ABSTRACT
KDM5B (JARID1B/PLU1) is a H3K4me2/3 histone demethylase that is implicated in cancer development and proliferation and is also indispensable for embryonic stem cell self-renewal, cell fate, and murine embryonic development. However, little is known about the role of KDM5B during preimplantation embryo development. Here we show that KDM5B is critical to porcine preimplantation development. KDM5B was found to be expressed in a stage-specific manner, consistent with demethylation of H3K4me3, with the highest expression being observed from the 4-cell to the blastocyst stages. Knockdown of KDM5B by morpholino antisense oligonucleotides injection impaired porcine embryo development to the blastocyst stage. The impairment of embryo development might be caused by increased expression of H3K4me3 at the 4-cell and blastocyst stages, which disturbs the balance of bivalent H3K4me3-H3K27me3 modifications at the blastocyst stage. Decreased abundance of H3K27me3 at blastocyst stage activates multiple members of homeobox genes (HOX), which need to be silenced for faithful embryo development. Additionally, the histone demethylase KDM6A was found to be upregulated by knockdown of KDM5B, which indicated it was responsible for the decreased abundance of H3K27me3 at the blastocyst stage. The transcriptional levels of Ten-Eleven Translocation gene family members (TET1, TET2, and TET3) are found to be increased by knockdown of KDM5B, which indicates cross talk between histone modifications and DNA methylation. The studies above indicate that KDM5B is required for porcine embryo development through regulating the balance of bivalent H3K4me3-H3K27me3 modifications.
Keywords: embryonic development, H3K4me3, histone demethylase, KDM5B, pig
INTRODUCTION
Embryonic development is a complicated process of lineage proliferation and cell differentiation, which is tightly regulated by transcription factors and chromatin-associated proteins [1]. Epigenetic mechanisms are one of the important means of controlling gene expression during embryonic cell differentiation [2–7]. This epigenetic control results in a unique pattern of gene expression between different cell types and stages of embryo development [8–10].
Dynamic methylation and demethylation of histones at specific residues is one of the most important posttranslational chromatin-associated modifications [11]. These modifications are known to regulate nuclear function, including transcriptional regulation, epigenetic inheritance, and maintenance of genome integrity [12–17]. Specifically, trimethylation at both H3K4 (H3K4me3) and H3K27 (H3K27me3) play a role in embryonic genome activation and blastocyst cell lineage segregation in the mouse [18–23]. The colocalization of H3K4me3 and H3K27me3, termed bivalent domains, was found in mouse embryonic stem cells (ESCs) by whole genome mapping [24–26]. This bivalent modification pattern is observed in clusters of homeobox (HOX) genes and other genes related to early embryonic development, such as POU5F1, NANOG, and SOX2 [25]. The bivalent domains are proposed to silence key developmental genes in ESCs while keeping them poised for later activation [27].
Lysine-specific histone demethylase 5B (KDM5B) (also known as JARID1B or PLU-1) can catalyze the demethylation of tri- and dimethylated H3K4 (H3K4me3 and H3K4me2) to the monomethylated form (H3K4me1) [28–30]. Several studies demonstrated that KDM5B is implicated in breast and prostate cancers as well as in melanoma maintenance, making it a potential drug target for cancers [31–35]. Kdm5b is critical for mouse ESC differentiation because depletion of the demethylase leads to increased ability of self-renewal in the absence of leukemia inhibitor factor [36]. However, the exact role of Kdm5b in mouse embryo development remains controversial. It was reported that Kdm5b−/− was embryonic lethal in mice between Embryonic Day 4.5 (E4.5) to E7.5 [35]; however, studies from Albert et al. [1] showed that Kdm5b−/− mice exhibited neonatal lethality due to several neural defects. Recently, Zou et al. [37] reported that Kdm5b−/− mice are viable beyond embryonic and neonatal stages, but they exhibit decreased body weight, premature mortality, decreased female fertility, and delayed mammary gland development. These results might suggest that Kdm5B is critical to ensure faithful murine embryonic development. The importance of KDM5B for porcine embryo development remains unknown, and, as such, the goal of this study was to investigate the function of KDM5B during porcine preimplantation embryonic development.
MATERIALS AND METHODS
Media and Reagents
All the chemicals were purchased from Sigma Chemical Company unless stated otherwise. All of the following solutions and media were filtered using a 0.22 μm filter.
Oocyte in vitro maturation (IVM) medium consisted of TCM 199 (Gibco BRL) supplemented with 0.1% (w/v) polyvinyl alcohol (PVA), 3.05 mM d-glucose, 0.91 mM sodium pyruvate, 1 μg/ml gentamicin, 0.57 mM cysteine, 0.5 μg/ml luteinizing hormone, 0.5 μg/ml follicle-stimulating hormone, and 10 ng/ml epidermal growth factor. Fusion medium contained 0.3 M mannitol, 1.0 mM CaCl2, 0.1 mM MgCl2, 0.5 mM Hepes, pH 7.0–7.4. The embryo culture medium was porcine zygote medium 3 (PZM3), pH of 7.4, supplemented with 3 mg/ml bovine serum albumin (BSA) [38]. Oocyte manipulation medium contained 9.5 g TCM-199 powder, 0.05 g NaHCO3, 0.75 g Hepes, 0.05 g penicillin, 0.06 g streptomycin, 1.755 g NaCl, 3.0 g BSA, and 1 L of Milli-Q (Millipore) water, pH at 7.2–7.4 [39].
