delayed at low salt medium and the highest PSI value at 24 h in samples obtained from medium with 1% salt. PSI values for O4 were inversely proportional to the GI, particularly when the added salt was higher than 1%.
Fig. 2.
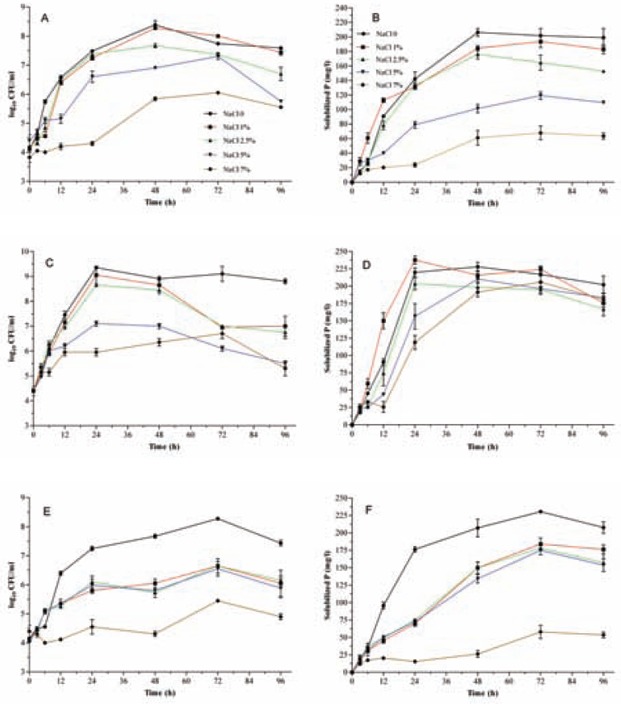
The effect of NaCl amounts added to minimal liquid medium on bacterial growth rates and Pi solubilization indices. Panels are arranged as Fig. 1. All data points are the means of three replicates. Standard errors are shown by vertical bars.
Medium inoculation at various initial pH values resulted in reduced bacterial growth and PSI of O2 and O6 isolates in both alkaline and acidic media. However, the PSI values for both isolates at acidic pH were higher than the alkaline pH (Fig. 3). While the growth range of O4 was different at various pH levels, the initial pH did not affect the PSI values of O4 isolate, except for alkaline broth. Maximum PSI was obtained when incubation was extending for more than 72 h. The highest PSI values in acidic and alkaline pH were for O4 strain (Fig. 3).
DISCUSSION
A diverse group of rhizobacteria, collectively called plant growth promoting rhizobacteria (PGPR), has been reported to enhance the growth of plants directly and/or indirectly. As one of the most known groups of PGPR, nitrogen fixing bacteria have ability of phosphate solublization considered to main constituents of biofertilizers (3, 9, 16). Of the most widely used method to study N2 fixation in asymbiotic systems, the ARA is simpler and faster than the other methods because it is easily measured by gas chromatography (20). The results presented in this paper are in agreement with the previous works. Rodelas et al.(33) reported the ARA results of pure cultures of strains were between 9.70 to 257.73nmol C2H4 h−1 vial−1. It is worth noting that A. chroococcum isolates gave high values of ARA. In another study, Tejera et al. (21) surveyed efficient nitrogen fixation A. chroococcum isolates from soil or rhizosphere samples with the range of 79.6 to 329.5 nmol C2H4 h−1 culture.
Although, most studies have focused on biological nitrogen fixation of Azotobacter but several researchers paid attention to other characteristic termed as PGPR. In order to minimize the use of expensive chemical fertilizers, in this study we investigated the potential of phosphate solubilization in these strains of Azotobacter. As previously reported by Kumar et al.(27, Garg et al.(16) and Farajzadeh et al.(22), the Azotobacter isolates were able to dissolve inorganic and organic phosphate compounds. The PSI results obtained in the solid medium was significantly higher than other observations and corroborated with Farajzadeh et al. (22), indicating that indigenous strains of Azotobacter isolated from alkaline soils are effective Pi solubilizers. The presented results showed that isolated P solubilizing and N-fixation Azotobacter could serve as efficient biofertilizer candidates for improving the N and P nutrition of crop plants simultaneously.
Nevertheless, the performance of PGPR as biofertilizers is severely influenced by both biotic and abiotic environmental conditions of the targeted regions. This has lead researchers to seek out isolation of PGPR from native soil and to explore methods for screening PGPR strains and subsequent evaluations for specific criteria particularly with respect to applications under various environmental conditions. Tolerances to extreme climates are of special interests for bacteria to be used as biofertilizer in arid and semi-arid regions. It has been evidenced that the members of the genus Azotobacter produce copious amount of capsular slime. This property allows adaptation of these species to the stressful environments. Besides, it is suggested that the cyst formation under unfavorable environmental conditions reduces oxygen transferring into the cell and consequently increases the nitrogenase activity (23, 34). Therefore, the isolates were intensively examined for tolerance toward high temperature, high concentration of NaCl and a wide range of pH (Figs. 1-3). All PSB strains survived under the examined conditions. Decreased GI and PSI at 45°C for the isolate are acceptable as in natural situation these bacteria survive hot noons in summer while continue growing on the rest of the times. All isolate tolerated the added NaCl to more than 2.5% indicating that these isolates would functionally be active in most cultivated lands in which the salinity of soil is usually below 2.5%. As a key feature of cultivation soils, we measured GI and PSI of bacteria grown in medium with various initial pH ranging from 5 to 9. The fact that the highest GI and PSI values for isolates were obtained at high pH suggests that these bacteria favor alkaline condition. These results indicate that the isolated Azotobacter could be used as biofertilizer in agricultural soils in Iran which are predominately calcareous and are characterized by a high pH and low amounts of plant available phosphorus (35).
