Abstract
Treatment of Hepatitis C virus (HCV) with pegylated interferon and ribavirin (IFN/RBV) can be associated with neuropsychiatric side effects, which may necessitate dose reductions or treatment discontinuation. This study aimed to characterize the time course and predictors of cognitive and affective/mood symptoms after IFN/RBV treatment initiation. Forty individuals enrolled in a longitudinal project underwent comprehensive cognitive, medical, and psychiatric assessment at baseline and 10 weeks, 6 months, 12 months, and 18 months after treatment initiation. Analyses were conducted to determine the prevalence of neurocognitive impairment over time, explicate the relationship between neurocognitive impairment, neuropsychiatric symptoms, and liver disease at each time point, and identify predictors of neurocognitive decline as well as cognitive effects of viral clearance. By 10 weeks after initiating IFN/RBV, the prevalence of neurocognitive impairment rose from 22.5% to 47.4% (p < 0.05). Infection with Genotype 1 and premorbid depression were associated with more severe declines (p < 0.05). After 18 months, 42.5% remained neurocognitively impaired, independent of viral clearance, severity of liver disease, and current depressive symptoms. Undetectable viral load was not associated with improvement 18 months after initiating treatment (p > .10). Results of the current study indicate that IFN/RBV treatment-emergent neurocognitive declines are significant, prevalent, and may persist long after treatment cessation. Clinicians should monitor cognition throughout the course of treatment for HCV, noting that early declines may indicate individuals at elevated risk for persistent neurocognitive impairment. Longer-term studies are needed to determine whether lasting declines may remit over longer intervals.
Keywords: Antiviral treatment, side effects (treatment), neuropsychological effects, cognitive dysfunction/impairment, depressive symptoms
Introduction
Hepatitis C Virus (HCV) primarily infects hepatocytes, and may result in inflammation, fibrosis, cirrhosis and hepatocellular carcinoma. However, HCV also causes extrahepatic disease (Sene et al. 2004) impacting numerous organ systems (e.g., endocrine, lymphatic, renal) including central nervous system (CNS) structure and function (Laskus et al. 2005; Wilkinson et al., 2009; Murray et al., 2008; Vargas et al., 2002; Schaefer et al., 2012). Whether CNS disturbances are due to primary neurotoxic effects of the virus, secondary effects of immune/glial activation and liver disease (Senzolo et al. 2011), or both, they are of sufficient magnitude to increase disease burden and impact patient management (Fried and Russo 2003). Many individuals with HCV report cognitive symptoms, which are often associated with objective evidence of neurocognitive impairment (Forton et al. 2005; Weissenborn et al. 2004; Hilsabeck et al. 2002) as well as psychiatric syndromes (Schaefer et al. 2012). These sequelae occur commonly, are often present prior to the development of clinically significant liver disease, and may be exacerbated by advanced liver disease and other common comorbidities (Pattulo et al. 2011; Cordoba et al. 2009). Although combination treatment with pegylated interferon and ribavirin (IFN/RBV) can result in viral eradication in over 50% of individuals who begin treatment (McNutt et al. 2012), this regimen is associated with numerous adverse effects of its own. Perhaps most notably, IFN/RBV is associated with prominent cognitive and neuropsychiatric effects (Raison et al. 2009), which are reported in up to 80% of patients after beginning treatment, and often necessitate dose reductions (40% of treated patients) or discontinuation of therapy (20% of treated patients) [McNutt et al. 2012; Fontana 2000; Valentine and Meyers 2005; Schaefer et al. 2002; de Knegt et al. 2011). Due to inconsistencies in the methodologies of existing studies, the predictors and long-term effects of these neurocognitive and neuropsychiatric complications are largely unknown. This is particularly the case in individuals with multiple risk factors for cognitive impairment and psychiatric symptomatology, as these are exclusion criteria in many treatment studies (Schmidt et al. 2009).
At present, IFN/RBV remains in widespread use despite a relatively high prevalence of treatment-related complications, although IFN-sparing regimens will soon become available. Some side effects (e.g., flu-like symptoms) appear to be dose-dependent and reversible upon cessation of IFN/RBV therapy (Fried and Russo 2003; Neri et al. 2010), while others (e.g., fatigue, mood changes, and cognitive impairment) may have a more insidious onset and more variable course (Reichenberg et al. 2005). For example, evidence suggests that depression, anxiety, neurovegetative symptoms, and cognitive dysfunction can increase within two weeks of initiating treatment, exhibiting a fluctuating and worsening course thereafter (Dusheiko 1997; Reichenberg et al. 2005). In turn, IFN-induced depression may reduce treatment efficacy by decreasing responsiveness to antiretroviral treatment. There is mixed evidence as to whether neuropsychiatric symptoms may resolve following treatment cessation (McNutt et al. 2012; Schmidt et al. 2009; Thein et al. 2007; McAndrews et al. 2005). In some studies, persistent depressive symptoms and cognitive disturbances have been detected after as long as 72 weeks even without premorbid risks, suggesting that it may be beneficial to monitor patients even after discontinuation of treatment, particularly in the presence of independent neurocognitive or neuropsychiatric risk factors (Reichenberg et al. 2005). However, precisely which factors may confer additional risk for incident or lasting neurocognitive and neuropsychiatric symptoms has not been well established. Identifying these relative risks and understanding their implications is critical, as a disproportionate number of HCV-infected individuals have multiple conditions (e.g., substance abuse, depression) that may produce or exacerbate neurocognitive impairment and mood disturbances, which in turn may contribute to functional disability and health-related quality of life.