Oocyte Collection, Oocyte IVM, Parthenogenetic Activation, In Vitro Fertilization, and Embryo In Vitro Culture
Ovaries were collected from prepubertal gilts at a local slaughter house, stored in saline, and transported to our laboratory at 37°C. Follicles between 3 and 6 mm in diameter were aspirated with an 18-gauge needle attached to a 10 ml syringe. Cumulus-oocyte complexes (COCs) within the follicular fluid were allowed to settle by gravity at 37°C. The COCs were rinsed three times in Hepes-buffered Tyrode medium (6.663 g NaCl, 0.237 g KCl, 0.168 g NaHCO3, 0.041 g NaH2PO4, 1.868 ml Na lactate, 0.102 g MgCl2·6H2O, 2.383 g Hepes, 0.065 g penicillin G, 0.010 g phenol Red, 0.294 g CaCl2·2H2O, 2.186 g sorbitol, 0.025 g gentamicin, 0.022 g sodium pyruvate, 0.100 g PVA, and 1000 ml MilliQ H2O [39]) containing 0.01% PVA in an incubator at 37°C. Only the COCs with multiple layers of intact cumulus cells and uniform ooplasm were selected for IVM. After washing three times in IVM medium, a group of 70–80 COCs were placed into wells of four-well cell culture plates (Nunc) containing 500 μl of IVM medium and 350 μl mineral oil per well. The COCs were cultured for 42–44 h at 38.5°C and 5% CO2 in air (100% humidity). Matured COCs were then vortexed in 0.1% hyaluronidase in Hepes-buffered Tyrode medium containing 0.01% PVA for 4 min to remove the cumulus cells. Only the matured oocytes having an extruded first polar body with uniform cytoplasm were used for in vitro development. Parthenogenetic activation of the matured oocytes were accomplished with two direct current pulses (1-sec interval) of 1.2 kV/cm for 30 μsec provided by a BTX Electro-cell Manipulator 200 (BTX) in the fusion medium. Then the activated oocytes were transferred and incubated in four-well plates containing 500 μl of PZM3 and 350 μl mineral oil per well at 38.5°C and 5% CO2 in humidified air. In vitro fertilization (IVF) was carried out as previously described. Briefly, metaphase II (MII) oocytes were washed three times in the modified fertilization Tris-buffered medium: 10 mM Tris, containing 2 mg/ml BSA and 2 mM caffeine. Approximately 30–35 oocytes were transferred into 50 μl droplets of fertilization medium covered with mineral oil that had been equilibrated for 4 h at 38.5°C in 5% CO2 in air. Porcine semen was washed three times by centrifugation with Dulbecco phosphate buffered saline (Gibco BRL) supplemented with 1 mg/ml BSA (pH 7.3) (1900 × g, 4 min), then spermatozoa were resuspended with modified fertilization Tris-buffered medium to a concentration of 1 × 106 cells/ml. Fifty microliters of the sperm suspension solution was added to the fertilization droplets, giving a final sperm concentration of 0.5 × 106 cells/ml. Oocytes were coincubated with sperm for 4–6 h. After fertilization, oocytes were washed three times and cultured in 500 μl PZM3 medium in four-well Nunclon dishes at 38.5°C in 5% CO2 in air.
Microinjection
For KDM5B knockdown, morpholino antisense oligonucleotides (MOs) (GENE-Tools) against porcine KDM5B were injected into the cytoplasm of matured MII stage oocytes using a FemtoJet microinjector (Eppendorf). MOs were designed to target porcine KDM5B at 362–384 bp (ENSSSCT00000029047) to block translation while water was injected as a negative control. Either the MOs or water was delivered into cytoplasm of matured porcine oocytes. Microinjection was performed in oocyte manipulation medium with 7.5 μg/ml cytochalasin B on the heated stage of a Nikon inverted microscope (Nikon Corporation). A number of control experiments (no injection, water injection, nonspecific MOs injection, and KDM5B-MOs injection) were designed to eliminate potential detrimental effects of the microinjection technique and morpholino toxicity on embryo development and to demonstrate that the morpholino was specifically blocking the KDM5B translation. Injected oocytes were then activated according to the protocol. The activated oocytes were transferred to PZM3 medium for culture.
Indirect Immunofluorescence
Embryos of every stage derived from parthenogenetic development were washed in PBS, fixed for 15 min in 4% paraformaldehyde in PBS, and permeabilized with 0.1% Triton X-100 in PBS for 30 min. After permeabilization, the embryos were then blocked in 5% BSA in PBS for 1 h at room temperature. The samples were stained with primary antibodies against H3K4me3 (1:1000; Abcam), or H3K27me3 (1:200; Abcam) according to the manufacturer's protocol overnight at 4°C. After extensive washing with PBS containing 0.1% PVA, samples were treated with a secondary antibody of Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG) (1:200) or Alexa Fluor 594 goat anti-mouse IgG (1:200) according to the manufacturer's protocol (ZSGB-Bio) for 1 h at room temperature. After washing three times with PBS containing 0.1% PVA, embryos were mounted on slides in mounting medium containing 15 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratory Inc.). Day 6 blastocysts with good morphology were selected for counting total number of nuclei. After being fixed in 4% paraformaldehyde in PBS for 15 min and permeabilized with 0.1% Triton X-100 in PBS for 30 min at room temperature, embryos were mounted on slides in mounting medium containing DAPI. Groups of embryos stained without primary antibody or secondary antibody or both antibodies were used as negative controls to examine the specificity of the reaction. At least 10 oocytes or embryos were processed for each treatment group, and the experiments were replicated three times. Slides were analyzed using an epifluorescence microscope (Nikon) equipped with a digital camera. Images were captured and quantified using Nikon NIS element software. To make relative comparisons, the settings for exposure and image capture remained constant, and all the images were assembled without any adjustment of contrast or brightness to the images.
Western Blot Analysis
A total of 400 porcine embryos per sample were mixed with SDS sample buffer and boiled for 5 min at 100°C. Western blot analysis was performed as described previously [40] and according the manufacturer's protocol. Briefly, protein samples were separated by SDS-PAGE and transferred onto nitrocellulose filter membrane. After blocking with 5% BSA for 1 h, the membranes were incubated with anti-KDM5B/H3K4me3/H3K27me3 (1:500) and anti-GAPDH (1:2000) antibody overnight at 4°C. After rinsing three times in PBS, the membranes were incubated at 37°C for 1 h with Alexa Fluor 680 goat anti-mouse IgG (A21057, Invitrogen) or IRDye 800CW goat anti-rabbit (926–32211; LI-COR Biosciences). After rinsing three times in PBS, the membrane was imaged with the ODYSSEY Sa Infrared Imaging System (LI-COR Biosciences).