Islam et al. (36) investigated the effects of pH, temperature and salt on growth of Azotobacter spp. isolated from crop rhizosphere. Even though no data was presented for PSI, they however showed tolerance for a number of their isolated strains toward different environmental factors such as NaCl (0-0.8%) and temperature (20– 40°C) while no isolate survived at 50°C. Rajeswari et al. (37) isolated Azotobacter species which showed tolerance toward the extra amounts of salt (3.5%)in an optimized medium. Tejera et al. (21) assessed growth rates at different initial pH values (4- 9) and showed that a lower number of isolates grew on N-free media at pH value as high as 8.7. Ravikumar et al. (31) investigated the effects of salinity on nitrogen fixation and also indole acetic acid (IAA) production They found a maximum level of IAA production and nitrogenease activity at the salinity of 20 g/l and 30 g/l NaCl, respectively.
A noticeable reduction in PSI during stationary phase of growth found in all cases supports the dependence of PSI on bacterial metabolism. It was also shown that the phosphatase activity of bacterial strain could synergistically enhance the release of Pi in the acidified medium (Fig. 3). The advantage of bacteria capable of phosphate solubilitizing with simultaneous secretion of organic acids and phosphatase activity on production and yield were shown in both green house and field trials (38).
In conclusion, we have isolated several Azotobacter strains, three of which were more promising for being used as N and P biofertilizer. These bacteria are well adapted to various environmental conditions.
References
- 1.Hofman G, van Cleemput O. Soil and Plant Nitrogen. 1. IFA; Paris: 2004. [Google Scholar]
- 2.Rees DC, Akif Tezcan F, Haynes CA, Walton MY, Andrade S, Einsle O, et al. Structural basis of biological nitrogen fixation. Phil Trans R Soc. 2011;363:971. doi: 10.1098/rsta.2004.1539. [DOI] [PubMed] [Google Scholar]
- 3.Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321:305–339. [Google Scholar]
- 4.Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60:579–598. [Google Scholar]
- 5.Burgmann H, Widmer F, Von Sigler W, Zeyer J. New molecular screening tools for analysis of free-living diazotrophs in soil. Appl Environ Microbiol. 2004;70:240–247. doi: 10.1128/AEM.70.1.240-247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol. 2004;2:621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
- 7.Halbleib CM, Ludden PW. Regulation of biological nitrogen fixation. The Journal of Nutrition. 2000;130:1081. doi: 10.1093/jn/130.5.1081. [DOI] [PubMed] [Google Scholar]
- 8.Raymond J, Siefert JL, Staples CR, Blankenship RE. The natural history of nitrogen fixation. Mol Biol Evol. 2004;21:541. doi: 10.1093/molbev/msh047. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 10.Deubel A, Merbach W. In: Influence of Microorganisms on Phosphorus Bioavailability in Soils Microorganisms in Soils: Roles in Genesis and Functions. 3. Varma A, Buscot F, editors. Springer; Berlin Heidelberg: 2005. pp. 177–191. [Google Scholar]
- 11.Mehta S, Nautiyal CS. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol. 2001;43:51–56. doi: 10.1007/s002840010259. [DOI] [PubMed] [Google Scholar]
- 12.Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, et al. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem. 2005;37:1970–1974. [Google Scholar]
- 13.Khan MS, Zaidi A, Wani PA. In: Role of Phosphate Solubilizing Microorganisms in Sustainable Agriculture Sustainable Agricultur. Lichtfouse E, Navarrete M, Debaeke P, Vinronique S, Alberola C, editors. Springer; Netherlands: 2009. pp. 551–570. [Google Scholar]
- 14.Perez E, Sulbaran M, Ball MM, Yarzabal LA. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem. 2007;39:2905–2914. [Google Scholar]
- 15.Sashidhar B, Podile AR. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol. 2010;109:1–12. doi: 10.1111/j.1365-2672.2009.04654.x. [DOI] [PubMed] [Google Scholar]
- 16.Garg SK, Bhatnagar A, Kalla A, Narula N. In vitro nitrogen fixation, phosphate solubilization, survival and nutrient release by Azotobacter strains in an aquatic system. Bioresource Technology. 2001;80:101–109. doi: 10.1016/s0960-8524(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Fischer S, Magris S, Mori G. Isolation and characterization of bacteria from the rhizosphere of wheat. World J Microbiol Biotechnol. 2007;23:895–903. [Google Scholar]