Published studies on IFN/RBV vary in their patient characteristics, control groups, methodology (e.g., time points and duration of follow-up), cognitive assessments, and consideration of potentially confounding factors such as liver disease, medical comorbidity, premorbid depression, and substance abuse/dependence (Valentine and Meyers 2005; Hilsabeck et al. 2002; McAndrews et al. 2005). In particular, many studies examine only patients within a narrow range of mild or severe liver disease, further clouding interpretation of relationships along a continuum of hepatic disease. For this reason, the current observational study endeavored to 1) determine the neurocognitive correlates of IFN/RBV treatment in a group of individuals inclusive of comorbidities commonly seen in HCV infected persons (e.g., histories of depression and substance abuse disorders, and a range of medical and psychological health at pre-treatment baseline), and if possible, to 2) identify predictors of decline and deficits in neurocognitive and neuropsychiatric functioning from among relevant clinical variables (e.g., failure to achieve sustained viral clearance, liver fibrosis stage).
Participants and methods
Participants
All participants were enrolled in a longitudinal National Institute on Drug Abuse (NIDA)-funded study at the University of California San Diego (UCSD) examining the neuropsychiatric effects of antiviral treatment for HCV disease. 174 individuals were screened for eligibility for this observational substudy (see flow chart depicting enrollment and selection of participants in Figure 1). Participants were selected if they met the following criteria: (1) were prescribed IFN/RBV and scheduled to receive care at the UCSD Hepatology Clinic, (2) had never received IFN therapy at the time of initial assessment, and (3) were available and interested in completing additional assessments at designated time points. Informed consent was obtained from each participant, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki by obtaining a priori approval by the UCSD Human Research Protection Program. HCV diagnoses were confirmed through detection of HCV immunoglobulin G (IgG) antibody in plasma by enzyme-linked immunosorbent assay. HCV RNA was quantified by real time polymerase chain reaction (PCR), and HCV genotyping was performed by a commercial lab. Liver biopsies were administered as clinically warranted (results available for 35 of the 40 participants). See Table 1 for HCV and fibrosis characteristics of the sample at baseline.
Fig. 1.
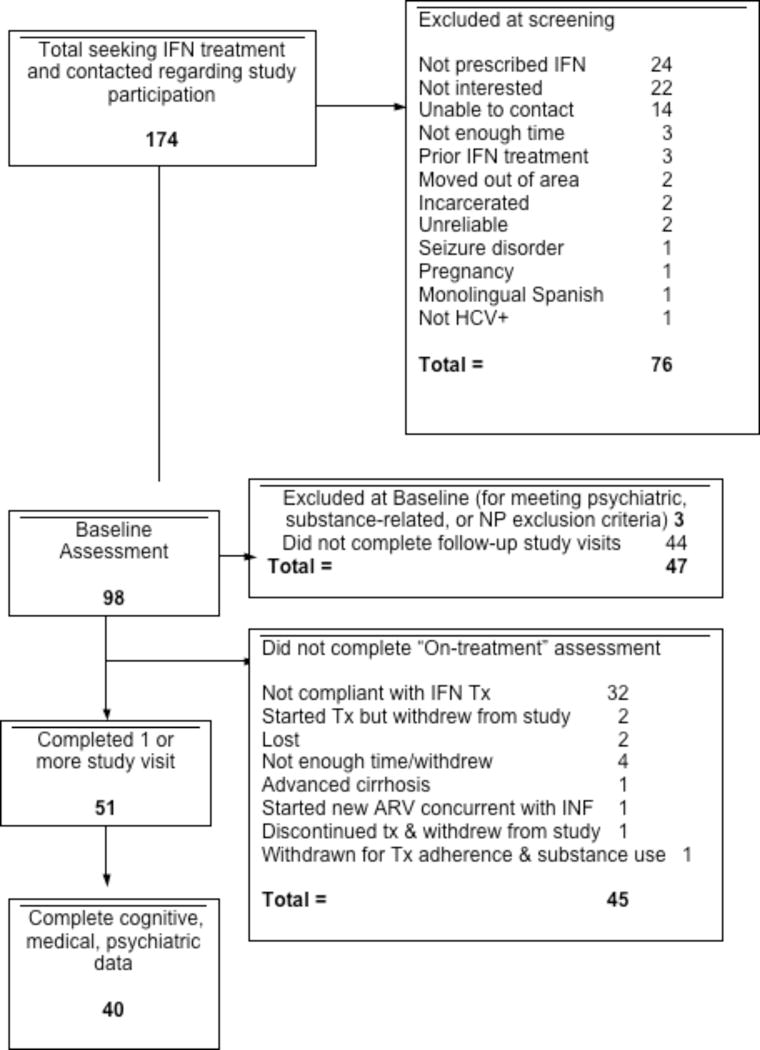
Study Recruitment, Screening, and Enrollment
Table 1.
