Abstract
A number of articles have dealt with the importance and mechanisms of the sympathetic nervous system alterations in experimental animal models of hypertension. This review addresses the role of the sympathetic nervous system in the pathophysiology and therapy of human hypertension. We first discuss the strengths and limitations of various techniques for assessing the sympathetic nervous system in humans, with a focus on heart rate, plasma norepinephrine, microneurographic recording of sympathetic nerve traffic, and measurements of radiolabeled norepinephrine spillover. We then examine the evidence supporting the importance of neuroadrenergic factors as “promoters” and “amplifiers” of human hypertension. We expand on the role of the sympathetic nervous system in two increasingly common forms of secondary hypertension, namely hypertension associated with obesity and with renal disease. With this background, we examine interventions of sympathetic deactivation as a mode of antihypertensive treatment. Particular emphasis is given to the background and results of recent therapeutic approaches based on carotid baroreceptor stimulation and radiofrequency ablation of the renal nerves.
Keywords: essential hypertension, sympathetic nervous system, human obesity, renal failure, renal denervation
The past 30 years have seen a renaissance of the interest of investigators and clinicians for the sympathetic nervous system, a development supported by multiple lines of evidence. First, sympathetic neural factors are involved in the genesis of life-threatening cardiac arrhythmias and sudden death1, particularly in patients with advanced heart failure, severe obesity or sleep apnea syndrome, i.e. clinical conditions which display as a common pathophysiological hallmark a marked activation of the sympathetic cardiovascular drive2–4. Second, abnormalities in sympathetic modulation of the cardiovascular function have been reported in metabolic disease, such as diabetes mellitus, obesity and metabolic syndrome, promoting cardiovascular complications and favoring directly or indirectly the disease progression3,5,6. Third, in a number of diseases, such as congestive heart failure, acute myocardial infarction, acute ischemic stroke, malignant cardiac arrhythmias, renal failure and chronic obstructive pulmonary disease, the sympathetic overdrive has been reported to be an independent predictor of mortality7–13. Finally, recent developments have suggested, although not univocally14–17, that in patients with true resistant hypertension radiofrequency ablation of bilateral sympathetic afferent and efferent renal nerves might trigger a blood pressure reduction, thereby emphasizing the importance of the adrenergic overdrive as a target of therapeutic blood pressure lowering interventions18–21.
The present paper will provide an in-depth review of the knowledge available on the behavior of the sympathetic nervous system in uncomplicated and complicated human hypertension. Following an introductory part critically addressing the pros and cons of the methodological approaches used in clinical research to assess human sympathetic neural function, the paper will examine the evidence available in favor or against the hypothesis that sympathetic activation may represent a hallmark of the essential hypertensive state. This will be followed by a discussion of sympathetic abnormalities in the escalating and challenging problems of hypertension associated with obesity or renal failure. This will allow to discuss the metabolic-sympathetic as well as the renal-sympathetic crosstalks which may concur at the marked potentiation of the sympathetic overdrive detectable in these diseases. Finally, we review the background and the results of recent clinical studies aimed at assessing the effects of carotid baroreceptor stimulation and renal denervation on cardiovascular sympathetic drive and blood pressure. The future perspectives of the research in the field of the adrenergic nervous system in human hypertension will be finally highlighted.
Methods to assess human sympathetic cardiovascular function
An understanding of methods for evaluating the sympathetic nervous system is facilitated by appreciation of two major features. The first is the profound regional differences in the characteristics, control, and function of the sympathetic nervous system. There are examples of qualitative, not simply quantitative, differences in sympathetic activity to various regional beds in both physiologic and pathologic states22,23. Second, there are multiple levels of the sympathetic nervous system. These include 1. central neural regulation of sympathetic nerve activity to different regions, 2. ganglionic transmission, 3. prejunctional modulation of the release of norepinephrine (and other postulated neurotransmitters) at the postganglionic neuroeffector junction, 4. clearance and reuptake of the neurotransmitter, 5. adrenergic receptors, and 6. the responsiveness of the effector22,23. As a result of these complexities, there is no such thing as "overall" or "generalized" sympathetic nerve activity or tone, and one cannot obtain an overall assessment of the sympathetic nervous system with any single method. Each method must be judged by its strengths and limitations and by the insight being sought.
Heart rate
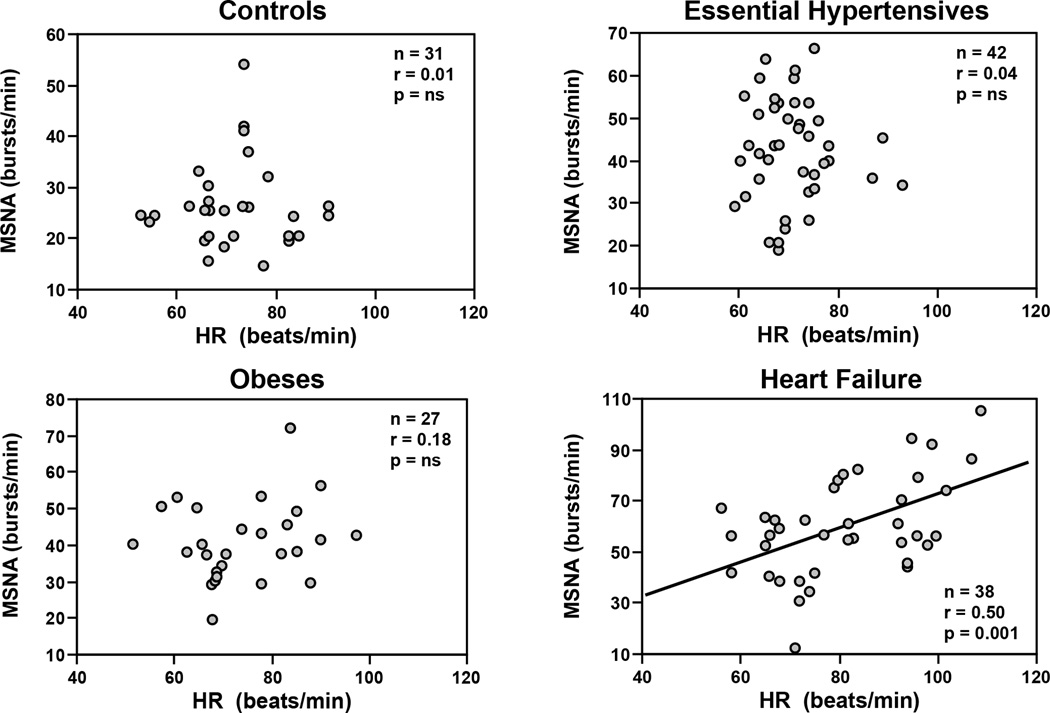
Throughout the years several techniques have been developed systemic and regional sympathetic neuroadrenergic activity22. One approach has been assessment of heart rate, based on the evidence that heart rate is regulated in part by the positive chronotropic effects of norepinephrine and epinephrine in addition to the negative chronotropic effects of vagal parasympathetic inhibitory influence on the sinus node1,22 There are, however, several limitations to heart rate as a specific and sensitive marker of sympathetic adrenergic drive. Heart rate is not a specific marker of cardiac adrenergic activity because it is also regulated substantially by vagal cholinergic influences so that changes in heart rate cannot be taken as a measure of adrenergic drive without the benefit of the response to beta adrenergic blockade to assess the sympathetic contribution. In addition, there are both quantitative and qualitative differences between sympathetic activity to the heart as compared with the kidney or skeletal muscle. For example in several conditions characterized by an increase in norepinephrine spillover rate or muscle sympathetic nerve traffic, heart rate may be within normal range22. In a study of baseline heart rate, muscle sympathetic nerve traffic and venous plasma norepinephrine in patients with essential hypertension, obesity or heart failure, the relationship between heart rate and muscle sympathetic nerve traffic was of moderate degree (heart failure) or absent (essential hypertension and obesity) (Figure 1)23. This suggests that heart rate may not reflect sympathetic overdrive to other important regional circulations in heart failure, hypertension, obesity and other conditions. These and other studies24,25 indicate that despite the ease of measurement, heart rate cannot be taken as either a marker of cardiac sympathetic adrenergic activity or a reliable indicator of sympathetic activity to other regional circulations.
Figure 1.
Relationships between heart rate (HR) and muscle sympathetic nerve traffic (MSNA), as assessed by the microneurographic technique in the peroneal nerve (MSNA, expressed as bursts frequency over time), in healthy controls, essential hypertensive patients, obese subjects and patients with heart failure. In each panel number of subjects (n), correlation coefficients (r) and P values are shown. Note that only in heart failure patients a significant relationship was found. Figure redrawn from data of Ref 23.
Power spectral analysis of heart rate
Given the limitations of heart rate per se as a marker of cardiac sympathetic activity, power spectral analysis of heart rate has gained popularity for assessment of cardiac sympathetic in addition to parasympathetic cholinergic influences on the sinus node22. The approach has had appeal because it is noninvasive and relatively easy and inexpensive to perform. Despite this appeal, the method has significant limitations, particularly as a quantitative and specific indicator of cardiac sympathetic as opposed to parasympathetic activity, and its insight does not extend beyond sympathetic control of heart rate26. The same reservations extend to power spectral analysis of arterial pressure as an indicator of generalized sympathetic activity26.
Plasma norepinephrine
A common and traditional approach for assessing adrenergic drive is measurement of plasma norepinephrine. The advantages of this approach are its relatively easy performance and wide applicability even in large scale studies. However, the sensitivity and reproducibility of the measurements are far from optimal22. The limited reproducibility can be circumvented by repeating the sampling several times and averaging the values, in which case, the results of the assay approach values of reproducibility displayed by microneurographic recording of efferent postganglionic muscle sympathetic nerve traffic22. The sensitivity of the method is more challenging as a result of several factors. First, circulating norepinephrine levels are only a minor fraction of the amount secreted from sympathetic nerve terminals27. As a result, plasma norepinephrine values may not reflect sympathetic neural drive and secrection of the neurotransmitter27,28. For example, Meredith and coworkers, demostrated that in patients with autonomic failure, elevated plasma norepinephrine responses to head-up tilt do not reflect an increase in the release of norepinephrine from the adrenergic nerve terminal, but rather a reduced tissue clearance process of norepinephrine dependent on a reduction in tissue perfusion29. Finally, there is a conceptual limitation to measurement of plasma norepinephrine as an indicator of sympathetic activity. Specifically, it treats the sympathetic nervous system as a humoral system that can be assayed with a single circulating marker. As mentioned earlier, the sympathetic nervous system has multiple levels and components. There can be profound regional differences in sympathetic nerve activity in physiologic and pathologic states. Thus, there is no such thing as “overall” or “generalized” sympathetic activity. Consequently, measurement of plasma norepinephrine (or any other single measurement) cannot be taken as a sensitive or specific marker of overall sympathetic activity and may miss alterations in sympathetic activity in various regions or levels.