Quantitative Real-Time PCR
To investigate the abundance of mRNA in porcine oocytes and embryos, 100–200 oocytes or 50–100 embryos were collected for each stage. Total RNA was extracted from the samples using the Qiagen AllPrep DNA/RNA Micro Kit (Qiagen) according to the manufacturer's protocol. After RNA isolation, the reverse transcriptase reaction was conducted using a TIANscript RT Kit (TIAGEN). The synthesized cDNA was used for quantitative real-time PCR. A housekeeping gene, H2AFZ, was used as the internal control (primer sequences are shown in Supplemental Table S1; Supplemental Data are available online at www.biolreprod.org). The PCR was conducted using TaKaRa SYBR Premix Ex Taq (TaKaRa). Primer validation tests were run for each designed primer to verify that the amplification efficiencies were similar for each cycle. The program used for the PCR included an initial temperature of 95°C for 30 sec followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. Real-time fluorescence data was collected during the extension time. The relative quantification method based on comparative threshold cycles (Ct) values was used to identify the abundance of message. The transcript abundance of each gene was then calculated relative to that of the internal control gene H2AFZ, and ΔCt was calculated by subtracting Ct values of each gene from the Ct of H2AFZ. Control group Ct values served as calibrators and were used subsequently to obtain ΔΔCt values. Fold differences in transcript abundance were obtained by using the equation2−ΔΔCt. At least three biological and three experimental replications were used for each assay. The quantitative real-time PCR results were compared by the general linear model (PROC GLM) of Statistical Analysis System (SAS Institute). Differences with P < 0.05 were considered significantly different.
TdT-Mediated dUTP Nick End Labeling Assay
TdT-mediated dUTP nick end labeling assays were carried out with In Situ Cell Death Detection Kit (Roche Diagnostics) according to the manufacturer's instructions.
Statistical Analysis
All the experiments were repeated at least three times. Differences in relative expression assayed by quantitative PCR (qPCR) and immunofluorescence as well as embryo nuclear number and percentage of apoptosis cells were tested for significance by Student t-test. Difference in cleavage rates and blastocyst rate were tested for significance by chi-square. The data were considered significant when the P value was less than 0.05 (*) or 0.01 (**).
RESULTS
Demethylation of H3K4me3 Is Temporally Related to Expression of KDM5B During Porcine Preimplantation Development
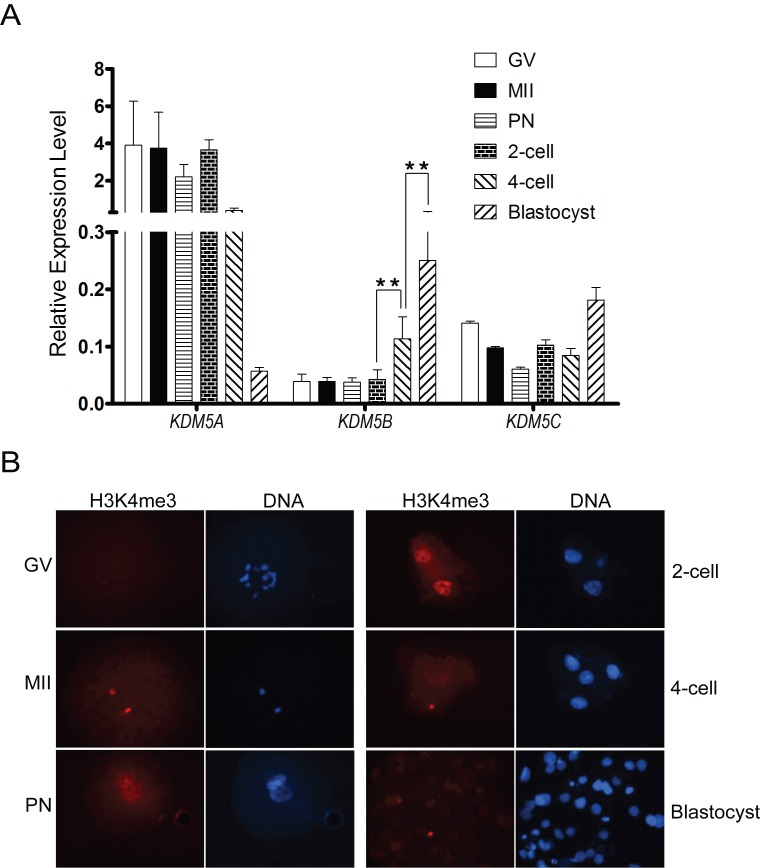
The four members of KDM5 histone demethylases are KDM5A, KDM5B, KDM5C, and KDM5D. KDM5D is encoded on the Y chromosome, and its mRNA expression is detectable in all male tissues [41]. In order to determine which histone demethylase is key during porcine embryogenesis, the abundance of three KDM5 family demethylases (KDM5A, KDM5B, KDM5C) in preimplantation porcine embryo were investigated by qPCR. KDM5B was the only transcript that was differentially expressed, with an increase in abundance from the 2- to 4-cell stage before reaching a peak at the blastocyst stage (P < 0.01; Fig. 1A).
Immunofluorescence staining of H3K4me3 from different development stage embryos was performed. H3K4me3 is not detectable in GV stage oocytes; however, starting at the MII stage, it is easily detectable through the 2-cell stage before decreasing at the 4-cell and blastocyst stages (Fig. 1B). The dynamic changes of KDM5B expression are consistent with demethylation of H3K4me3 during porcine embryo development.
FIG. 1.
Temporal and spatial abundance of histone demethylases KDM5B and H3K4me3 in porcine oocytes and embryonic development. A) Transcriptional profile of KDM5A, KDM5B, and KDM5C using real-time PCR. The levels of the transcripts were normalized against H2AFZ. Note that KDM5B was stage specifically expressed and increased from 4-cell stage to blastocyst stage (**P < 0.01). Data are presented as the mean ± SEM. B) Immunofluorescence detection of H3K4me3 protein in porcine oocytes and early embryonic stages. H3K4me3 protein (red) was probed with rabbit anti-H3K4me3 antibodies (1:1000) and detected by using Alexa 594-conjugated goat anti-rabbit antibodies (1:200). Nuclei (blue) were labeled with DAPI stain. Original magnification was ×400 for embryos. Note that H3K4me3 was decreased at the 4-cell and blastocyst stages.