Demographic, Psychiatric, and Substance Use Characteristics (n = 40)
| Demographics | |
| Age (years) | 47.8 (8.5) |
| Education (years) | 12.9 (2.0) |
| Sex+ | 20 (50.0) |
| Caucasian+ | 28 (70.0) |
| African-American | 3 (7.5) |
| Hispanic | 8 (20.0) |
| Other | 1 (2.5) |
| Reading literacy (WRAT3), mean (SD) | 96.0 (12.5) |
| Affective distress | |
| Profile of Mood States totala | 38 (23, 60) |
| Beck Depression Inventory-II | |
| Lifetime Psychiatric/Substance disorders++ | |
| Major depressive disorder+ | 13 (32.5) |
| Alcohol+ | 15 (37.5) |
| Cannabis+ | 15 (37.5) |
| Methamphetamine+ | 17 (42.5) |
| Cocaine+ | 16 (40.0) |
| Opioid+ | 10 (25.0) |
| Any substance+ | 25 (62.5) |
Note.
Number of cases (%)
None met criteria for current abuse/dependence
Total = total mood disturbance
Exclusion criteria included inability to provide informed consent, history of severe psychiatric (e.g., psychosis) or neurological (e.g., seizures) disorders, and active substance use. Our sample included conditions that are commonly associated with HCV. Specifically, seven individuals tested positive for human immunodeficiency virus (HIV), and as HIV is another common comorbidity of HCV disease, HIV-infected subjects were included in the analysis after confirming that their demographic, psychiatric, liver fibrosis, and HCV disease characteristics did not significantly differ from HIV- participants at baseline. Demographic, HCV, HIV, psychiatric, and neuropsychological characteristics for this sample are summarized in Table 2.
Table 2.
Baseline Medical Characeristics (n = 40)
| Characteristic | Mean (SD) |
|---|---|
| Hemoglobin | 14.2 (1.6) |
| Platelet count | 213.0 (77.2) |
| Albumin | 4.0 (0.4) |
| Alanine transaminase (ALT) | 85.2 (59.9) |
| Aspartate transaminase (AST) | 73.2 (48.7) |
| Bilirubin, total | 1.0 (0.2) |
| AST-to-platelet ratio index (APRI) | 0.8 (0.5) |
| HCV RNA (log10 IU/mL) | 5.8 (0.7) |
| HCV Genotype | n (%) |
| 1 | 28 (70.0) |
| 2 | 6 (15.0) |
| 3 | 5 (12.5) |
| 4 | 1 (2.5) |
| Fibrosis stage at baseline* | n (%) |
| 0 | 10 (28.5) |
| 1 | 12 (34.2) |
| 2 | 2 (5.7) |
| 3 | 5 (14.3) |
| 4 | 6 (17.1) |
Liver biopsy results available for 35 participants
Materials and Procedure
All participants provided written, informed consent prior to completing a comprehensive neuropsychological, medical, and psychiatric research evaluation at baseline (prior to initiating treatment) and at four follow-up visits. Follow-up visits occurred at approximately 10 weeks, 6 months, 12 months, and 18 months after initiating treatment for HCV. Participants received financial compensation for completing each visit.
Medical Assessment and Treatment
All participants were evaluated using structured medical history, physical examination, and laboratory tests at each time point in conjunction with their treatment for HCV. Liver biopsy was conducted for 35 of the 40 (87.5%) subjects. Treatment regimens followed standard recommendations, i.e., peg-IFN α-2a (180 mcg via subcutaneous injection/week) as well as daily oral doses of ribavirin (400–600 mg depending on body weight and HIV status). The mean treatment duration was 36.6 weeks (SD = 18.4) and varied by HCV genotype, such that individuals with genotypes 2 and 3 were treated for approximately 24 weeks (mean= 27.6, SD = 13.4) and individuals with genotype 1 were treated for approximately 48 weeks (mean= 40.4, 19.1). Individual regimens were altered as indicated by treating physicians. Aspartate transaminase (AST) to platelet ratio index (APRI) was calculated as a noninvasive proxy for liver fibrosis at each study visit. Due to the study visit schedule, sustained virologic response (SVR) data (undetectable HCV RNA a full 6 months after completion of therapy) was not available for all participants. Therefore, we instead report the proportion of individuals with undetectable HCV RNA in the serum at their final study visit. Undetectable HCV RNA in serum at the final study visit was achieved by 40.0% of the sample.
Neuropsychological Assessment
At each visit, participants were administered a comprehensive neuropsychological test battery, which included a measure of estimated premorbid verbal intelligence (i.e., reading portion of the Wide Range Achievement Test – Third Edition [Wilkinson and Robertson 2006]) as well as a relatively brief but robust assessment of the cognitive domains commonly affected by HCV (i.e., learning, memory, verbal fluency, speed of information processing, executive functions, attention/working memory, and motor performance). The neuropsychological battery is detailed in Table 3. Raw scores from individual neuropsychological tests were converted to demographically (e.g., age, education) corrected T scores. Wherever possible, neuropsychological test data was also adjusted for ethnicity, utilizing published normative data. These demographically corrected T scores were then transformed into deficit scores, which account for both the number and severity of impaired scores in an individual’s neuropsychological profile. Domain-level deficit scores were then averaged to yield a global deficit score (GDS), or summary index indicating degree of cognitive impairment (Heaton et al. 1994), for each individual at each time point. A cutoff of 0.5 was used to classify individuals as neurocognitively impaired (Carey et al. 2004). In order to assess meaningful global neurocognitive change across several study visits (including corrections for practice effects and regression toward the mean), we used regression-based change scores as continuous variables in our analyses. See (Cysique et al. 2009) for a detailed algorithm and validation of this method.