This brings us to a discussion of the strengths and limitations of the two methods that have greatly advanced understanding of the sympathetic nervous system in humans in the past 35 years: 1. Norepinephrine isotope dilution method for measurement of regional and total norepinephrine spillover and 2. Direct intraneural microneurographic recordings of postganglionic efferent sympathetic nerve activity to skeletal muscle or cutaneous circulations. Although each of these methods has its limitations, the two methods have proven highly complementary and have been primarily responsible for enormous advances in understanding sympathetic nervous system alterations in human hypertension, often in ways not currently realized in experimental animals using available techniques.
Regional and total norepinephrine spillover
The norepinephrine isotope dilution method involving infusion of small doses of radiolabeled norepinephrine represents an approach that overcomes several limitations related to plasma norepinephrine measurement22,27,28. This method permits precise quantification of the “net” release of the adrenergic neurotransmitter from sympathetic nerves and the amount of norepinephrine undergoing “clearance” from the bloodstream27,28. Given the regional differences in sympathetic regulation, an advantage of the norepinephrine spillover method is its ability to quantity sympathetic drive selectively to several regional circulations22,27,28. Measurement of regional norepinephrine spillover to kidney and heart is the distinctive strength of this method. No other method currently available provides direct quantitative measurement of sympathetic adrenergic drive to the kidney or heart in humans. As mentioned later, an example of this advantage is the evidence that in normotensive obese humans, there are marked increases in renal norepinephrine spillover, but decreases in cardiac norepinephrine spillover despite the frequent presence of elevated heart rate30. Despite its impressive advantages, there are several practical limitations to widespread use of this method for assessing regional norepinephrine spillover. The measurements require the use of radiolabeled norepinephrine in humans and catheterization of renal vessels and the coronary sinus for assessment of renal and cardiac norepinephrine spillover, respectively. These requirements limit widespread use of this method. Fortunately, the investigators at the Baker Institute in Melbourne, Australia who developed the method, have mastered its use. As a result of their work, this method has yielded valuable insights into the sympathetic nervous system in humans, including human hypertension.
Microneurography
The microneurographic method for direct intraneural recording of efferent postganglionic sympathetic nerve activity to skeletal muscle and skin has contributed greatly to study of sympathetic cardiovascular drive in humans. There are profound differences in the characteristics and control of sympathetic nerve activity to skeletal muscle and skin, and the focus in most microneurographic studies of cardiovascular control has been on sympathetic activity to skeletal muscle The technique is “minimally” invasive, requiring the percutaneous insertion and positioning of a 200 micron tungsten recording electrode in superficial nerves (usually peroneal or radial nerve), allowing to directly record efferent postganglionic sympathetic neural bursts22. The advantages of the approach include: 1) direct measurement of central nervous system sympathetic neural outflow to either the skeletal muscle circulation or to skin; 2) a continuous moment-to-moment recording of sympathetic nerve activity, allowing a dynamic assessment of sympathetic drive in a given experimental session; 3) the ease of performing repeated recordings over time; and 4) the high reproducibility of the activity between recordings in two different nerves and over time22. The limitations are two-fold. First, postganglionic sympathetic nerve traffic may not always reflect the release of the neurotransmitter at the neuroeffector junction. Second, microneurographic recordings do not provide direct information on sympathetic activity to visceral tissues such as the kidney and heart. In healthy humans there are good relationships between the microneurographic data collected in the peroneal nerve and the ones characterizing the cardiac or the renal neural network, but this may not pertain in pathologic states22,.
Sympathetic adrenergic neuroimaging
One further approach of investigating sympathetic activity in humans deserves to be briefly mentioned, namely the so-called “neuroimaging technique”, which employs radiolabeled sympathetic amines (specifically 123-metaiodobenzylguanidine) to provide imaging of innervation of a given organ, most frequently the heart22. The cost of the method and its inability to provide dynamic, rather than static, assessment of adrenergic cardiovascular drive have limited its widespread use in clinical research.
In summary, measurements of regional norepinephrine spillover to kidney and heart and direct microneurographic recordings of central neural sympathetic traffic to the skeletal muscle circulation represent the gold standards for evaluating the sympathetic nervous system in humans today. They have complementary strengths and limitations and together have greatly advanced understanding of sympathetic cardiovascular regulation in human hypertension and beyond. The current review draws mainly on the results of studies employing these two state of the art methods.
Evidence in favor and against adrenergic overdrive as hallmark of human hypertension
Studies examining indirect and direct markers of sympathetic function have provided compelling evidence in the early stages of hypertension or in young hypertensives, the sympathetic nervous system is activated. In classic studies by Julius et al31, young subjects with hyperkinetic borderline hypertension, had increased heart rate which was frequently associated with an elevation in plasma norepinephrine. Subsequent studies employing microneurography or measurements of norepinephrine spillover in young adults with borderline or established hypertension have also demonstrated marked sympathetic activation28,32,33. Although there are several exception34,35, the weight of evidence suggest that in the early stages of hypertension, particularly in young patients, there is marked adrenergic overdrive, a finding that supports the hypothesis that sympathetic overactivity occurs in the clinical phases of essential hypertension. It is more challenging to know: 1) if the adrenergic activation precedes and promotes the elevation of blood pressure and 2) if the sympathetic overdrive is also present in stable hypertensive state of middle age or elderly patients. As far as the first issue is concerned, old data from the Framingham study seem to support the possibility that adrenergic overdrive precedes the elevation of blood pressure, by showing that young adults who display a resting tachycardia are more prone to develop an elevation in blood pressure over years than are age-matched controls with normal heart rate36. This finding is supported by the data collected in a longitudinal study in which arterial epinephrine (but not norepinephrine) predicted the development of hypertension during the twenty year follow-up37. As far as the second issue is concerned, microneurographic studies almost universally shown that sympathetic nerve traffic is potentiated in established hypertension in middle age and in elderly as well as young patients38–46. This finding suggests that neuroadrenergic factors may contribute to the maintenance and progression as well as the development of the hypertensive state. Interestingly, studies of elderly hypertensives have not always documented an increase in renal norepinephrine spillover in contrast to the finding of increased sympathetic nerve traffic to the skeletal muscle circulation22,28,39,47. This highlights the regional differences in sympathetic nerve activity in some pathologic states and emphasizes the value of direct measurements of sympathetic activity to specific regional circulations.
Adrenergic neural factors may also contribute to the development and progression of target organ damage, as documented by the pronounced increase in sympathetic nerve traffic and cardiac norepinephrine spillover rate in patients in which hypertension is complicated by left ventricular hypertrophy or left ventricular diastolic dysfunction40,42,44,45. A similar potentiation has been described when renal damage complicates the hypertensive state (see below)48–51. Finally, the arteriolar remodeling that characterizes hypertension and makes the elevation of total peripheral resistance partly dependent on structural factors, ie, an increased wall-to-lumen ratio, has been found to be in large part mediated by sympathetic neural influences throughout direct effects on the different components of the arterial vascular wall52,53.
In discussing the evidence in favor and against adrenergic overdrive as hallmark of the hypertensive state it is worthy to mentioning that sympathetic activation may not be confined to the essential or idiopathic hypertensive state. Some studies have reported that patients with hypertension secondary to renal artery stenosis or primary aldosteronism also display an increase in sympathetic nerve traffic to skeletal muscle circulation54–56. In contrast, other microneurographic studies failed to show an increase in sympathetic nerve traffic in these forms of secondary hypertension38,54.
In summary, measurements of sympathetic nerve traffic to skeletal muscle and of norepinephrine spillover from kidney and heart in the past several decades provide direct evidence for sympathetic activation to skeletal muscle, kidney and heart in the early as well as established stages of human hypertension. This evidence supports the concept that the sympathetic nervous system contributes to the development and maintenance of hypertension and to end organ damage. The sympathetic overactivity is not, however, uniform or universal. It does not, for example, extend to skin sympathetic activity57. Because of limited methodology, there is much less information about sympathetic activity to the splanchnic circulation in humans. Given the recent interest in the role of the splanchnic circulation in animal models of hypertension, this highlights the need for advances in methodology for direct measurement of sympathetic activity to splanchnic circulation in humans.
Sympathetic nervous system in obesity-related hypertension and the metabolic syndrome: Insights from studies in humans
During the past 20 years, there has been burgeoning interest in the role of the sympathetic nervous system in obesity, hypertension, and the metabolic syndrome. Drawing frequently on the power of microneurographic recordings58 and measurements of regional norepinephrine spillover59–60, studies of humans have provided unique insights not feasible in studies of experimental animals. These studies have demonstrated that while obesity and essential hypertension are both characterized by sympathetic overactivity, there are features of the altered sympathetic nervous system in human obesity and obesity-associated hypertension that are distinct from those in essential hypertension. Most notably, elevated sympathetic activity in obesity often occurs in the absence of hypertension3,59,60–61.
Sympathetic nervous system in obese normotensive and hypertensive humans
There have been two major competing concepts regarding the role of the sympathetic nervous system in obesity. The first, introduced by Landsberg62, postulated that obesity produces insulin resistance and hyperinsulinemia which stimulates the sympathetic nervous system and promotes thermogenic metabolism. The second, advanced by Bray63, proposed that most obese states are characterized by underactivity of the sympathetic nervous system that decreases thermogenesis and predisposes to obesity. Sophisticated studies using microneurography and/or radiolabeled norepinephrine spillover have unequivocally supported Landsberg’s concept of sympathetic overactivity in most human obesity3,60–61,64. In an exception, however, there is evidence that lean Pima Indians have lower muscle sympathetic nerve traffic than lean Caucasians65. According to Bray’s hypothesis63, this could contribute to the known predisposition of Pima Indians to obesity. It has also been speculated that the lower sympathetic activity may explain why Pima Indians have a low prevalence of obesity-associated hypertension66.