- 18.Aquilanti L, Favilli F, Clementi F. A Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol Biochem. 2004;36:1475–1483. [Google Scholar]
- 19.Staley J, Brenner D, Krieg N. The Proteobacteri, Part B The Gammaproteobacteria. In: Garrity GM, editor. Bergey’s manual of systematic bacteriology. 2. Springer; Michigan: 2005. pp. 384–402. [Google Scholar]
- 20.Sar bay GF. Growth and nitrogen fixation dynamic of Azotobacter chroococcum in nitrogen-free and omw containing medium [ Department of Food Engineering. The Middle East Technical University; 2003. [Google Scholar]
- 21.Tejera N, Lluch C, Martinez-Toledo MV, Gonzalez-Lopez J. Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere. Plant Soil. 2005;270:223–232. [Google Scholar]
- 22.Farajzadeh D, Yakhchali B, Aliasgharzad N, Sokhandan-Bashir N, Farajzadeh M. Plant Growth Promoting Characterization of Indigenous Azotobacteria Isolated from Soils in Iran. Curr Microbiol. 2012;64:397–403. doi: 10.1007/s00284-012-0083-x. [DOI] [PubMed] [Google Scholar]
- 23.Sabra W, Zeng AP, Lunsdorf H, Deckwer WD. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl Environ Microbiol. 2000;66:4037. doi: 10.1128/aem.66.9.4037-4044.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daiz-Barrera A, Soto E. Biotechnological uses of Azotobacter vinelandii: Current state, limits and prospects. Afr J Biotechnol. 2010;9:5240–5250. [Google Scholar]
- 25.Reid NM, Bowers TH, Lloyd-Jones G. Bacterial community composition of a wastewater treatment system reliant on N2 fixation. Appl Microbiol Biotechnol. 2008;79:285–292. doi: 10.1007/s00253-008-1413-6. [DOI] [PubMed] [Google Scholar]
- 26.Malboobi MA, Owlia P, Behbahani M, Sarokhani E, Moradi S, Yakhchali B, et al. Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World Journal of Microbiology and Biotechnology. 2009;25:1471–1477. [Google Scholar]
- 27.Kumar V, Narula N. Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum mutants. Biol Fertil Soils. 1999;28:301–305. [Google Scholar]
- 28.Hardy RWF, Holsten RD, Jackson EK, Burns RC. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiology. 1968;43:1185. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthukumarasamy R, Revathi G, Lakshminarasimhan C. Influence of N fertilisation on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp. from Indian sugarcane varieties. Biol Fertil Soils. 1999;29:157–164. [Google Scholar]
- 30.Natheer SE, Muthukkaruppan S. Assessing the in vitro zinc solubilization potential and improving sugarcane growth by inoculating Gluconacetobacter diazotrophicus. Ann Microbiol. 2012;62:435–441. [Google Scholar]
- 31.Ravikumar S, Kathiresan K, Ignatiammal S, Babu Selvam M, Shanthy S. Nitrogen-fixing Azotobacters from mangrove habitat and their utility as marine biofertilizers. J Exp Mar Biol Ecol. 2004;312:5–17. [Google Scholar]
- 32.Methods for studing biological soil crusts. Methodology Paper of the 4th International Conference on IL TER East Asian and Pacific Region; 2001. [Google Scholar]
- 33.Rodelas B, Gonzalez-Lopez J, Pozo C, Salmeron V, Martinez-Toledo MV. Response of faba bean (Vicia faba L.) to combined inoculation with Azotobacter and Rhizobium leguminosarum bv. viceae. Appl Soil Ecol. 1999;12:51–59. [Google Scholar]
- 34.Nosrati R, Owlia P, Saderi H, Olamaee M, Rasooli I, Akhavian Tehrani A. Correlation between nitrogen fixation rate and alginate productivity of an indigenous Azotobacter vinelandii from Iran. Iran J Microbiol. 2012;4:153–159. [PMC free article] [PubMed] [Google Scholar]
- 35.Alikhani HA, Saleh-Rastin N, Antoun H. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil. 2006;287:35–41. [Google Scholar]
- 36.Islam M, Sharif D, Hossain M. A Comparative Study Of Azotobacter spp. From Different Soil Samples. J Soil Nat. 2008;2:16–19. [Google Scholar]
- 37.Rajeswari K, Kasthuri M. Molecular characterization of Azotobacter spp. nifH gene Isolated from marine source. Afr J Biotechnol. 2009;8:6850–6855. [Google Scholar]
- 38.Malboobi MA, Behbahani M, Madani H, Owlia P, Deljou A, Yakhchali B, et al. Performance evaluation of potent phosphate solubilizing bacteria in potato rhizosphere. World J Microbiol Biotechnol. 2009;25:1479–1484. [Google Scholar]