Table 3.
Neuropsychological Test Battery By Cognitive Domain
| Premorbid Verbal Intelligence |
| WRAT-III reading |
| Attention/working memory |
| PASAT 50 |
| WMS-III spatial span |
| Speed of information processing |
| WAIS-III digit symbol |
| WAIS-III symbol search |
| Executive functioning |
| WCST-64 |
| TMT Part B |
| Learning |
| HVLT-R Trials 1–3 |
| BVMT-R Trials 1–3 |
| Memory |
| HVLT-R delayed recall |
| BVMT-R delayed recall |
| Motor |
| Grooved Pegboard |
| Verbal fluency |
| Letter (FAS) |
| Category (animals) |
Note. BVMT-R: Brief Visuospatial Memory Test—Revised; HVLT-R: Hopkins Verbal Learning Test—Revised; PASAT; Paced Auditory Serial Addition Test; TMT: Trail Making Test; WAIS-III: Wechsler Adult Intelligence Scale—Third Edition; WCST-64: Wisconsin Card Sorting Test—64 card version.
Psychiatric and Substance Use Assessment
Participants underwent comprehensive psychiatric assessment at each time point using the Composite International Diagnostic Interview (CIDI version 2.1) (World Health Organization 1998) to generate lifetime diagnoses of mood and substance-related disorders per Diagnostic and Statistical Manual of Mental Disorders (4th ed.) criteria (American Psychiatric Association, 2000). Participants also completed the Beck Depression Inventory-II (BDI-II; Beck 1987) at each study visit. Substance use assessment was augmented at each time point by a separate semi-structured follow-back interview.
Analytic Plan
Using the above methodology (Cysique et al. 2009), regression-based change scores were calculated in order to identify reliable changes in neurocognitive functioning over time. From these change scores (indicating severity of declines), global and domain-level impairment rates were calculated at each time point in order to estimate the prevalence of clinically meaningful neurocognitive deficits at baseline, 10 weeks, and 18 months after beginning treatment. In order to accommodate departures from normality in individual variable distributions, nonparametric statistical tests were employed, including 1) Wilcoxon signed-rank tests to identify significant changes in cognition, depressive symptoms, and liver fibrosis relative to baseline levels, and 2) Spearman’s ρ to calculate correlations between cognitive performance, depressive symptoms, fatigue, and liver fibrosis at each time point. Finally, clinical and medical variables associated with cognitive decline severity at the univariable level were entered into a least squares regression analysis to identify significant independent predictors of neurocognitive worsening. A critical alpha level of .05 was set for all analyses.
Results
Degree and Persistence of Neurocognitive Change Over Time
At baseline (prior to IFN/RBV treatment), 22.5% of the cohort was classified as globally neurocognitively impaired (GDS ≥50), which significantly increased to 47.4% of patients by 10 weeks after initiating treatment (p < .05; see Fig. 2A). Nearly all of these patients (42.9%) remained classified as globally impaired after 18 months, and mean global deficit scores remained significantly elevated relative to baseline levels (p < .05) at the final study visit. More specifically, these effects were driven by declines within the domains of working memory, learning, and executive functions (see Fig. 2B). Within the domain of attention/working memory, impairment rates increased from 30.0% (baseline) to 52.6% (10 wks), reaching 46.4% at 18 months. Executive impairment rates increased from 30.0% (baseline) to 47.4% (10 wks) and 42.9% at 18 months. Learning impairment rates rose from 42.5% (baseline) to 63.2% (10 wks) and 53.6% at 18 months. Recall impairment rates rose from 42.5% (baseline) to 52.6% (10 wks), resolving to 39.3% at baseline. Motor impairment rates rose from 30.8% (baseline) to 38.9% (10 wks), resolving to 33.3% at 18 months. Verbal impairment rates were 12.5% at baseline, 21.1% at 10 weeks, and 32.1% after 18 months. With regard to speed of information processing, impairment rates were 22.5% at baseline and 10 weeks, rising to 28.0% after 18 months. Figure 3 depicts changes in cognitive performance over time relative to changes in depressive symptoms and liver fibrosis, recorded at each time point. BDI scores at 18-month follow-up did not significantly differ from baseline (p > .10), and estimated liver fibrosis (APRI) had decreased to significantly below baseline levels by 12 months after treatment initiation (following a mean treatment duration = 36.6 weeks; p < .05). Cognition (GDS) was not significantly associated with fatigue (POMS subscale) or depressive symptoms (BDI) at any study visit (all ps > .10). However, baseline BDI score was positively associated with fatigue ratings at 10 weeks and 18 months (ρ = .54 and .60, respectively).
Fig. 2. Global and Domain-Level Impairments During and After IFN/RBV treatment.