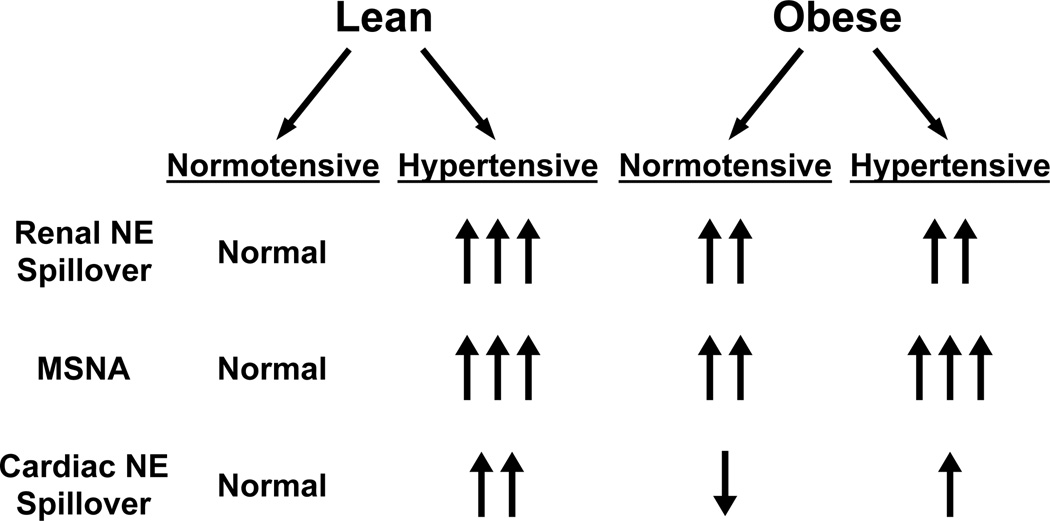
A remarkable feature of sympathetic overactivity in human obesity is that it often occurs in the absence of hypertension3,59,64. Furthermore, the neurophysiologic alterations in the sympathetic nervous system in obese normotensive and hypertensive humans differ from those in lean hypertensives (Figure 2). In obese hypertensive humans, there is enhanced muscle sympathetic nerve activity, but the neurophysiologic basis of this increase is different in obese vs lean hypertensive subjects67. In lean hypertensive patients, the increased sympathetic activity is related to increased firing frequency of single nerve fibers. In contrast, in obese normotensive and hypertensive subjects, the increased activity is due to recruitment of additional fibers and not to increasing firing frequency of single nerve fibers67. The precise mechanistic and hemodynamic significance of increased single fiber vs multifiber sympathetic activity is not clear, but the contrast between the neurophysiologic pattern of increased muscle sympathetic nerve traffic in obese vs lean hypertensives suggests that the mechanisms of adrenergic activation differ in lean and obese human hypertensives.
Figure 2.
Summary of differences in sympathetic activity in obese and lean normotensive and hypertensive subjects. NE: norepinephrine, MSNA: muscle sympathetic nerve activity.
There are two other notable features of sympathetic activity in obese normotensive and hypertensive humans. First, cardiac norepinephrine spillover is decreased in obese normotensives and is only marginally increased in obese hypertensives59,60–61. This contrasts with marked elevation in cardiac norepinephrine spillover in lean hypertensives60. Second, in obese humans renal norepinephrine spillover is not significantly higher in hypertensive than it is in normotensive subjects60–61. In contrast, in lean humans renal norepinephrine spillover is significantly higher in hypertensive vs normotensive subjects27,28. Given the view that sympathetic activity to the kidney is critical to the pathogenesis of hypertension, the finding that renal norepinephrine spillover is not different in obese normotensives and hypertensives suggests that increased renal sympathetic activity is not a sufficient explanation for the development of obesity-associated hypertension in humans61. The contribution of renal sympathetic activity to the development of hypertension appears to be greater in lean vs obese individuals61.
Two studies using pharmacologic antagonists to evaluate the contribution of the sympathetic nervous system to obesity-associated hypertension merit discussion. Wofford et al68 compared the antihypertensive effect of combined alpha and beta adrenergic receptor blockade for one month in obese vs lean hypertensive patients with comparable baseline blood pressure. Based on the previous discussion, one might predict that adrenergic blockade would produce a greater fall in blood pressure in lean hypertensive patients. Surprisingly, this was not the result. Adrenergic blockade produced a significantly greater decrease in blood pressure in obese vs lean hypertensive patients. The effect of adrenergic blockade on blood pressure in obese normotensive subjects was not studied.
Shibao et al69 evaluated the sympathetic contribution to blood pressure in obesity using a different strategy. These investigators employed acute ganglionic blockade to reversibly disrupt sympathetic activity in lean and obese normotensive subjects and in obese hypertensive subjects. There were three key findings. First, ganglionic blockade produced an exaggerated fall in vascular resistance and blood pressure in obese compared with lean normotensive subjects. This indicates that even in the absence of overt obesity-associated hypertension, obesity is associated with an exaggerated sympathetic contribution to blood pressure in humans. Among other implications, this may help explain why weight loss tends to decrease blood pressure in obese individuals even without a history of hypertension. Second, the fall in blood pressure with ganglionic blockade in the obese hypertensives was double that in the obese normotensives, consistent with an important role for the sympathetic nervous system in maintaining blood pressure in obese hypertensives. Third, after ganglionic blockade, blood pressure was normalized in the obese normotensives. Blood pressure also decreased in the obese hypertensives but it did not completely indicating that factors other than the sympathetic nervous system contribute to obesity-associated hypertension. This study supports an exaggerated sympathetic contribution to blood pressure in both normotensive and hypertensive obese humans.
So there are two seemingly conflicting observations. First, renal norepinephrine spillover, although elevated, is not higher in hypertensive vs normotensive obese humans. Second, pharmacologic studies indicate that the sympathetic contribution to blood pressure is greater in hypertensive vs normotensive obese humans. What is the explanation(s) for these seemingly conflicting observations. The most likely is a prohypertensive contribution of increased muscle sympathetic nerve activity which is higher in hypertensive vs normotensive obese humans67. This explanation is consistent with the finding that ganglionic blockade produced an exaggerated fall in vascular resistance in obese hypertensives69. An additional explanation may be that heightened sympathetic activity is a necessary but not sufficient cause for the development of hypertension in obesity61. The residual elevation in blood pressure in obese hypertensives after ganglionic blockade in the study by Shibao et al69 indicates, not surprisingly, that factors other than the sympathetic nervous system contribute to the hypertension in obesity. The interaction with these factors may enhance the prohypertensive influence of the sympathetic nervous system on blood pressure and the development of obesity-associated hypertension.
Sympathetic nervous system in the metabolic syndrome
Obese humans with the metabolic syndrome have elevated muscle sympathetic nerve traffic values even in the absence of hypertension6. During diet-induced weight loss, the decreases in sympathetic activity are accompanied by reduction in all components of the metabolic syndrome70. Muscle sympathetic nerve activity correlates directly with waist circumference in subjects with the metabolic syndrome6. This is consistent with evidence that muscle sympathetic nerve traffic correlates with visceral but not subcutaneous fat in obesity71–72. Visceral adiposity is a key driver of the metabolic syndrome.
Huggett et al73 demonstrated that patients with metabolic syndrome without hypertension had increased muscle sympathetic nerve activity (specifically increased single fiber firing) when compared to a control group matched for body weight and lacking the metabolic syndrome. The presence of hypertension in addition to other components of the metabolic syndrome was associated with further increases in single fiber firing and multifiber muscle sympathetic nerve activity. This suggests that the metabolic syndrome is accompanied by greater sympathetic drive than is obesity in the absence of metabolic syndrome. We speculate that this may reflect the influence of visceral as opposed to subcutaneous fat.
Mechanisms of sympathetic overactivity and hypertension in human obesity
Since the discovery of leptin in 1994, there has been an explosion of research on the genetic neurobiologic mechanisms of obesity. This in turn has fueled mounting interest and insights, particularly in experimental animals, on the mechanisms of obesity-associated hypertension74–77. Many of these mechanisms such as the role of the renin-angiotensin system, the kidney, reflex mechanisms, obstructive sleep apnea, and brain oxidative and endoplasmic stress relate to mechanisms in experimental models of essential hypertension and are not discussed here, but several are distinct to obesity-associated hypertension. In 1999, it was suggested that the emerging biology of obesity prompted two new concepts regarding obesity-associated hypertension78. First, the effect of obesity on blood pressure may depend critically on the genetic-neurobiologic mechanisms underlying the obesity. Second, obesity is not consistently associated with increased blood pressure. We focus here on evidence from humans relating to three possible mechanisms that are of particular relevance to the role of sympathetic nervous system in to obesity-associated hypertension and to the role of the genetic-neurobiologic mechanisms of obesity in the regulation of the sympathetic nervous system and blood pressure.
Interaction of insulin, the sympathetic nervous system and insulin sensitivity
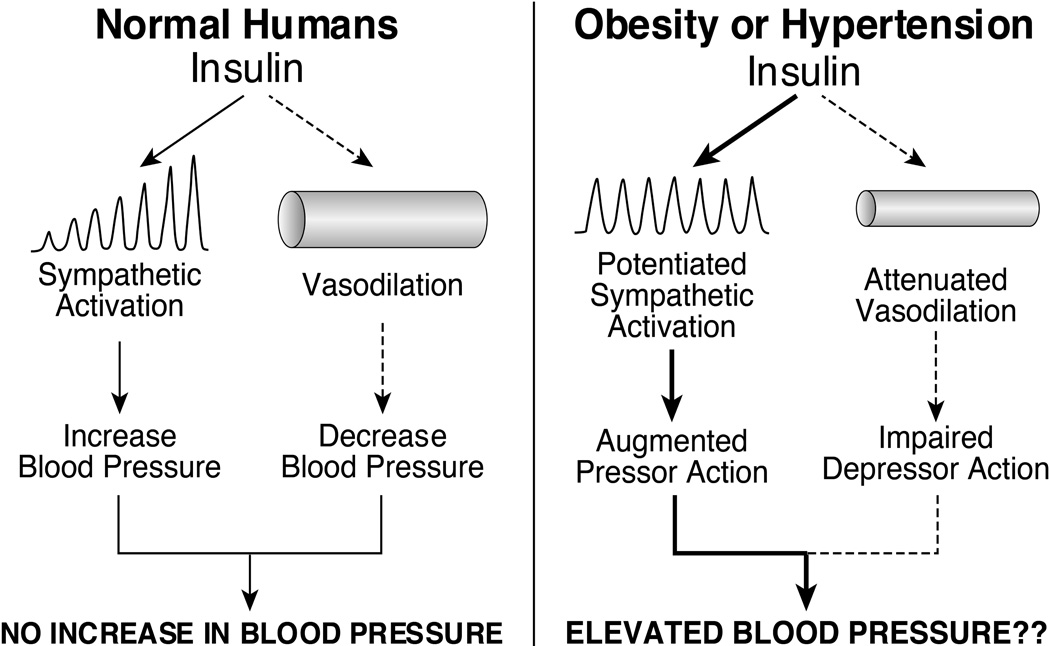
Landsberg62 proposed that the sympathoexcitatory response to obesity is triggered by hyperinsulinemia and that the sympathetic activation in turn increases vascular resistance. Indeed, the concept that insulin-induced sympathetic activation produces vasoconstriction is a linchpin of the hypothesis that hyperinsulinemia is a major contributor to obesity-associated hypertension. Two decades ago, studies from several laboratories demonstrated that hyperinsulinemic, euglycemic clamp produced striking increases in muscle sympathetic nerve traffic and plasma norepinephrine in healthy lean subjects25–79–80. The surprising finding was that despite insulin-induced sympathetic activation, insulin failed to increase blood pressure because the sympathetic vasoconstriction was opposed and overridden by a vasodilator action of insulin25. These findings were consistent with studies in dogs demonstrating that physiological increases in plasma insulin failed to increase blood pressure because increases in cardiac output were offset by substantial decreases in vascular resistance81. Thus, in normal humans, hyperinsulinemia increases sympathetic nerve activity, but does not increase blood pressure because the sympathetic activation is offset by a direct vasodilator action of insulin82 (Figure 3, left panel). There is, however, evidence that the balance between the vasodilator and sympathetic actions of insulin are altered in obesity and hypertension in favor of the sympathetic pressor action. Laakso et al83 reported that insulin-induced vasodilation in skeletal muscle is reduced in obese, insulin resistant humans. In contrast, Lembo et al84 demonstrated that insulin-induced increases in sympathetic activity and norepinephrine release in skeletal muscle are substantially exaggerated in hypertensive patients (Figure 3, right panel). These observations raise the possibility, not yet established experimentally, that in obese, hypertension-prone individuals, chronic hyperinsulinemia might contribute to increases in blood pressure as well as sympathetic nerve activity.