KDM5B Is Required for Early Porcine Embryo Development
Conservation of KDM5B across species was analyzed using the amino acid sequences of KDM5B from pig (XM_005668019.1), mouse (NM_152895.2), and human (NM_006618.3). They were aligned using DNAman software. Porcine KDM5B is 91.4% and 89.6% homologous to human and mouse, respectively, which indicates that KDM5B is highly conserved across these species (Fig. 2).
FIG. 2.
Protein sequence of KDM5B from porcine, mouse, and human were aligned using DNAman software. KDM5B was conserved in these species, with the porcine sequence being 91.4% and 89.6% homologous to human and mouse, respectively.
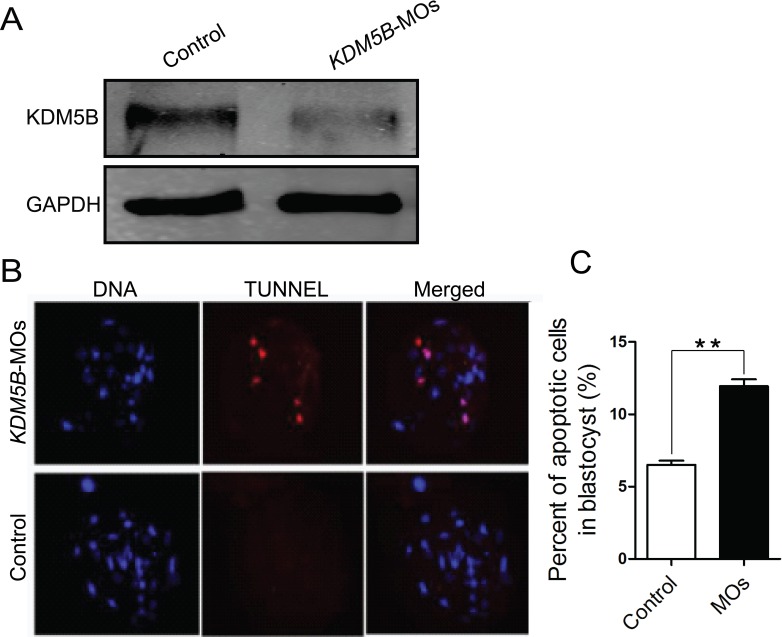
To investigate the potential biological function of KDM5B in porcine early embryonic development, 10 ng of MOs designed against KDM5B-coding sequence were microinjected into MII oocytes. Several experiments detected no significant difference in embryo developmental competence and embryo quality when using water or nonspecific MOs as negative controls (Table 1). Oocytes that survived microinjection were parthenogenetically activated or in vitro fertilized to initiate embryo development. As shown in Figure 3A, we confirmed by Western blot analysis that the expression of KDM5B in Day 4 porcine embryos is notably reduced.
TABLE 1.
Effect of KDM5B knockdown on porcine parthenogenetic and in vitro fertilized embryo development.
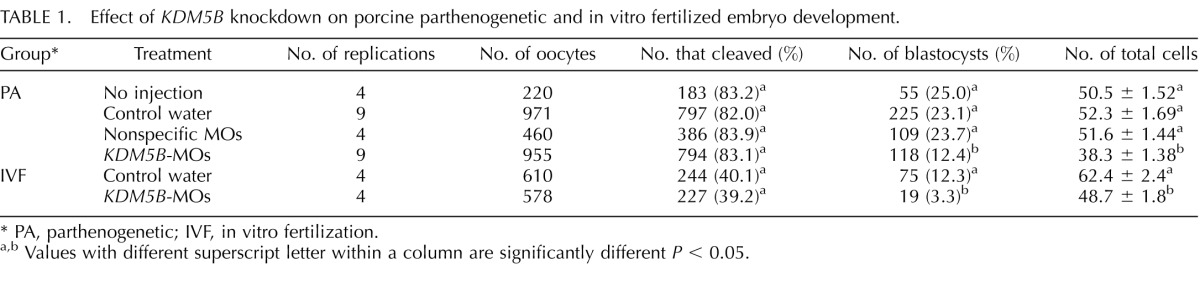
PA, parthenogenetic; IVF, in vitro fertilization.
Values with different superscript letter within a column are significantly different P < 0.05.
FIG. 3.
Knockdown of KDM5B resulted in increasing apoptotic cells in the blastocysts. A) Western blot analysis of KDM5B expression after knockdown using MOs blocking KDM5B translation. Embryos were collected 96 h after injection and parthenogenetic development. The levels of the translation were normalized against GAPDH. B, C) Staining for cell death (using TdT-mediated dUTP nick end labeling) showed that KDM5B-knockdown blastocysts had more apoptotic cells; 30 embryos of each group were detected. Original magnification was ×200 for embryos. Data are presented as the mean ± SEM; **P < 0.01.
There is no difference in cleavage rate (as defined by the number of embryos cleaved on Day 2 over the total number of oocytes subjected to parthenogenesis or IVF) between KDM5B-MOs zygotes and the negative control (Table 1). However, in the KDM5B-MOs group, the developmental competence of parthenogenetic (12.3%) and IVF (3.3%) embryos reaching the blastocyst stage was significantly reduced when compared to the control group, which is 23.1% and 12.3%, respectively (P < 0.01, Table 1). Total cell number was significantly decreased in the KDM5B-MOs group when compared with the control cohort in both parthenogenetic and IVF embryos (Table 1). The percentage of apoptotic cells in blastocysts from the KDM5B-MOs parthenogenetic development group was increased compared to the control-injected embryos (12.5% vs. 6.5%, P < 0.01; Fig. 3, B and C).