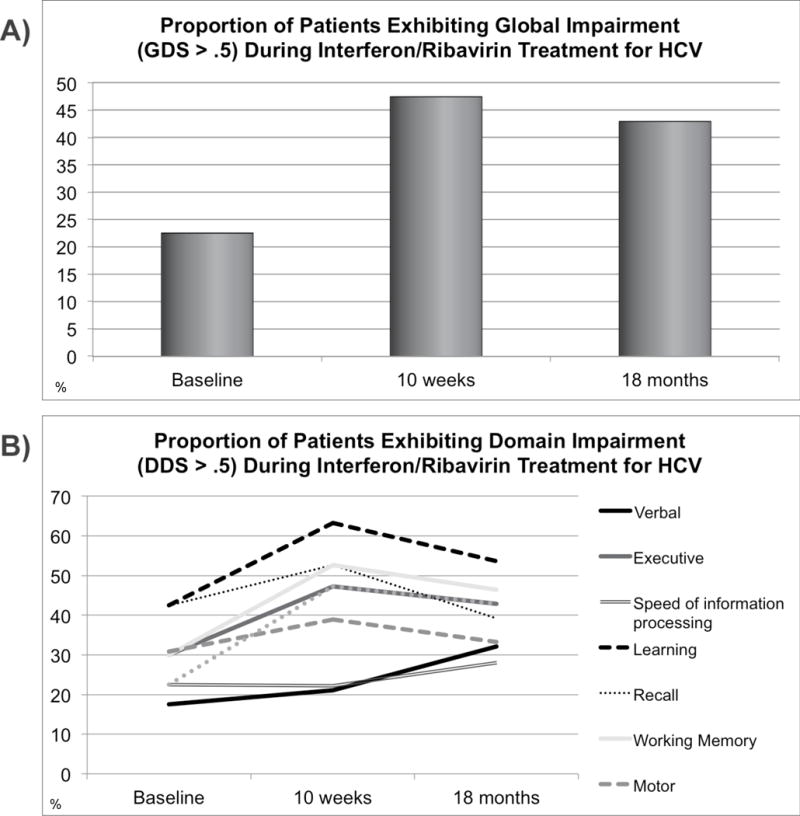
(A) Global neurocognitive impairments. After initiating IFN/RBV treatment, the mean global deficit score (indicating severity of global cognitive impairment) significantly rose between baseline (mean = .31) and 10 weeks (mean = .47), remaining significantly elevated 18 months (mean = .43) after treatment initiation.
(B) Domain-level neurocognitive impairments. The prevalence and magnitude of incident impairments (during IFN/RBV treatment), and the extent to which these impairments resolved over time, varied substantially by domain. The largest and most persistent declines were observed in the domain of working memory, which remained impaired relative to baseline at 18 months. At 18 months, impaired learning performance was the most prevalent deficit, present in over 50% of the sample.
Fig. 3.
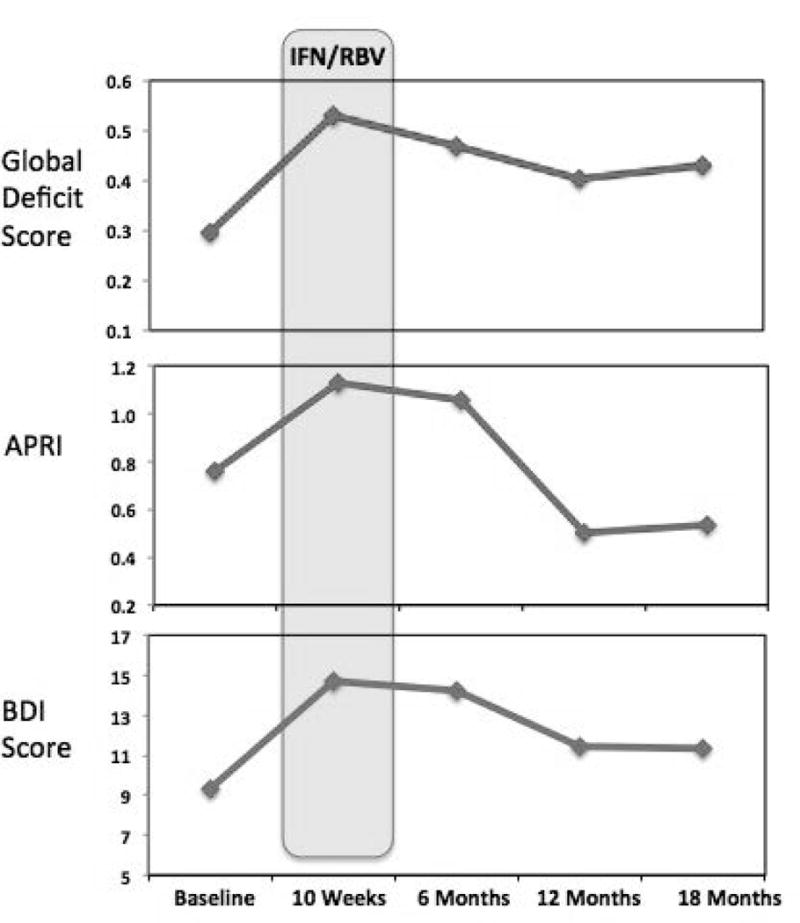
Cognition, Liver Fibrosis, and Depression Before, During, and Following IFN/RBV Treatment
Predictors of Neurocognitive Decline
Demographic factors (age, gender, education), current depressive symptoms (BDI), and most medical characteristics (fatigue rating, liver fibrosis, HIV status) were not significantly associated with severity of acute neurocognitive decline (10 weeks) at the univariable level (all ps > .10). However, infection with HCV Genotype 1 and baseline BDI score were significantly associated with acute decline severity (ps < .05). After meeting this threshold for inclusion in a least squares regression, the resulting model was significant (Adjusted R2 = .30, p = .02), indicating that infection with HCV genotype 1 significantly and independently predicted more severe global neurocognitive decline at 10 weeks (standardized β = −.42, p < .05), while baseline BDI-II scores approached significance (standardized β = .36, p = .08). Higher baseline BDI-II scores were associated with more severe acute declines (Spearman’s ρ = .52, p < .05). No demographic, medical (treatment duration, undetectable HCV RNA at final study visit, fatigue rating, liver fibrosis, HIV status), or psychiatric characteristics were significantly associated with decline severity after 18 months (all ps > .10).