Figure 3.
Schematic diagram depicting the balance between the sympathetic and vasodilator actions of insulin in the regulation of blood pressure normal humans (left panel) and the potential effect on blood pressure of potentiated sympathetic action and/or attenuated vasodilator action of insulin in obesity and hypertension (right panel).
An iconoclastic and major development in the insulin-sympathetic story was the demonstration that reflex sympathetic activation promotes insulin resistance in skeletal muscle. In studies of insulin-induced vasodilation in humans, Laakso et al83 introduced the concept that insulin-induced increases in skeletal muscle blood flow play an important role in glucose delivery and uptake, i.e. impaired insulin-induced vasodilation in skeletal muscle contributes importantly to reduced glucose uptake in skeletal muscle and to insulin resistance. Shortly thereafter, two key studies demonstrated that reflex sympathetic vasoconstriction in the forearm of normal subjects decreased glucose uptake during hyperinsulinemia85,86. Recently, Gamboa et al87 expanded on this discovery by demonstrating that ganglionic blockade improved insulin-glucose metabolism in obese insulin resistant subjects with elevated baseline muscle sympathetic nerve activity but not in obese insulin sensitive subjects. These studies advanced the concept that the sympathetic nervous system promotes insulin resistance in skeletal muscle through hemodynamic mechanisms and that the interaction of insulin, the sympathetic nervous system and insulin sensitivity is a two way street88. Insulin increases sympathetic activity. In turn, increased sympathetic vasoconstriction decreases glucose uptake in skeletal muscle and thereby promotes insulin resistance and compensatory hyperinsulinemia.
Brain melanocortin pathway, sympathetic activity and obesity-associated hypertension
The brain melanocortin pathway has emerged from studies in both experimental animals74,75 and humans89,90 as a crucial pathway in the regulation of sympathetic activity and BP in obesity. In addition to inhibiting appetite and increasing metabolism, stimulation of hypothalamic melanocortin-4 receptors increases sympathetic activity and blood pressure74,75. Rodents with loss of function mutations in the hypothalamic melanocortin-4 receptors are obese but lack sympathetic activation and obesity-associated hypertension74,75. There are rare patients with obesity caused by loss of function mutations in hypothalamic melanocortin-4 receptors. These individuals have lower sympathetic activity and blood pressure than control, weight-matched patients with common human obesity89,90. In addition, administration of an hypothalamic melanocortin-4 receptors agonist in healthy subjects increases blood pressure89. These data support the concept, discussed above, that the genetic neurobiologic basis of obesity can critically influence the sympathetic and blood pressure response to obesity78, and demonstrate that hypothalamic melanocortin-4 receptors contribute to regulation of sympathetic activity and blood pressure in obese humans.
Leptin, the sympathetic nervous system and obesity-associated hypertension
In addition its effects on appetite and metabolism, leptin acts in the brain to produce receptor-mediated increases in regional sympathetic activity91. A large body of evidence has demonstrated that leptin contributes to sympathetic overactivity and hypertension in a number of animal models of monogenic and diet-induced obesity74–77. Surprisingly then, evidence that leptin contributes to obesity-associated hypertension in humans is inconclusive77. Ozata et al92 reported that three adults and one child with severe obesity and complete leptin deficiency had sympathetic hypofunction manifest by orthostatic hypotension and an attenuated cold pressor test. Baseline blood pressures were not presented, but the patients reportedly did not have hypertension92. These observations suggest that loss of physiologic leptin action in humans is associated with decreases in sympathetic activity and blood pressure. Machleidt et al93 demonstrated that a bolus injection of leptin in healthy lean men produced an acute increase in muscle sympathetic nerve activity but blood pressure did not increase. The vexing question is whether hyperleptinemia contributes to obesity-associated hypertension in humans. The lack of a safe, effective, reversible leptin antagonist for studies in humans has greatly impeded evaluation of the contribution of leptin to obesity-associated hypertension in humans77. There are, however, data from several studies in relatively large numbers of lean and obese subjects that have failed to show an increase in blood pressure with chronic or acute administration of leptin77. This contrasts with increases in blood pressure with administration of a hypothalamic melanocortin-4 receptors agonist in humans89. We conclude that studies in humans have not yet convincingly demonstrated that hyperleptinemia contributes significantly to obesity-associated human hypertension.
Summary: the sympathetic nervous system in obesity, hypertension and the metabolic syndrome
Obesity-associated hypertension in humans is characterized by sympathetic overactivity, but there are features of the sympathetic nervous system in obesity that are distinct from those in lean hypertensives. Cardiac norepinephrine spillover is lower in obese normotensive and hypertensive patients than in their lean counterparts. Elevated renal norepinephrine spillover and muscle sympathetic nerve traffic are observed in normotensive as well as hypertensive obese subjects. Muscle sympathetic nerve activity is higher in hypertensive vs normotensive obese subjects, but renal norepinephrine spillover is not different in hypertensive vs normotensive obese subjects. This contrasts with essential hypertension where renal norepinephrine spillover is significantly higher in lean hypertensive vs normotensive humans. These observations suggest that renal sympathetic activity is more important in the development of hypertension in lean vs obese individuals. In juxtaposition to the neurophysiologic studies, experiments with pharmacologic antagonists indicate that the sympathetic contribution to vascular resistance and blood pressure is exaggerated in obesity-associated hypertension in humans. These observations suggest an important contribution of elevated muscle sympathetic nerve traffic to obesity-associated hypertension. Muscle sympathetic nerve activity is higher in hypertensive vs normotensive obese subjects. In addition, other factors mediating obesity-associated hypertension may interact to enhance the prohypertensive contribution of elevated sympathetic activity.
In the past 20 years, extensive research in experimental animals has implicated multiple and mounting mechanisms in the pathogenesis of sympathetic activation and hypertension in obesity. In contrast, experimental studies in humans have not yet conclusively identified the major mediators of obesity-associated sympathetic activation and hypertension in humans. This difference between the abundant and compelling data on the mechanisms of obesity-associated sympathetic activation and hypertension in experimental animals and the paucity of conclusive evidence in humans highlights the need for mechanistic, state of the art patient oriented research on this topic.
The renal sympathetic nervous system and kidney-related hypertension
The nerves of the kidney occupy a special place in the panoply of hypertension. The renal sympathetic nerves, through their multiple influences on tubular processing of sodium, on renin secretion and on renal vascular resistance94,95, with activation of the renal sympathetic outflow in essential hypertension as the driver, are pivotal in hypertension pathogenesis. Beyond that, nociceptive sensory nerves of the kidneys which detect renal injury, through projection to the central nervous system, contribute to the systemic sympathetic nervous system activation of severe essential hypertension, renal hypertension and end-stage renal disease.
Renal efferent sympathetic nerves
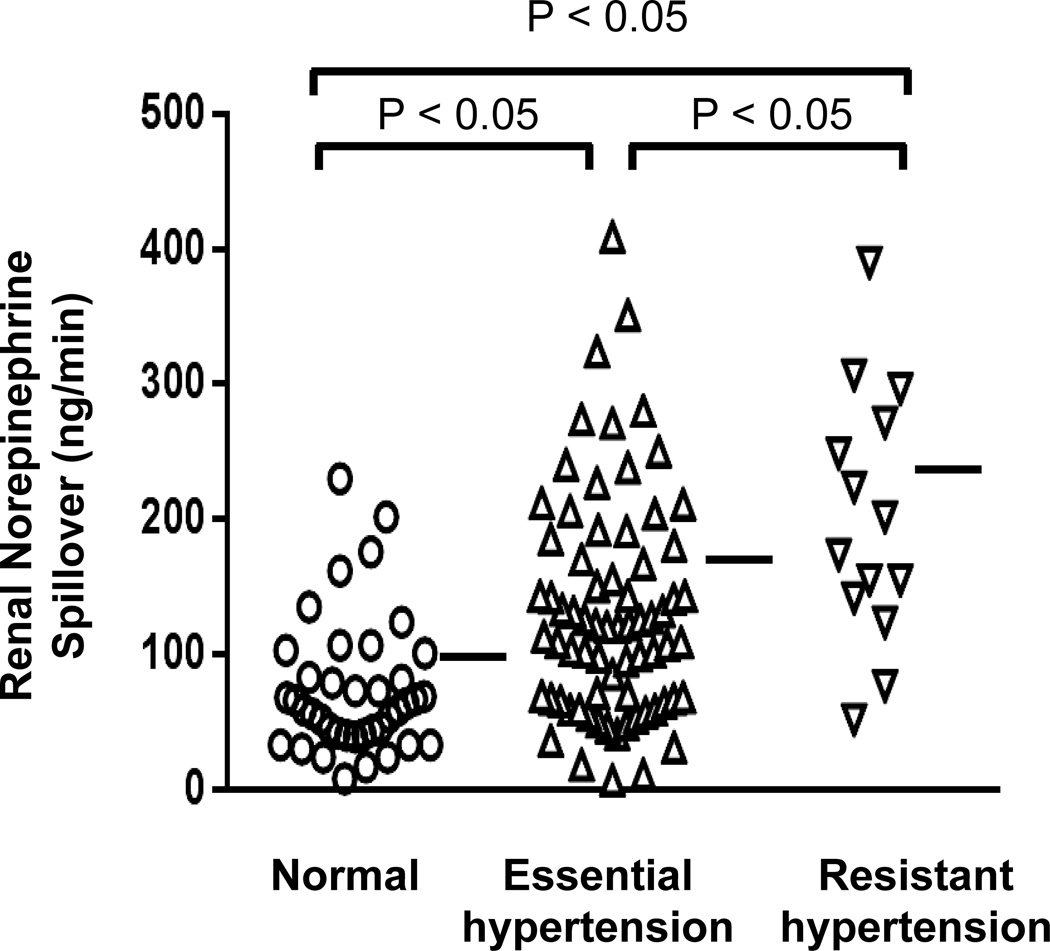
Renal sympathetic activation is thought to be central to the pathogenesis of essential hypertension94–96. In untreated essential hypertensive patients, the application of regional norepinephrine isotope dilution methodology27 demonstrates that a high level of activation of the renal sympathetic outflow is present27 (Figure 4). The sympathetic neural outflow is commonly activated also to the heart, shown with selective cardiac norepinephrine spillover measurements28, and to the skeletal muscle vasculature, demonstrated with microneurographic nerve traffic recording32,33,38–46, but it is the renal sympathetic activation which is central to hypertension pathogenesis96–97.