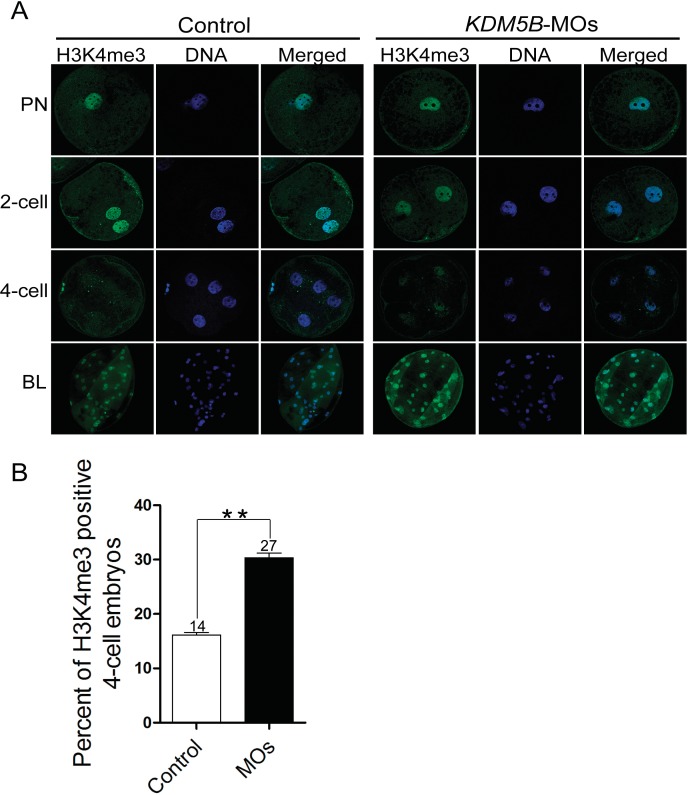
KDM5B Knockdown Stimulated the Trimethylation of H3K4 in 4-Cell Stage Embryos and Increased the Abundance of H3K4me3 at the Blastocyst Stage
To address the exact mechanism of KDM5B knockdown during porcine embryogenesis, the abundance of H3K4me3 was examined. Immunofluorescence using an antibody against H3K4me3 revealed that pronuclear and 2-cell stages embryos from both KDM5B knockdown and control groups had robust abundance of H3K4me3 (Fig. 4A). However, at the 4-cell stage there were a higher number of embryos that were positively stained with H3K4me3 observed in the KDM5B-MOs injected group compared with the control group (16% vs. 30%, P < 0.01; Fig. 4B). Additionally, KMD5B knockdown increased the abundance of H3K4me3 at the blastocyst stage (P < 0.01, Figs. 4A and 5C).
FIG. 4.
Knockdown of KDM5B stimulated H3K4me3 in 4-cell stage embryos and increased the abundance of H3K4me3 at the blastocyst stage. A) Immunofluorescence staining of H3K4me3 protein during embryonic development in KDM5B-knockdown and control groups. H3K4me3 protein (green) was probed with rabbit anti-H3K4me3 antibodies (1:1000) and detected by use of Alexa 488-conjugated goat anti-rabbit antibodies (1:200). Nuclei (blue) were labeled with 15 μg/ml DAPI. Original magnification was ×400 for pronuclear and 2- and 4-cell embryos and ×200 for blastocyst embryos. B) Fluorescence intensity analysis of H3K4me3 abundance in the 4-cell stage embryos. In the KDM5B-knockdown group, 16% (14/87) of H3K4me3-positive 4-cell stage embryos were observed, and in the control group, 30% (27/90) of H3K4me3-positive 4-cell stage embryos were found. Images were captured and quantified using Nikon NIS element software. To make relative comparisons, the settings for exposure and image capture remained constant, and all the images were assembled without any adjustment of contrast or brightness to the images. Data are presented as the mean ± SEM; **P < 0.01.
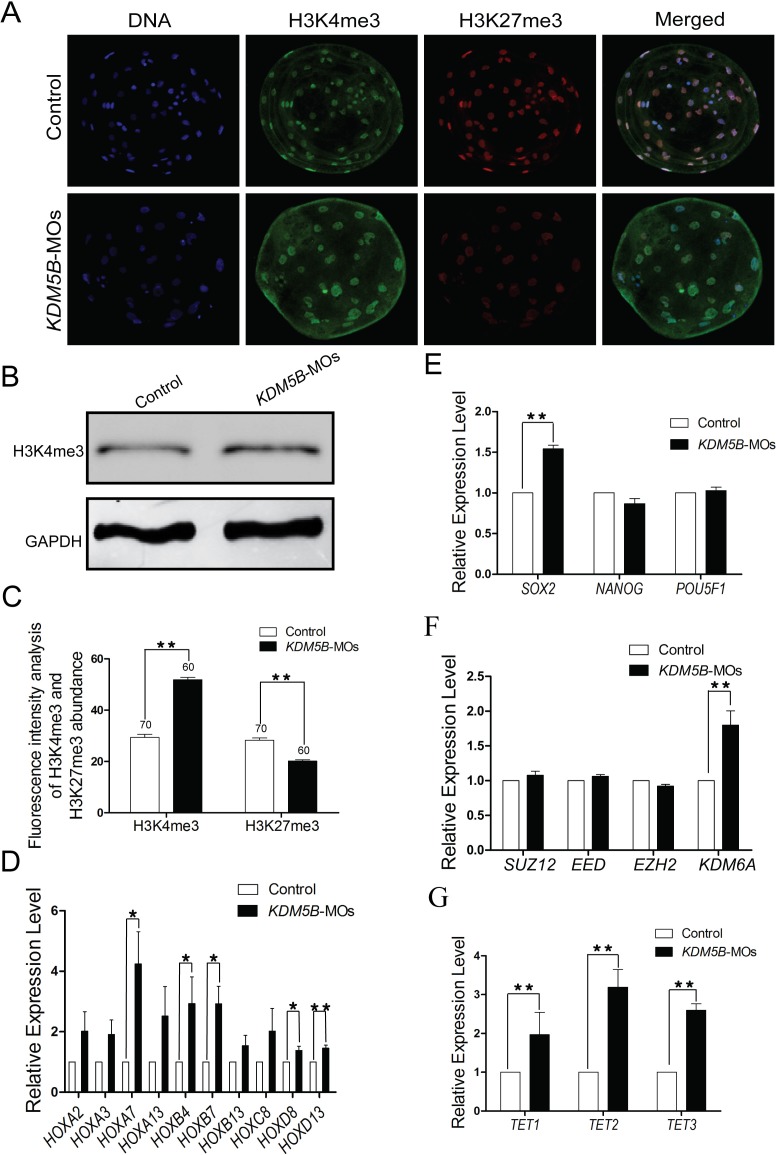
FIG. 5.