Conclusions
In this observational study, IFN/RBV-related cognitive declines were prevalent, clinically significant, and most notably, persistent. Nearly half of this naturalistic sample receiving IFN/RBV treatment for HCV exhibited global neurocognitive impairments soon thereafter. While liver function gradually improved and depressive symptoms were alleviated over time, all but one individual exhibiting incident impairment remained globally impaired 6–12 months after completing IFN/RBV treatment. The high frequency of these persistent declines suggests that such impairments may be more commonplace than expected in community samples, in contrast to the widely held belief that neurocognitive symptoms may remit soon after IFN-RBV treatment cessation, or after achieving a favorable virologic response. In this sample, the observed deficits persisted for months after treatment cessation, even in individuals who had achieved virologic suppression (undetectable HCV). Although the observed neurocognitive decrements were modest, the elevated proportion of globally impaired individuals and the persistence of these deficits over time raises the possibility that these declines may be indicative of CNS effects that are slow to reverse, and which may have impacts on quality of life and everyday functioning.
The impact of Genotype 1 on cognitive impairments may be imparted via several possible mechanisms, including differences in treatment regimen (e.g., higher cumulative dosage and longer duration). However, these findings are unlikely to be an artifact of dose or duration, which were not significantly associated with genotype or neurocognitive performance, respectively. We also considered whether hallmark symptoms of IFN treatment (e.g., fatigue, depression) may have been elevated in this group, which may have disproportionately impacted cognitive performance in these individuals. However, this did not appear to be the case, as levels of reported fatigue and depressive symptoms were comparable between genotypes at baseline, on treatment, and after treatment (ps > .10). After ruling out these alternative explanations, we hypothesized that differences in viral genetics or suppression in genotype 1 (potentially related to its frequently chronic course and relative resistance to IFN treatment) may account for the disproportionate association between Genotype 1 and neurocognitive impairments observed in our sample. This hypothesis is strengthened by the high prevalence of Genotype 1 (88% and 80%) in several studies that have noted more severe and enduring cognitive effects of IFN treatment for HCV [e.g., 25, 33].
Dose-dependent neurological and neuropsychiatric toxicity has been reported in up to 40% of patients treated with combination therapy (Fontana 2000); however, risk factors remain poorly defined (McAndrews et al. 2005). One aim of the current study was to identify predictors of lasting neurocognitive impairment in a sample inclusive of several comorbid risk factors. Our results identified HCV Genotype 1 and higher self-reported depressive symptoms at baseline to be predictive of declines at 10 weeks, which is an earlier time point than has been included in most other IFN/RBV studies to date. This may explain why our results (specifically, elevated impairment rates) differ somewhat from investigations that included less acute follow-up timepoints. Importantly, however, these short-term declines in cognitive functioning do appear to presage longer-term neurocognitive impairment. Our results indicated that cognitive status was not significantly associated with liver disease, a finding that differs from some prior studies (Thein et al. 2007). However, only 11 of 35 (31.4%) subjects had stage 3 or 4 liver fibrosis and this may have limited our ability to establish associations. While premorbid depression was identified as a risk factor for more severe early declines, severity of depressive symptoms were not associated with cognitive impairment at any study visit. Thus, having more severe depressive symptomatology at baseline does appear to represent a vulnerability to early and significant adverse treatment effects, whereas depressive symptoms which emerge later in the course of treatment may not necessarily increase risk for persistent cognitive symptoms.
A number of limitations of the current study warrant mention. First, contrary to the results of the current study, other groups have observed improvements or stable performances (Hilsabeck et al. 2005) in certain measures of cognitive function associated with sustained virologic response. Further studies with longer post-treatment follow-up are needed to determine whether, when, and to what extent, these incident impairments may dissipate. Second, while this study provided comprehensive assessment of cognitive, psychiatric, and medical outcomes over 18 months, it was not a clinical trial (i.e., the study did not control the intervention). Thus, participants ended treatment when medically indicated, rather than at equivalent, predetermined study intervals. In addition, we were not able to include a randomized control (no treatment) group in the study; as such, interpretation of these results should take into account the lack of a directly comparable but untreated group. Third, despite limited exclusion criteria, over half of participants screened for participation were either noncompliant with treatment, ineligible for participation or lost to follow-up, thereby reducing our sample sizes and restricting our analytic plan. However, it stands to reason that the large number of noncompliant individuals may be related to the very side effects we observed in our sample. Fourth, whereas we were able to evaluate the relationships between cognition, depression, and estimated liver fibrosis over time to a large extent, many subgroup analyses were not adequately powered due to small sample sizes.