Figure 4.
Measurements of renal norepinephrine spillover to plasma, used to assess sympathetic activity in the kidneys in healthy volunteers and patients with arterial hypertension. Renal sympathetic activation was commonly evident in hypertensive patients. In untreated patients with mild-moderately severe essential hypertension (middle column), renal norepinephrine spillover was increased overall, and elevated in approximately 50%. In drug-resistant hypertension, with patients administered on average five antihypertensive drug classes, renal norepinephrine spillover was higher again, attributable to their hypertension, and perhaps its treatment. From unpublished data by Murray Esler, Markus Schlaich, Gavin Lambert and Dagmara Hering.
The renal tubules receive a dense sympathetic innervation, at all tubular levels. A specific and important relation of the renal sympathetic nerves to renal tubular sodium reabsorption, key to hypertension pathogenesis, concerns pressure natriuresis, the normal capacity of the kidneys to excrete sodium at higher arterial perfusion pressures98. Impairment of pressure natriuresis is believed to be a central element in the development of hypertension94,95,8. Renal sympathetic denervation shifts the renal pressure-natriuresis curve to the left, promoting urinary sodium excretion and lowering of blood pressure94–98. Surgical sympathectomy in experimental hypertension abolishes the hypertension, or prevents its development94–95.
Renal afferent sympathetic nerves
Renal sensory nerves are of two principal types. The first, of the renal parenchyma and bearing nociceptive receptors, respond to a renal injury signal by projecting to the hypothalamus to increase central sympathetic outflow95–99. The second are pressure-sensitive receptors in the renal pelvis; these are sympathoinhibitory and important in mediating reno-renal reflexes95. The nociceptive renal afferents have been implicated in generating increased systemic sympathetic activity in drug-resistant essential hypertension and chronic kidney disease.
What the renal injury signal is in these two contexts is not known. Renal ischemia has been invoked, but this proposition is unproven. Chemical irritation, from intra-renal phenol injection, can act as a potent stimulus in experimental studies in rats99. In patients with drug-resistant hypertension there are few if any clinical markers of injury, glomerular filtration rate is commonly normal and proteinuria is often absent. But these clinical indices are insensitive, and belie the fact that an injury signal must be operating. Ablation of the renal afferent nerves with endovascular delivery of radiofrequency energy in patients with drug-resistant hypertension causes inhibition of central sympathetic outflow21.
Chronic kidney disease
Sympathetic nervous system activation is present in patients with chronic kidney disease, intimated first with the finding of elevated plasma norepinephrine concentrations100–101, then more definitively with demonstration of whole-body norepinephrine spillover measurements and increased sympathetic nerve firing recorded with clinical microneurography48–51,102. Consistent with this, pronounced blood pressure lowering is observed with pharmacological adrenergic inhibition by clonidine and debrisoquine103. Sympathetic activation is present in the early phases of renal disease, in the absence of uremia, being seen in patients with nephrotic syndrome and with autosomal dominant polycystic kidney disease despite normal renal function, and increases with disease progression103. The mechanism of sympathetic activation appears to be complex, no doubt involving afferent renal nerve signaling, but also perhaps other mechanisms103.
End-stage renal disease
The highest level of systemic sympathetic nervous activation is seen in patients with end-stage renal disease maintained on hemodialysis, equal to or exceeding that seen in New York Heart Association class IV cardiac failure48,101. This has been conclusively demonstrated, in elegant clinical studies, to be generated by renal afferent nerve signaling, not systemic uremic toxins48. The sympathetic activation of end-stage renal disease, and commonly also the hypertension, is not reversed by successful renal transplantation49, but is reversed by bilateral removal of the diseased native kidneys48, the surgery removing the influence of the afferent renal nerves. These clinical observations have a parallel in experimental studies in rats with 5/6 nephrectomy103, where the hypertension and sympathetic activation in this renal failure model is abolished by dorsal rhizotomy, which abolishes renal afferent nerve input to the central nervous system.
The influence of sympathetic activity, and its inhibition on kidney disease progression and clinical outcome
Sympathetic nervous system activation in renal hypertension and end-stage renal disease contributes to the blood pressure elevation, to kidney disease progression and to cardiovascular complications and clinical outcomes. Sympathetic activation aggravates existing hypertension, proteinuria, interstitial fibrosis and glomerulosclerosis104. A striking observation is that of Zoccali and colleagues, who demonstrated the plasma concentration of norepinephrine to predict the incidence of cardiovascular events, and survival, in patients with end-stage renal disease10. High sympathetic activity in the heart is arrhythmogenic, providing a partial explanation for the very high rate of sudden death in chronic renal failure. Some of these effects can be blocked by pharmacological sympathetic inhibition. The central sympatholytic drug, moxonidine has been demonstrated to reduce urinary protein excretion inpatients with type I diabetes, at a dose not influencing blood pressure105, and in another study in patients with chronic renal failure106, to minimize progression of renal disease compared with a comparator drug, nitrendipine, an effect not explained by the small falls in blood pressure.
Catheter-based renal denervation in renal hypertension
Given the importance of the renal afferent nerves in generating high sympathetic nervous activity in renal hypertension and end-stage renal disease, thereby contributing to the blood pressure elevation, on theoretical grounds there might be a special place for catheter-based renal denervation in the treatment of the hypertension of renal disease. To this point only two small pilot studies have been conducted, both uncontrolled but successful, in renal hypertension, and in end-stage renal disease107–108. In any definitive trials, yet to be conducted, there are challenging technical matters which will need to be overcome. The first is to preserve renal function during the necessary angiographic imaging, avoiding radiocontrast nephropathy by, for example, the use of carbon dioxide angiographic imaging. The second technical difficulty, in end-stage renal disease, is that the denervation procedure will often need to be performed on small diameter renal arteries with low blood flow, which increases the risk of damaging the artery. As bilateral nephrectomy is sometimes performed for uncontrollable hypertension in end-stage renal disease, exploring the possible option of renal denervation through a well designed clinical trial is warranted.
Sympathetic deactivation as a goal of antihypertensive treatment: the long and circular path from surgical sympathectomy on the “pressor nerves” to catheter-based renal denervation
Thomas Willis and the seventeenth century London neuroanatomical school he led provided the first accurate depictions of the sympathetic nervous system109. Stimulation of the sympathetic nerves, by Claude Bernard and Charles Brown-Sequard demonstrated them to be vasoconstrictor, and to elevate blood pressure, leading to their designation as the “pressor nerves”110. By the first years of the twentieth century this information, and his own clinical observatons, led Geisbock111 to propose that human hypertension was caused by the influence of the brain on the sympathetic nervous system.
Surgical sympathectomy
In this era no treatment of hypertension was available until the introduction of surgical sympathectomy112. The aim was to surgically sever sections of the sympathetic chain, and all accessible sympathetic nerves of the thorax and abdomen, cutting as many “pressor nerves” as possible to remove their systemic vasoconstrictor influence. Around this time, the first measurements of cardiac output demonstrated that blood pressure elevation in severe hypertension was directly attributable to increased total peripheral resistance, plausibly thought to derive from the pressor nerves, which were now targeted. Selective renal sympathectomy was not performed, as no theory existed suggesting specific importance of the sympathetic nerves of the kidneys in hypertension pathogenesis, although surgical sympathectomy no doubt often interrupted the sympathetic outflow to the kidneys. Surgical sympathectomy for the treatment of hypertension, applied in the years 1935–1960112 took many forms, with the various surgeries being demonstrably of value in lowering blood pressure and prolonging life in patients with severe and malignant hypertension, but at the cost of disabling side effects, most notably postural and postprandial hypotension and syncope, and sexual dysfunction.
Anti-adrenergic drugs
Ganglionic blocking drugs, discovered by Paton and Zaimis113, ended the period of surgical sympathectomy for hypertension, and ushered in the era of antiadrenergic drugs. Ganglion blockers constituted the first antiadrenergic pharmacotherapy for hypertension, and could achieve what surgery sympathectomy achieved minus surgical risk, but regrettably not minus complications, which as expected were almost identical with those of sympathectomy. But the new concept of antiadrenergic antihypertensive pharmacotherapy had been established. Based on identification of the sympathetic neurotransmitter as norepinephrine, documentation of central neural mechanisms controlling sympathetic outflow, and categorization of adrenergic receptors,, centrally-acting sympathetic nervous inhibitors including methyldopa and clonidine, neurone-blocking drugs such as guanethidine and debrisoquine, beta-adrenergic receptor blocking drugs including propranolol, and alpha-adrenergic receptor blockers were developed in quick succession114. Ganglion blockers rapidly became a footnote to history. Antiadrenergic drugs, coupled with diuretics and direct-acting vasodilators such as hydralazine became the preferred antihypertensive therapy for thirty years115.
Drug-resistant hypertension
From the 1990s, drugs antagonizing the renin-angiotensin system have become the dominant antihypertensive therapy. Angiotensin converting enzyme-inhibitor drugs and angiotensin receptor blocking drugs gradually replaced antiadrenergic drugs as the preferred antihypertensive agents because they were at least equally efficacious, and substantially better tolerated. Subsequently joined by dihydropyridine calcium channel blocking drugs, the anti-renin drugs, calcium channel blockers and diuretics came to occupy the top rung of hypertension treatment international guidelines lists115, with antiadrenergic antihypertensive drugs drifting towards the bottom. But there was a problem. Despite the widespread availability and prescribing of angiotensin converting enzyme-inhibitors, angiotensin receptor blockers, diuretics and calcium channel blockers, in a substantial minority of patients with essential hypertension, perhaps 10%116, goal blood pressure were not achieved. In these drug-resistant hypertensives a new strategy was needed, and in fact, devised. This was the development of two device-based therapies targeting the sympathetic nervous system, the surgically implanted barostimulator device117 and catheter-based renal denervation20,118. These new therapies were developed on the well established premise that activation of the sympathetic nervous system commonly initiates and sustains the blood pressure elevation in essential hypertension.