Knockdown of KDM5B impairs the balance of bivalent H3K4me3-H3K27me3 modifications at the blastocyst stage. A) Immunofluorescence staining results of the H3K4me3 and H3K27me3 in the blastocyst from the control (n = 70) and KDM5B-knockdown groups (n = 60). Protein (green) was probed with rabbit anti-H3K4me3 antibodies (1:1000) and detected by use of Alexa 488-conjugated goat anti-rabbit antibodies (1:200). Protein (red) was probed with mouse anti-H3K27me3 antibodies (1:200) and detected by use of Alexa 594-conjugated goat anti-mouse antibodies (1:200). Nuclei (blue) were labeled with 15 μg/ml DAPI. Original magnification was ×200 for the embryos. B) Western blot analysis of H3K4me3 abundance after knockdown using MOs blocking KDM5B translation. Ninety-six hours after injection and parthenogenetic development, 400 live embryos per sample were collected. The levels of the translation were normalized against GAPDH. C) Fluorescence intensity analysis of H3K4me3 and H3K27me3 abundance in the blastocyst stage of the control and KDM5B-knockdown groups. D) Real-time PCR results of the HOX family genes in the blastocyst from the control and KDM5B-knockdown groups. E) Real-time PCR results of SOX2, NANOG, and POU5F1 in the blastocysts from the control and KDM5B-knockdown groups. F) Real-time PCR results of PRC2 (EED, SUZ12, and EZH2) and KDM6A of blastocyst from the control and KDM5B-knockdown groups. G) Real-time PCR results of the TET family genes in the blastocyst from the control and KDM5B-knockdown groups. The abundance of the transcripts was normalized against H2AFZ. Data are presented as the mean ± SEM; *P < 0.05, **P < 0.01.
Knockdown of KDM5B Impairs the Balance of H3K4me3-H3K27me3 at the Blastocyst Stage
H3K27me3 and H3K4me3 are important repressive and permissive histone modifications that can repress and activate, respectively, gene expression during embryonic development [25, 42]. Observation of enhanced H3K4me3 expression following the knockdown of KDM5B (Figs. 4A and 5A–C), warranted further examination of the abundance levels for H3K27me3. Immunofluorescence results showed that knockdown of KDM5B can not only increase the trimethylation of H3K4 but also decrease the abundance of H3K27me3 at the blastocyst stage (Fig. 5, A and C, and Supplemental Fig. S1). The increase of H3K4me3 after KDM5B knockdown was also confirmed by Western blot analysis (Fig. 5B). The antibody against H3K27me3 we used for Western blot analysis was not specific enough to make good quality images, however, the results still indicated a decrease in H3K27me3 after KDM5B knockdown (Supplemental Fig. S1). The dynamic change in methylation and demethylation of H3K27me3 was coordinated by KDM6A and Polycomb Repressive Complex 2 (PRC2). The qPCR results indicated that the increased expression of H3K27m3 at the blastocyst stage might be caused by KDM6A but not PRC2 (Fig. 5F).
Knockdown of KDM5B Increased the Expression Level of HOX Genes and TETs
The colocalization of H3K4me3 and H3K27me3, the bivalent domains, was found in mouse ESCs by whole genome mapping [24–26]. This modification pattern is observed in clusters of HOX genes in addition to other genes corresponding to early embryonic development, such as POU5F1, NANOG, and SOX2 [25]. The transcript level of HOX genes (HOXA2, HOXA3, HOXA7, HOXA13, HOXB4, HOXB7, HOXB13, HOXC8, HOXD8, and HOXD13) and pluripotency genes (POU5F1, NANOG, and SOX2) were analyzed. The qPCR results showed that the expression of multiple members of the HOX family (HOXA7, HOXB4, HOXB7, HOXD8, and HOXD13) and SOX2 are greatly elevated (P < 0.01; Fig. 5, D and E). We found that the abundance of TET1, TET2, and TET3 in the blastocysts from the KDM5B-knockdown group are all increased compared to controls (Fig. 5G).
DISCUSSION
Here we demonstrate an essential role of KDM5B in porcine embryogenesis. Knockdown of KDM5B by microinjection of MOs at the MII stage resulted in a lower rate of oocytes that developed to the blastocyst stage and fewer cells within the resulting blastocysts. The studies in mice have suggested that Kdm5b is important for faithful mouse embryonic development because Kdm5b−/− embryos (deletion of exon 1) fail to develop beyond the preimplantation stage (E4.5–E7.5), and deletion of exon 6 results in neonatal lethality [1, 9, 35]. The embryonic lethality in the Kdm5b−/− mouse might be due to the method used to generate the embryonic stem line by disrupting the Kdm5b locus with the neomycin cassette or by the genetic background of mice [43]. Additionally, the delayed mammary gland development defect observed in a Kdm5b −/− mice [43] is similar to the phenotype of a different mouse model that carries a deletion for ARID domain of Kdm5b [35]. The above studies highlight the importance of the function of Kdm5b in mouse embryonic development. Furthermore, ESCs could not be generated from mouse Kdm5b−/− blastocysts successfully, and mouse embryo ESCs do not survive upon abolishing Kdm5b expression by small hairpin RNA [28].