Understanding the course of IFN/RBV-related adverse effects has become especially important in recent years, in the context of novel and emerging therapeutics that may hold promise in mitigating neurocognitive and neuropsychiatric sequelae (Forton and Karayiannis 2006). For example, triple combinations involving protease inhibitors (e.g., boceprevir, telaprevir) offer potential improvements in response with shorter treatment latencies, which may potentially reduce cognitive side effects (Zeuzem et al. 2012). While prophylactic antidepressant treatment has been reported to reduce psychiatric symptoms accompanying IFN-based treatments (Kraus et al. 2002; Asnis and De La Garza 2006), the relevance of these data to neurocognitive impairment is not known. Finally, whether novel non-inteferon based therapies (e.g., GS-7977) may improve long-term neurocognitive outcomes will need to be determined (Gane et al. 2012). While these are important directions for future work, it remains to be seen whether decreased treatment durations may mitigate the impacts on cognition that may be sustained by patients with certain risk factors (e.g., HCV genotype 1, premorbid depression). In the meantime, our results suggest that particularly in the context of multiple risk factors, clinicians should monitor cognition throughout the course treatments for HCV, noting that early declines may identify individuals at elevated risk for persistent neurocognitive impairment.
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Cristian L. Achim, M.D., Ph.D., and Scott L. Letendre, M.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Leader), Clint Cushman (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Leader), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Leader), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
Financial support: NIH (P50-DA026306), P01 DA12065, T32-DA031098, P30-MH062512, R01-MH073419.
List of abbreviations
- HCV
Hepatitis C Virus
- IFN/RBV
interferon and ribavirin
- CNS
central nervous system
- NIDA
National Institute on Drug Abuse
- UCSD
University of California San Diego
- IgG
immunoglobulin G
- ELISA
enzyme-linked immunosorbent assay
- HIV
human immunodeficiency virus
- MDD
major depressive disorder
- GDS
global deficit score
- SD
standard deviation
- BDI-II
Beck Depression Inventory-II
- APRI
Aspartate aminotransferase to platelet ratio index
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
We have no commercial affiliations or consultancies which might be construed as conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; [Google Scholar]
- Asnis GM, De La Garza R. Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck Depression Inventory. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsy. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Córdoba J, Flavià M, Jacas C, Sauleda S, Esteban JI, Vargas V, Esteban R, Guardia J. Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol. 2003;39:231–238. doi: 10.1016/s0168-8278(03)00189-2. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Knegt RJ, Bezemer G, Van Gool AR, Drenth JP, Hansen BE, Droogleever Fortuyn HA, Weegink CJ, Hengeveld MW, Janssen HL. Randomised clinical trial: escitalopram for the prevention of psychiatric adverse events during treatment with peginterferon-alfa-2a and ribavirin for chronic hepatitis C. Alimentary pharmacology & therapeutics. 2011;34:1306–1317. doi: 10.1111/j.1365-2036.2011.04867. [DOI] [PubMed] [Google Scholar]
- Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997;26:S112S–121. doi: 10.1002/hep.510260720. [DOI] [PubMed] [Google Scholar]
- Fontana RJ. Neuropsychiatric toxicity of antiviral treatment in chronic hepatitis C. Digestive Diseases. 2000;18:107–116. doi: 10.1159/000051384. [DOI] [PubMed] [Google Scholar]
- Forton DM, Allsop JM, Cox IJ, Hamilton G, Wesnes K, Thomas HC, Taylor-Robinson SD. A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection. AIDS. 2005;19:S53–63. doi: 10.1097/01.aids.0000192071.72948.77. [DOI] [PubMed] [Google Scholar]
- Forton D, Karayiannis P. Established and emerging therapies for the treatment of viral hepatitis. Digestive Diseases. 2006;24:160–173. doi: 10.1159/000090319. [DOI] [PubMed] [Google Scholar]
- Gane E, Stedman C, Hyland R, Sorensen R, Symonds W, Hindes R, Berrey M. Once daily GS-7977 plus ribavirin in HCV genotypes 1–3: the ELECTRON trial. Hepatology. 2012;56:306A–307A. [Google Scholar]
- Heaton RK, Kirson D, Velin RA, Grant I, The HNRC Group . The utility of clinical ratings for detecting cognitive change in HIV infection. In: Grant I, Martin A, editors. Neuropsychology of HIV Infection. Oxford University Press; New York: 1994. pp. 188–206. [Google Scholar]