Arterial baroreceptor stimulation
Devices stimulating the human carotid baroreflex were first developed five decades ago119, and after a prolonged absence have had a resurgence. In experimental models of hypertension, activation of central baroreflex pathways by continuous electrical stimulation of the nerves of the carotid sinus baroreceptors reduces sympathetic outflow from the central nervous system and lowers blood pressure. The Rheos implantable carotid sinus stimulator (CVRx, Minneapolis, MN, USA) has been studied in patients with severe drug-resistant hypertension117. In a large-scale, double-blind study, the Rheos device was implanted in 265 patients who were subsequently randomised (2:1) to immediate baroreceptor stimulation for the first 6 months (n=181), or deferred stimulation after 6 months (n=84)117. At 6 months, blood pressure fell in the group receiving immediate treatment, but the primary efficacy endpoint was not reached, partly because blood pressure likewise decreased in many control subjects117. The future of this procedure is therefore uncertain, to be determined by an ongoing clinical trial utilizing a less cumbersome electrode than that used previously, and with unilateral rather than bilateral stimulation of the carotid sinus.
Catheter-based renal denervation
Three facts provided the knowledge base for the development of catheter-based renal denervation for treatment of essential hypertension: 1) the presence of an activated renal sympathetic outflow in hypertensive patients, 2) the blood-pressure lowering effect of surgical renal denervation in experimental models of hypertension, and 3) the anatomy of the postganglionic renal sympathetic nerves in their passage to the kidneys94–96. In humans, the nerves pass from the sympathetic chain and ganglia to the kidneys via the outer adventitia of the renal arteries, or just beyond in perirenal adipose tissue and connective tissue, potentially within reach of radiofrequency energy delivered by a catheter in the artery lumen120–124. Drawing on these concepts, the first to suggest that essential hypertension might be treated with a renal nerve ablation catheter were Howard Levin and Mark Gelfand in United States provisional patents 60/370190 [April, 2002], 60/415575 [October, 2002] and 60/442970 [January, 2003]. The California start-up company, Ardian, acquired the Levin and Gelfand patent rights, and commenced a developmental program to design a radiofrequency ablation catheter suitable for human use, testing this purpose-designed catheter for safety and renal denervation capacity in pigs. The first-in-man studies were conducted in Melbourne, in patients with drug-resistant hypertension. This patient class, of resistant hypertension, was selected because of the evident clinical need, and because the potential benefit-risk balance made the study defensible ethically. Only recently has it been shown that among hypertensive patients, activation of the renal sympathetic outflow is at its highest in drug-resistant hypertension (Figure 4). This trial, commencing in June 2007, is now known as SYMPLICITY HTN-120.
Renal denervation antihypertensive trials
The SYMPLICITY trials in endovascular renal nerve ablation20,118, their subsequent continuation to specified endpoints, accompanying renal denervation registry files, trials with other, newly engineered radiofrequency renal denervation devices122 and application of denervating ultrasonic energy123 have established these therapeutic principles:
Efferent sympathetic renal denervation can be achieved with luminal delivery of radiofrequency and ultrasonic energy.
Blood pressure reduction across the trials shows consistency, office systolic blood pressure falling on average by 20–30 mm Hg.
The blood pressure reduction is durable, persisting beyond 3 years, with no evidence that renal nerve regeneration occurs, to cancel out blood pressure lowering.
The development of renal artery stenosis in the region of radiofrequency energy delivery is very uncommon.
Testing for achieved renal denervation
The belief that renal denervation has been achieved in clinical trials of catheter-based renal denervation is almost invariably based on trust rather than testing. This contrasts with the studies of surgical renal denervation in experimental hypertension, where good experimental design demands that the effectiveness of denervation is always confirmed, typically by documenting 90–95% reduction in the kidney content of norepinephrine.
Among clinical studies of endovascular renal denervation only in the SYMPLICITY HTN-1 trial20 was renal denervation confirmed, by measurements of norepinephrine spillover (release of the transmitter from the renal nerves to plasma). Measurement of regional norepinephrine spillover is well established as a valid test for sympathetic denervation, having been applied for two decades in the diagnosis of pure autonomic failure124, a disorder characterized by spontaneous degeneration of postganglionic sympathetic nerve fibers, and denervation of the heart, kidneys and other organs. The degree of renal denervation achieved in the SYMPLICITY HTN-1 trial was less than expected (on average 47%)20, but did seem to be sufficient, in that the antihypertensive response was very adequate.
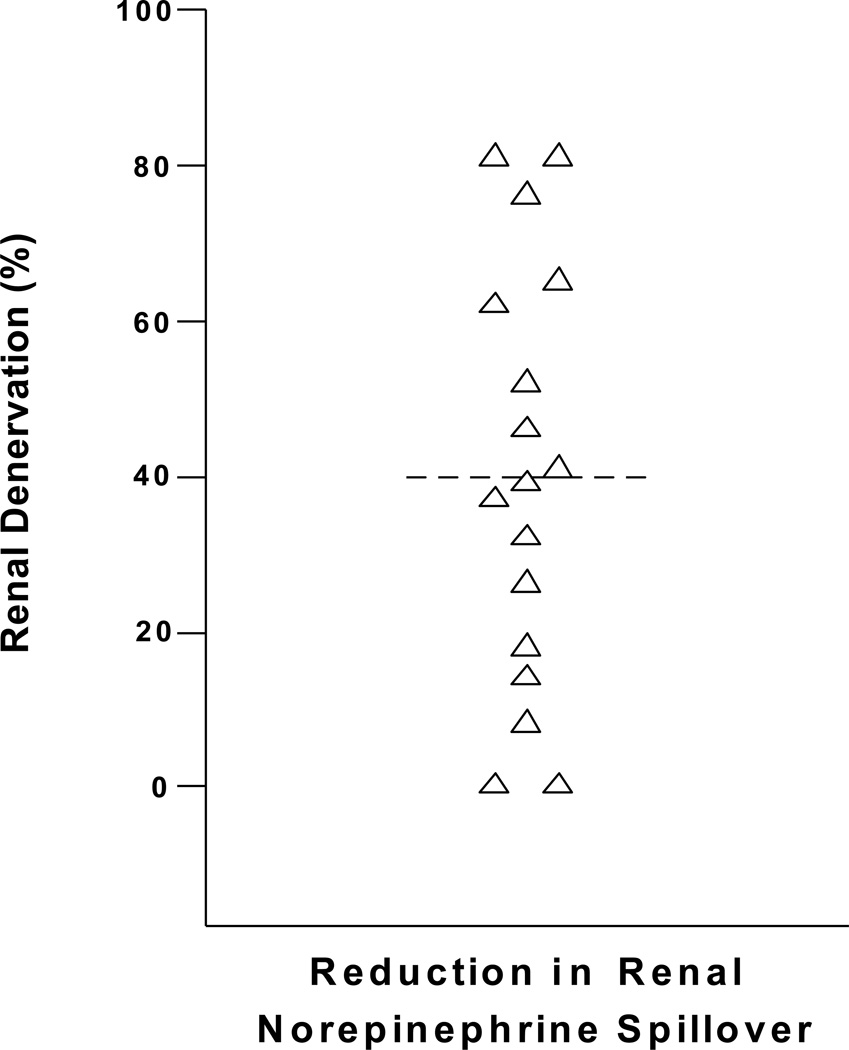
Subsequent analyses in Melbourne confirm that denervation is often very incomplete, and surprisingly non-uniform from patient to patient (Figure 5). Achieved denervation can be less than 25%, no doubt inadequate for a full therapeutic effect. Although the denervation catheter technique might look “easy”, compared to many other interventional cardiologic and radiologic procedures, achieving denervation is difficult. Illustrations showing the ready proximity of renal sympathetic nerves to the renal artery lumen were misleading. Older surgical anatomies of the renal nerves121 demonstrated that at the origin of the renal artery from the aorta the sympathetic nerves were more remote from the artery, but they converged on the renal arteries distally, near the renal artery branch point. Contemporary anatomical studies confirm this120. Clearly ablative energy should be preferentially focused on the distal renal artery.
Figure 5.
Regional norepinephrine spillover to plasma is a validated test for sympathetic denervation, being used in the diagnosis of patients with pure autonomic failure who have sympathetic denervation due to disease. Testing was done before and 30 days after the procedure. SYMPLICITY Arch and Flex catheters were used in approximately equal numbers, with similar results. The renal sympathetic denervation was incomplete, sometimes markedly so, with pronounced non-uniformity between individual patients, contradicting the idea that achieving sympathetic denervation is technically “easy”. Blood pressure non-response to renal denervation must commonly be due to suboptimal denervation, not only to the absence of sympathetic nervous activation in individual patients, often invoked as the determinant of non-responder status. From unpublished data by Murray Esler, Markus Schlaich, Gavin Lambert and Dagmara Hering.
Renal denervation “defrocked”? The SYMPLICITY HTN-3 trial
A challenge to the percutaneous renal denervation treatment of resistant hypertension came with the 9 January 2014 press release concerning the SYMPLICITY HTN-3 trial in drug-resistant hypertension, the pivotal study for United States Food and Drug Administration licensure, and in the subsequent New England Journal of Medicine publication on 29 March125, indicating that the primary efficacy endpoint had not been reached in the trial. A lot was expected of the SYMPLICITY HTN-3 study. Five times larger than the first two SYMPLICITY renal denervation trials, and incorporating a blinded sham design, this trial was expected to provide the definitive statement on the value of renal denervation in the treatment of patients with severe hypertension. To many it did –“renal denervation does not work!”126. The sham design was lauded126. How was it possible to argue against the findings of the SYMPLICITY HTN-3 trial?
But the perfection of the trial is an illusion, only apparent if no discrimination is attempted between its “form” and its “substance”. The form of the trial is represented by its generic strengths (large patient enrolment, blinding, sham control), applicable to any blood pressure trial. But this is a neuroscience trial in hypertension, based on a neuroscience theory of the pathogenic mechanism of severe hypertension (activation of the renal sympathetic outflow), on detailed knowledge of neuroanatomy (of the renal efferent and afferent nerves) and on the engineering of nerve ablative devices. This essence of the trial received inadequate attention, and its execution ultimately failed. Energy delivery was not preferentially to the distal renal artery, where it should have been, but inexplicably was more typically to the proximal renal artery; the renal nerves are, in fact, closer to the distal artery120. It is now a matter of record that the denervation procedure fared badly in SYMPLICITY HTN-3 (Kandzari D, presented on behalf of the SYMPLICITY HTN-3 Investigators. EuroPCR 2014, Paris, France). Retrospective analysis of stored angiographic and procedural records of all radiofrequency energy applications demonstrated that in 74% of patients not even one fully circumferential renal artery application of energy was achieved, when it is mandatory that this be achieved bilaterally, making effective nerve ablation impossible.