The data from the current study shows that expression of KDM5B but not KDM5A or KDM5C is stage specific, being highly expressed at the 4-cell and blastocyst stages, which is in accordance with the dynamic change of H3K4me3. Therefore, we hypothesized that KDM5B would be critical for embryo development and regulation of H3K4me3. Our results showed that knockdown of KDM5B decreased developmental competence to the blastocyst stage and markedly increased H3K4me3 at the 4-cell and blastocyst stages of porcine embryos in accordance with a previous study that showed KDM5B is responsible for demethylation of the tri- and dimethylation states of H3K4 (H3K4me3 and H3K4me2) to the mono form (H3K4me1) [30, 31, 44]. Kdm5b is reported to be a barrier to the reprogramming process because genes associated with epithelial to mesenchymal transition lose H3K4me3/2 during the early reprogramming process. A global analysis of H3K4me3/2 reveals that enhancers of fibroblast-specific genes are rapidly deactivated in the absence of Kdm5b [36]. Additionally, a study reported that a Kdm5b−/− mouse model exhibited neonatal lethality due to several neural defects, which was caused by aberrant H3K4me3 and activation of normally inactive genes encoding developmental regulators such as Pax6 and Otx2 during embryogenesis [1]. These results suggest that aberrant abundance of H3K4me3 in the 4-cell and blastocyst stage embryos might be harmful to porcine embryonic development.
Knockdown KDM5B could indirectly lead to lower abundance of H3K27me3 in the blastocysts compared to the control group, which is consistent with a previous finding in ESCs where there was a bivalent balance between H3K4me3 and H3K27me3 [25, 45]. H3K4me3 positively regulates transcription by recruiting nucleosome-remodeling enzymes and histone acetylases, while H3K27me3 negatively regulates transcription by promoting a compact chromatin structure. Bivalent modifications of H3K4me3 and H3K27me3 at the same area of one gene are proposed to play a pivotal role related to pluripotency in ESCs and is observed in clusters of HOX genes and other genes related to early embryonic development [25, 45]. The analysis between ESCs and differentiated cell types suggests that bivalent domains are characteristic of pluripotent cells and that they silence developmental genes while keeping them poised for activation [25]. Disturbance of the bivalent modifications of H3K4me3 and H3K27me3 might activate expression of the HOX family and other genes related to early embryonic development [46]. In this study, the abundance of HOXA7, HOXB13, HOXB4, HOXD8, and HOXD13 was found to significantly increase in the KDM5B-knockdown blastocysts. Previous research has demonstrated a direct interaction of methylases and demethylases of H3K27 at the promoter sites of HOXA7, HOXB1, and HOXA10 [46–48]. The HOX family genes are critical to the mammalian body plan and are expressed in a restricted anterior/posterior pattern that coincides with their physical position in the cluster. Tight regulation of HOX gene expression boundaries and dosage are critical for embryonic development [49] with the repressed status being necessary for normal embryo development.
The dynamic change of H3K27me3 is coordinated with histone methylation transferase PRC2 and histone demethylase KDM6A [46, 50–53]. We noticed that the abundance of H3K27me3 was decreased after KDM5B deletion. This might be caused by KDM6A, which was found to be overexpressed in blastocysts as determined by qPCR. However, this result needs to be further confirmed by demonstration of overexpression of KDM6A.
KDM5B knockdown also affects the expression of SOX2 in the blastocysts stage. This result is consistent with studies in ESCs that showed that H3K4me3 could regulate the expression of POU5F1, NANOG, and SOX2, three pluripotency genes that regulate differentiation of ESCs [54, 55].
Cross talk between histone modification and DNA methylation is important to keep in mind for future manipulations of gene regulation in induced pluripotent stem cells and cancer treatment [56]. Tets are responsible for the cross talk between H3K27me3 and DNA methylation in mouse ESCs [56]. Increased expression of TET1, TET2, and TET3 by knockdown of KDM5B was observed and could be a reason for the decreased embryo development competency. This is supported by a study that showed the dynamics of TET family expression in porcine preimplantation embryos was related to zygotic genome activation and required for the maintenance of NANOG [57].
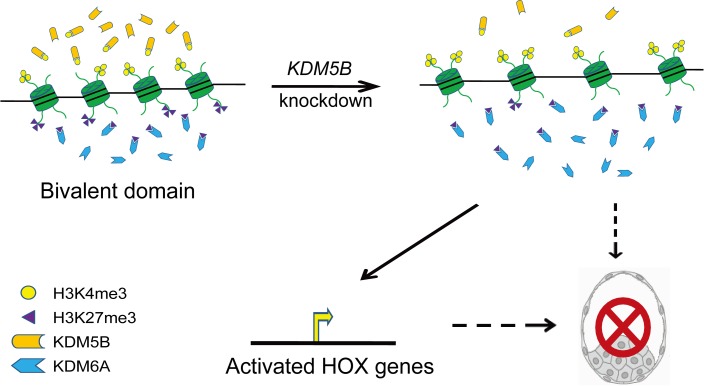
In summary, knockdown of KDM5B impaired early embryonic development and decreased the quality of the embryo. Deletion of KDM5B disturbed the bivalent balance between H3K4me3 and H3K27me3, which was indicated by an increase in H3K4me3 abundance at the 4-cell and blastocyst stages in conjunction with decreased abundance of H3K27me3. The disturbed bivalent balance of H3K4me3 and H3K27me3 caused activation of HOX genes, whose repressed status is necessary for normal embryo development. KDM5B might be a key epigenetic regulation-related factor that is crucial for the normal growth and development of porcine embryos (Fig. 6). More studies are needed for further understanding of the gene expression network regulated by KDM5B in early embryonic development.
FIG. 6.
Speculative model for the role of KDM5B knockdown in impairing embryo development. Knockdown KDM5B disturbs the bivalent balance between H3K4me3 and H3K27me3 and then activates the expression of HOX genes, which impairs embryo development.
ACKNOWLEDGMENT
We thank Shan Ye and Jinwei Zhou for embryo culture assistant and Shiwen Li for confocal microscopic studies.
Footnotes
This work was supported by grants from the National Natural Science Foundation of China (31172281, 31272440), the National High Technology Research and Development Program of China (2012AA020602), and the National Basic Research Program of China (2011CBA0100, 2011CB944100) to J.G.Z. and from the National Institutes of Health (U42 RR018877, U42 OD011140) to R.S.P.