- Hilsabeck RC, Hassanein TI, Ziegler EA, Carlson MD, Perry W. Effect of Interferon-[alpha] on cognitive functioning in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2005;11:16–22. doi: 10.1017/S1355617705050022. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–446. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- Kramer L, Bauer E, Funk G, Hofer H, Jessner W, Steindl-Munda P, Wrba F, Madi C, Gangl A, Ferenci P. Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol. 2002;37:349–354. doi: 10.1016/s0168-8278(02)00172-1. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schäfer A, Faller H, Csef H, Scheurlen M. Paroxetine for the treatment of interferon- α-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- Laskus T, Radkowski M, Adair DM, Wilkinson J, Scheck AC, Rakela J. Emerging evidence of hepatitis C virus neuroinvasion. AIDS. 2005;19:S140–144. doi: 10.1097/01.aids.0000192083.41561.00. [DOI] [PubMed] [Google Scholar]
- McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- McNutt MD, Liu S, Manatunga A, Royster EB, Raison CL, Woolwine BJ, Demetrashvili MF, Miller AH, Musselman DL. Neurobehavioral effects of Interferon-alpha in patients with Hepatitis-C: Symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology. 2012;37:1444–1454. doi: 10.1038/npp.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Fishman SL, Ryan E, Eng FJ, Walewski JL, Branch AD, Morgello S. Clinicopathologic correlates of hepatitis C virus in brain: a pilot study. J Neurovirology. 2008;14:17–27. doi: 10.1080/13550280701708427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri S, Bertino G, Petralia A, Giancarl C, Rizzotto A, Calvagno GS, et al. A Multidisciplinary therapeutic approach for reducing the risk of psychiatric side effects in patients with chronic hepatitis C treated with pegylated Interferon and Ribavirin. J Clin Gastroenterol. 2010;44:210–217. doi: 10.1097/MCG.0b013e3181d88af5. [DOI] [PubMed] [Google Scholar]
- Pattullo V, McAndrews MP, Damyanovich A, Heathcote EJ. Influence of hepatitis C virus on neurocognitive function in patients free from other risk factors: validation from therapeutic outcomes. Liver Int. 2011;31:1028–1038. doi: 10.1111/j.1478-3231.2011.02549. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GL, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19:S174–178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- Russo MA, Fried MW. Side effects of antiviral therapy for hepatitis C. Gastroenterology. 2003;124:1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- Sene D, Limal N, Cacoub P. Hepatitis C virus-associated extrahepatic manifestations: a review. Metab Brain Dis. 2004;19:357–381. doi: 10.1023/b:mebr.0000043982.17294.9b. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Capuron L, Friebe A, Diez-Quevedo C, Robaeys G, Neri S, Foster GR, Kautz A, Forton D, Pariante CM. Hepatitis C infection, antiviral treatment and Mental Health: A European Expert Consensus Statement. J Hepatol. 2012;57:1379–1390. doi: 10.1016/j.jhep.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Engelbrechta MA, Gut O, Fiebich BL, Bauer J, Schmidt F, Grunze H, Lieb K. Interferon alpha (IFNα) and psychiatric syndromes: a review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Janssen G, Martin G, Lorenzs R, Loeschkes K, Soyka M, Folwaczny C, Schaefer M. Factors influencing long-term changes in mental health after interferon-alpha treatment of chronic hepatitis C. Aliment Pharmacol Ther. 2009;30:1049–1059. doi: 10.1111/j.1365-2036.2009.04123. [DOI] [PubMed] [Google Scholar]
- Senzolo M, Schiff S, D’Aloiso CM, Crivellin C, Cholongitas E, Burra P, Montagnese S. Neuropsychological alterations in hepatitis C infection: The role of inflammation. World J Gastroenterology. 2011;17:3369. doi: 10.3748/wjg.v17.i29.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein HH, Maruff P, Krahn MD, Kaldor JM, Koorey DJ, Brew BJ, Dore GJ. Improved cognitive function as a consequence of hepatitis C virus treatment. HIV Med. 2007;8:520–528. doi: 10.1111/j.1468-1293.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA. Neurobehavioral effects of interferon therapy. Curr Psychiatry Rep. 2005;7:391–395. doi: 10.1007/s11920-005-0042-3. [DOI] [PubMed] [Google Scholar]
- Vargas HE, Laskus T, Radkowski M, Wilkinson J, Balan V, Douglas DD, Harrison ME, Mulligan DC, Olden K, Adair D, Rakela J. Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation. Liver transpl. 2002;8:1014–1019. doi: 10.1053/jlts.2002.36393. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schüler A, Ennen JC, Ahl B, Manns MP, Boker KW. Hepatitis C virus infection affects the brain—evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatology. 2004;41:845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test-4 (WRAT-4) Lutz. FL: Psychological Assessment Resources Inc; 2006. [Google Scholar]
- Wilkinson J, Radkowski M, Laskus T. Hepatitis C virus neuroinvasion: Identification of infected cells. J Virol. 2009;83:1312–1319. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite international diagnostic interview (CIDI, version 2.1) World Health Organization; Geneva: 1998. [Google Scholar]
- Zeuzem S, Buggisch P, Agarwal K, Marcellin P, Sereni D, Klinker H, Moreno C, Zarski JP, Horsmans Y, Mo H, Aterburn S, Knox S, Oldach D, McHutchison JG, Manns MP, Foster GR. The protease inhibitor, GS-9256, and non-nucleoside polymerase inhibitor tegobuvir alone, with ribavirin, or pegylated interferon plus ribavirin in hepatitis C. Hepatology. 2012;55:749–758. doi: 10.1002/hep.24744. [DOI] [PubMed] [Google Scholar]