It should be noted that the field of renal denervation for experimental hypertension is active, in fact energized by the clinical studies. Experimental surgical and catheter-based denervation for hypertension still works! Why should renal denervation, in four mammalian species (rats, dogs, rabbits pigs) invariably be antihypertensive95–96, but not in the human mammal, in SYMPLICITY HTN-3. In the United States Pivotal study, a failure to achieve renal denervation comes first to mind (Esler M, Louis F Bishop Lecture, American College of Cardiology 63rd Annual Scientific Session, 29 March 2014, Washington DC, USA).
The future of renal denervation in treating severe hypertension
The SYMPLICITY HTN-3 trial has inflicted a “flesh wound” on the renal denervation field, but this is not fatal. In the pause that this trial created world-wide, it is now time to reflect on what future science is needed. Failure to test for renal denervation, as an intrinsic component of all trials but one, represents the Achilles heel of the field. It is probable that in some negative trials, and with non-response in some patients, failure to achieve adequate denervation was responsible. The commonly attributed reason for renal denervation to fail to lower blood pressure, that in such patients sympathetic activity was not increased, has no authenticity in studies where denervation was not tested; failure to denervate is the ever-present confounder. Planned studies, including the second Medtronic US Pivotal Trial in hypertension, will need to incorporate denervation testing. As renal denervation is incomplete and non-uniform between patients, utilization of higher radiofrequency and ultrasonic energy doses in the future is envisaged (the established safety margin in renal denervation will allow this), with multielectrode radiofrequency catheters used. Energy may need to be delivered to both the aortic and the distal ends of the renal artery, to target both sympathetic and afferent nerves; after emerging from the kidneys, the afferent nerves travel rostrally at some distance from the renal arteries, but converge on the arteries near their origin from the aorta120. Preselection of patients will remain problematic, until the influence on the responder status of the two determinants, effectiveness of denervation and hypertension pathophysiology can be discriminated. Will renal denervation show a “class effect”, with all energy forms for denervation being equally efficacious? Direct comparison will be needed.
Conclusions: the future of research on the sympathetic nervous system in human hypertension
Since this review has focused on the sympathetic nervous system in human hypertension, we reflect in closing on the future of research on the sympathetic neural mechanisms in human hypertension. The past 30 years witnessed enormous progress in understanding the role and importance of the sympathetic nervous system in the pathogenesis of human hypertension and its consequences. These advances resulted substantially from hypothesis based, mechanistic, patient-oriented research drawing on the sophistication and power of measurements of regional norepinephrine spillover and direct microneurographic recordings of sympathetic nerve activity. This progress reflects the continuing vitality and importance of patient oriented integrative neural and cardiovascular physiology in an era of molecular biology and genetics and clinical trials. What is needed to renew that vitality in the coming years? Ingenious new methods. A coupling of patient-oriented research to basic research and advances in human genetics and neuroscience. Most of all, a serious commitment, not just lip service introduced with the word translational, to the training and careers of physician-scientists pursuing mechanistic patient-oriented research.
Supplementary Material
Acknowledgments
Research support. Research of Prof Allyn Mark is supported by research grant HL-84207 from the NHLBI(National Heart, Lung, Blood Institute) of the NIH. Prof Murray Esler research is supported by National Health and Medical Research Council research fellowships.
Prof Guido Grassi has received consulting and lectures fees from Medtronic. Prof Murray Esler has received consulting fees and research support from Medtronic Ardian and Kona Medical.
Footnotes
Nonstandard Abbreviations and Acronyms:
None
Disclosures section. Prof Allyn Mark: none.
References
- 1.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 2.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 3.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 4.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, Gamba PL, Mancia G. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–1365. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 8.Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J. 2001;22:1136–1143. doi: 10.1053/euhj.2000.2407. [DOI] [PubMed] [Google Scholar]
- 9.Sander D, Winbeck K, Klingelhöfer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57:833–838. doi: 10.1212/wnl.57.5.833. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 11.Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, de Matos LNJ, Braga AMW, Middlekauff HR, Negrao CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Intern J Cardiol. 2009;135:302–307. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Andreas S, Haarmann H, Klarner S, Hasenfuß G, Raupach T. Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung. 2014;192:235–241. doi: 10.1007/s00408-013-9544-7. [DOI] [PubMed] [Google Scholar]
- 13.Penne EL, Neumann J, Klein IH, Bots ML, Blankestein PJ. Sympathetic hyperactivity and clinical outcome in chronic kidney disease during standard treatment. J Nephrol. 2009;22:208–215. [PubMed] [Google Scholar]
- 14.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60:1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 15.Fadl Elmula FE, Hoffmann P, Fossum E, Brekke M, Gjønnæss E, Hjørnholm U, Kjær VN, Rostrup M, Kjeldsen SE, Os I, Stenehjem AE, Høieggen A. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532. doi: 10.1161/HYPERTENSIONAHA.113.01452. [DOI] [PubMed] [Google Scholar]
- 16.Fadl Elmula FE, Hoffmann P, Larstorp AC, Fossum E, Brekke M, Kjeldsen SE, Gjønnæss E, Hjørnholm U, Kjær VN, Rostrup M, Os I, Stenehjem AE, Høieggen A. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63:991–999. doi: 10.1161/HYPERTENSIONAHA.114.03246. [DOI] [PubMed] [Google Scholar]
- 17.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Roche-Singh K, Bakris GL the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1340. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 18.Schlaich MP, Schmieder RE, Bakris G, et al. International Expert Consensus statement: percutaneous transluminal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62:2031–2045. doi: 10.1016/j.jacc.2013.08.1616. [DOI] [PubMed] [Google Scholar]
- 19.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 21.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, Vailati S, Bertinieri G, Seravalle G, Stella ML, Dell’Oro R, Mancia G. Heart rate as marker of sympathetic activity. J Hypertens. 1998;16:1635–1639. doi: 10.1097/00004872-199816110-00010. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. 1994;90:248–253. doi: 10.1161/01.cir.90.1.248. [DOI] [PubMed] [Google Scholar]
- 25.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckberg DL. Sympathovagal balance. A critical appraisal. Circulation. 1997;96:3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 27.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 28.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. The assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Meredith IT, Eisenhofer G, Lambert GW, Jennings GL, Thompson J, Esler MD. Plasma norepinephrine responses to head-up tilt are misleading in autonomic failure. Hypertension. 1992;19:628–633. doi: 10.1161/01.hyp.19.6.628. [DOI] [PubMed] [Google Scholar]
- 30.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous system activity and oxygen consumption in obese normotensive subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 31.Julius S, Krause L, Schork NJ, Majia AD, Jones KA, van der Ven C, Johnson EH, Sekkarie MA, Kjieldsen SE, Petrin J. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. 1991;9:77–84. doi: 10.1097/00004872-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension. 1988;14:1277–1283. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 33.Floras JS, Hara K. Sympathoneural and haemodynamic characteristics of young subjects with mild essential hypertension. J Hypertens. 1993;11:647–655. doi: 10.1097/00004872-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Rea RF, Hamdan M. Baroreflex control of muscle sympathetic nerve activity in borderline hypertension. Circulation. 1990;82:856–862. doi: 10.1161/01.cir.82.3.856. [DOI] [PubMed] [Google Scholar]
- 35.Schobel HP, Heusser K, Schmieder RE, Veelken R, Fischer T, Luft FC. Evidence against elevated sympathetic vasoconstrictor activity in borderline hypertension . J Am Soc Neprol. 1998;9:1581–1587. doi: 10.1681/ASN.V991581. [DOI] [PubMed] [Google Scholar]
- 36.Levy RL, White PD, Stroud WD, Hillman CC. Transient tachycardia: prognostic significance alone and in association with transient hypertension. JAMA. 1945;129:585–588. [PubMed] [Google Scholar]
- 37.Gudmundsdottir H, Strand AH, Høieggen A, Reims HM, Westheim AS, Eide IK, Kjeldsen SE, Os I. Do screening blood pressure and plasma catecholamines predict development of hypertension? Twenty-year follow-up of middle-aged men. Blood Press. 2008;17:94–103. doi: 10.1080/08037050801972923. [DOI] [PubMed] [Google Scholar]
- 38.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;81:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- 39.Grassi G, Seravalle G, Bertinieri G, Turri C, Dell'Oro R, Stella ML, Mancia G. Sympathetic and reflex alterations in systo-diastolic and systolic hypertension of the elderly. J Hypertens. 2000;18:587–593. doi: 10.1097/00004872-200018050-00012. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood JP, Scott EM, Stoker JB, Mary DASG. Hypertensive left ventricular hypertrophy: relationship to peripheral sympathetic drive. J Am Coll Cardiol. 2001;38:1711–1717. doi: 10.1016/s0735-1097(01)01600-x. [DOI] [PubMed] [Google Scholar]
- 41.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol. 2002;40:126–133. doi: 10.1016/s0735-1097(02)01931-9. [DOI] [PubMed] [Google Scholar]
- 42.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 43.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Burns J, Sivananthan MU, Ball SG, Mackintosh AF, Mary DASG, Greenwood JP. Relationship between central sympathetic drive and magnetic resonance imaging-determined left ventricular mass in essential hypertension. Circulation. 2007;115:1999–2005. doi: 10.1161/CIRCULATIONAHA.106.668863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Arenare F, Spaziani D, Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension. 2009;53:205–209. doi: 10.1161/HYPERTENSIONAHA.108.121467. [DOI] [PubMed] [Google Scholar]
- 46.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–1814. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- 47.Esler M, Lambert G, Jennings G. Regional norepinephrine turnover in human hypertension. Clin Exp Hypertens. 1989;11(Suppl 1):75–89. doi: 10.3109/10641968909045414. [DOI] [PubMed] [Google Scholar]
- 48.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 49.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 50.Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol. 2003;14:3239–3244. doi: 10.1097/01.asn.0000098687.01005.a5. [DOI] [PubMed] [Google Scholar]
- 51.Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell’Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 52.Folkow B. The Fourth Volhard Lecture: cardiovascular structural adaptation: its role in the initiation and maintenance of primary hypertension. Clin Sci. 1978;4(suppl.3):3S–22S. doi: 10.1042/cs055003s. [DOI] [PubMed] [Google Scholar]
- 53.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 54.Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension. 1991;17:1057–1062. doi: 10.1161/01.hyp.17.6.1057. [DOI] [PubMed] [Google Scholar]
- 55.Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99:2537–2542. doi: 10.1161/01.cir.99.19.2537. [DOI] [PubMed] [Google Scholar]
- 56.Kontak AC, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Nesbitt SD, Victor RG, Vongpatanasin W. Reversible sympathetic overactivity in hypertensive patients with primary aldosteronism. J Clin Endocrinol Metab. 2010;95:4756–4761. doi: 10.1210/jc.2010-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grassi G, Colombo M, Seravalle G, Spaziani G, Mancia G. Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity and congestive heart failure. Hypertension. 1998;31:84–87. doi: 10.1161/01.hyp.31.1.64. [DOI] [PubMed] [Google Scholar]
- 58.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 59.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous system activity and oxygen consumption in obese normotensive subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 60.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-LaRocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 61.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related Hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 62.Landsberg L. Diet, obesity, and hypertension: an hypothesis involving insulin, the sympathetic nervous system and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- 63.Bray G A. Obesity, a disorder of nutrient partitioning: the MONA LISA hypothesis. J. Nutr. 1991;121:1146–1162. doi: 10.1093/jn/121.8.1146. [DOI] [PubMed] [Google Scholar]
- 64.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 65.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous system activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–1735. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension. 2000;36:531–537. doi: 10.1161/01.hyp.36.4.531. [DOI] [PubMed] [Google Scholar]
- 67.Lambert E, Straznicky N, Schlaich M, Esler M, Dawood T, Hotchkin E, Lambert G. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–8628. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 68.Wofford MR, Anderson DC, Jr, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14:694–698. doi: 10.1016/s0895-7061(01)01293-6. [DOI] [PubMed] [Google Scholar]
- 69.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 70.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler M, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 72.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–H418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 73.Huggett RJ, Burns J, Mackintosh AF, Mary DA. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44:847–852. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 74.Rahmouni K. Obesity-associated hypertension. Recent progress in deciphering the pathogenesis. Hypertension. 2014;64:215–221. doi: 10.1161/HYPERTENSIONAHA.114.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 77.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305:R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: obesity induced hypertension: new concepts from emerging biology of obesity. Hypertension. 1999;33:537–541. doi: 10.1161/01.hyp.33.1.537. [DOI] [PubMed] [Google Scholar]
- 79.Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode recordings in healthy subjects. Diabetologia. 1992;35:873–879. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- 80.Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest. 1994;93:2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA, Hall JE. The hemodynamic response to chronic hyperinsulinemia in conscious dogs. Am J Hypertens. 1991;4:164–168. doi: 10.1093/ajh/4.2.164. [DOI] [PubMed] [Google Scholar]
- 82.Anderson EA, Mark AL. The vasodilator action of insulin. Implications for the insulin hypothesis of hypertension. Hypertension. 1993;21:136–141. doi: 10.1161/01.hyp.21.2.136. [DOI] [PubMed] [Google Scholar]
- 83.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man: a novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lembo G, Napoli R, Capaido B, Rendina V, Iaccarino G, Volpe M, Trimarco B, Sacca L. Abnormal sympathetic overactivity evoked by insulin in skeletal muscle of patients with essential hypertension. J Clin Invest. 1992;90:24–29. doi: 10.1172/JCI115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brandt DO. Reflex sympathetic activation induces acute insulin resistance in the forearm. Hypertension. 1993;21:618–623. doi: 10.1161/01.hyp.21.5.618. [DOI] [PubMed] [Google Scholar]
- 86.Lembo G, Capaldo B, Rendina V, Iaccarino G, Napoli R, Guida R, Trimarco B, Sacca L. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol Endo Met. 1994;266:E242–E247. doi: 10.1152/ajpendo.1994.266.2.E242. [DOI] [PubMed] [Google Scholar]
- 87.Gamboa A, Okamoto LE, Arnold AC, Figueroa RA, Diedrich A, Raj SR, Paranjape SY, Farley G, Abumrad N, Biaggioni I. Autonomic blockade improves insulin sensitivity in obese subjects. Hypertension. 2014;64:867–874. doi: 10.1161/HYPERTENSIONAHA.114.03738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Julius S, Jamerson K. Sympathetics, insulin resistance and coronary risk in hypertension: the ‘chicken and egg’ question. J Hypertens. 1994;12:494–502. [PubMed] [Google Scholar]
- 89.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Camerson GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi S. Modulation of blood pressure by central melanocortinergic pathways. N Eng J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 90.Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic function in human carriers of melancortin-4 receptor gene mutations. J Clin Endocrinol Metab. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 91.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WL. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 93.Machleidt F, Simon P, Krapalis AF, Hallschmid M, Lehnert H, Sayk F. Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J Clin Endocrinol Metab. 2013;98:E491–E496. doi: 10.1210/jc.2012-3009. [DOI] [PubMed] [Google Scholar]
- 94.DiBona GF. The functions of the renal nerves. Rev Physiol Biochem Pharmacol. 1982;94:75–181. [Google Scholar]
- 95.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 96.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2012;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 97.Esler M, Lambert E, Schlaich M. Point:Counterpoint. Chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J Appl Physiol. 2010;109:1996–1998. doi: 10.1152/japplphysiol.00182.2010. [DOI] [PubMed] [Google Scholar]
- 98.Roman RJ, Cowley AW. Characterisation of a new model for the study of pressure natriuresis in the rat. Am J Physiol Renal Physiol. 1985;248:F190–F198. doi: 10.1152/ajprenal.1985.248.2.F190. [DOI] [PubMed] [Google Scholar]
- 99.Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis. 1995;26:861–865. doi: 10.1016/0272-6386(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 100.Brecht HM, Ernst W, Koch KM. Plasma noradrenaline levels in regular haemodialysis patients. Proc Eur Dial Transplant Assoc. 1976;12:281–290. [PubMed] [Google Scholar]
- 101.McGrath BP, Ledingham JG, Benedict CR. Catecholamines in peripheral venous plasma in patients on chronic haemodialysis. Clin Sci Mol Med. 1978;55:89–96. doi: 10.1042/cs0550089. [DOI] [PubMed] [Google Scholar]
- 102.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 103.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25:878–882. doi: 10.1161/01.hyp.25.4.878. [DOI] [PubMed] [Google Scholar]
- 104.Adamczak M, Zeier M, Dikow R, Ritz E. Kidney and hypertension. Kidney Int. Suppl. 2002;61:S62–S67. doi: 10.1046/j.1523-1755.61.s80.28.x. [DOI] [PubMed] [Google Scholar]
- 105.Strojek K, Grzeszczak W, Gorska J, Leschinger MI, Ritz E. Lowering of microalbuminuria in diabetic patients by a sympathicoplegic agent: Novel approach to prevent progression of diabetic nephropathy? J Am Soc Nephrol. 2001;12:602–605. doi: 10.1681/ASN.V123602. [DOI] [PubMed] [Google Scholar]
- 106.Vonend O, Marsalek P, Russ H, Wulkow R, Oberhauser V, Rump LC. Moxonidine treatment of hypertensive patients with advanced renal failure. J Hypertens. 2003;21:1709–1717. doi: 10.1097/00004872-200309000-00021. [DOI] [PubMed] [Google Scholar]
- 107.Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka P, Bohm M, Cremers B, Esler MD, Schlaich MP. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. doi: 10.1681/ASN.2011111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, Bohm M, Lambert EA, Krum H, Sobotka P, Schmieder RE, Ika-Sara C, Eikelis N, Straznicky N, Lambert GW, Esler MD. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiology. 2013;168:2214–2220. doi: 10.1016/j.ijcard.2013.01.218. [DOI] [PubMed] [Google Scholar]
- 109.Zimmer K. Soul Made Flesh. London: The Random House (Arrow Books); 2004. pp. 188–207. [Google Scholar]
- 110.Hamilton WF, Richards DW. Output of the heart. In: Fishman AP, Richards DW, editors. Circulation of the Blood. Men and Ideas. Bethesda: American Physiological Society; 1982. pp. 87–90. [Google Scholar]
- 111.Julius S, Esler M, editors. The Nervous System in Arterial Hypertension. Springfield, Illinois: Charles C. Thomas; 1976. [Google Scholar]
- 112.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Ass. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 113.Paton WDM, Zaimis EJ. Paralysis of autonomic ganglia by methonium salts. Brit J Pharmacol. 1951;6:155–168. doi: 10.1111/j.1476-5381.1951.tb00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Zwieten PA. Development of antihypertensive drugs: from the bench to the clinic. In: Birkenhager WH, Robertson JIS, Zanchetti A, editors. Handbook of Hypertension, Hypertension in the Twentieth Century: Concepts and Achievements. Amsterdam: Elsevier; 2004. pp. 457–486. [Google Scholar]
- 115.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 116.De la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 117.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, De Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension. J Am Coll Cardiol. 2011;58:765–773. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 118.Symplicity HTN-2 Investigators. Renal sympathetic denervationin patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet. 2010;376:1903–1309. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 119.Brest AN, Wiener L, Bachrach B. Bilateral carotid sinus nerve stimulation in the treatment of hypertension. Am J Cardiol. 1972;29:821–825. doi: 10.1016/0002-9149(72)90502-4. [DOI] [PubMed] [Google Scholar]
- 120.Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Kolodgie F, Joner M, Virmani R. Anatomical distribution of human renal sympathetic nerves: Pathological study. J Am Coll Cardiol. 2014;64:635–643. doi: 10.1016/j.jacc.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 121.Oldham JB. Denervation of the kidney. Hunterian Lecture of the Royal College of Surgeons, England. 9th March, 1950. Ann R Coll Surg Engl. 1950;7:222–245. [PMC free article] [PubMed] [Google Scholar]
- 122.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mabin T, Sapoval M, Cabane V, Stemmet J. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2013;8:57–61. doi: 10.4244/EIJV8I1A10. [DOI] [PubMed] [Google Scholar]
- 124.Meredith IT, Esler MD, Cox HS, Lambert GW, Jennings GL, Eisenhofer G. Biochemical evidence of sympathetic denervation of the heart in pure autonomic failure. Clin Auton Res. 1991;1:187–194. doi: 10.1007/BF01824986. [DOI] [PubMed] [Google Scholar]
- 125.Bhatt D, Kandzari D, O’Neill, D’Agostino R, Flack J, Katzen B, Leon M, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Towsend RR, Bakris GL SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. New Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 126.Shun-Shun M, Howard J, Francis D. Removing the hype from hypertension. Br Med Journal. 2014;348:g1937. doi: 10.1136/bmj.g1937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.