REFERENCES
- Albert M, Schmitz SU, Kooistra SM, Malatesta M. Morales Torres C, Rekling JC, Johansen JV, Abarrategui I, Helin K. The histone demethylase Jarid1b ensures faithful mouse development by protecting developmental genes from aberrant H3K4me3. PLoS Genet. 2013;9:e1003461. doi: 10.1371/journal.pgen.1003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean W, Santos F, Reik W. Epigenetic reprogramming in early mammalian development and following somatic nuclear transfer. Semin Cell Dev Biol. 2003;14:93–100. doi: 10.1016/s1084-9521(02)00141-6. [DOI] [PubMed] [Google Scholar]
- Dyban AP, Dyban PA. Theoretical and applied aspects of epigenetic reprogramming in mammalian development [in Russian] Genetika. 2006;42:1615–1620. [PubMed] [Google Scholar]
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Santos F, Peters AH, Otte AP, Reik W, Dean W. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol. 2005;280:225–236. doi: 10.1016/j.ydbio.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- Surani MA, Hayashi K, Hajkova P. Genetic and epigenetic regulators of pluripotency. Cell. 2007;128:747–762. doi: 10.1016/j.cell.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pacheco-Trigon S, Hennequet-Antier C, Oudin JF, Piumi F, Renard JP, Duranthon V. Molecular characterization of genomic activities at the onset of zygotic transcription in mammals. Biol Reprod. 2002;67:1907–1918. doi: 10.1095/biolreprod67.6.1907. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell. 2004;6:117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- Turner BM. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- Xu Y, Fang F. Histone methylation and transcriptional regulation in cardiovascular disease. Cardiovasc Hematol Disord Drug Targets. 2014;14:89–97. doi: 10.2174/1871529x14666140505122144. [DOI] [PubMed] [Google Scholar]
- An W. Histone acetylation and methylation: combinatorial players for transcriptional regulation. Subcell Biochem. 2007;41:351–369. [PubMed] [Google Scholar]
- Miller JL, Grant PA. The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell Biochem. 2013;61:289–317. doi: 10.1007/978-94-007-4525-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Xu M, Zhu B. Epigenetic inheritance mediated by histone lysine methylation: maintaining transcriptional states without the precise restoration of marks? Philos Trans R Soc Lond B Biol Sci. 2013;368:20110332. doi: 10.1098/rstb.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Gurard-Levin ZA, Almouzni G, Loyola A. Histone lysine methylation and chromatin replication. Biochim Biophys Acta. 2014;1839:1433–1439. doi: 10.1016/j.bbagrm.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Blackledge NP, Klose RJ. Histone lysine methylation: an epigenetic modification? Epigenomics. 2010;2:151–161. doi: 10.2217/epi.09.42. [DOI] [PubMed] [Google Scholar]
- Ostrup O, Reiner AH, Alestrom P, Collas P. The specific alteration of histone methylation profiles by DZNep during early zebrafish development. Biochim Biophys Acta. 2014;1839:1307–1315. doi: 10.1016/j.bbagrm.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Aday AW, Zhu LJ, Lakshmanan A, Wang J, Lawson ND. Identification of cis regulatory features in the embryonic zebrafish genome through large-scale profiling of H3K4me1 and H3K4me3 binding sites. Dev Biol. 2011;357:450–462. doi: 10.1016/j.ydbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Lv Z, Wang Y, Hai T, Huo R, Zhou Z, Zhou Q, Sha J. WDR82, a key epigenetics-related factor, plays a crucial role in normal early embryonic development in mice. Biol Reprod. 2011;84:756–764. doi: 10.1095/biolreprod.110.084343. [DOI] [PubMed] [Google Scholar]
- Dahl JA, Reiner AH, Klungland A, Wakayama T, Collas P. Histone H3 lysine 27 methylation asymmetry on developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation embryos. PLoS One. 2010;5:e9150. doi: 10.1371/journal.pone.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Hyttel P, Hall VJ. Regulation of H3K27me3 and H3K4me3 during early porcine embryonic development. Mol Reprod Dev. 2010;77:540–549. doi: 10.1002/mrd.21180. [DOI] [PubMed] [Google Scholar]
- Gao Y, Hyttel P, Hall VJ. Dynamic changes in epigenetic marks and gene expression during porcine epiblast specification. Cell Reprogram. 2011;13:345–360. doi: 10.1089/cell.2010.0110. [DOI] [PubMed] [Google Scholar]
- Gan Q, Yoshida T, McDonald OG, Owens GK. Concise review: epigenetic mechanisms contribute to pluripotency and cell lineage determination of embryonic stem cells. Stem Cells. 2007;25:2–9. doi: 10.1634/stemcells.2006-0383. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Azuara V, Perry P, Sauer S, Spivakov M, Jorgensen HF, John RM, Gouti M, Casanova M, Warnes G, Merkenschlager M, Fisher AG. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, Brockdorff N, Fisher AG, Pombo A. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- Dey BK, Stalker L, Schnerch A, Bhatia M, Taylor-Papidimitriou J, Wynder C. The histone demethylase KDM5b/JARID1b plays a role in cell fate decisions by blocking terminal differentiation. Mol Cell Biol. 2008;28:5312–5327. doi: 10.1128/MCB.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang H, Guo X, Rong N, Song Y, Xu Y, Lan W, Zhang X, Liu M, Xu Y, Cao C. The PHD1 finger of KDM5B recognizes unmodified H3K4 during the demethylation of histone H3K4me2/3 by KDM5B. Protein Cell. 2014;5:837–850. doi: 10.1007/s13238-014-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Korbel C, Laschke MW, Gimotty PA, Philipp SE, Krause E, Patzold S, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, Madsen B, Copier J, Lu PJ, Cooper L, Scibetta AG, Burchell J, Taylor-Papadimitriou J. PLU-1 nuclear protein, which is upregulated in breast cancer, shows restricted expression in normal human adult tissues: a new cancer/testis antigen? Int J Cancer. 2002;101:581–588. doi: 10.1002/ijc.10644. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Sundquist K, Baeckstrom D, Poulsom R, Hanby A, Meier-Ewert S, Jones T, Mitchell M, Pitha-Rowe P, Freemont P, Taylor-Papadimitriou J. A novel gene (PLU-1) containing highly conserved putative DNA/chromatin binding motifs is specifically up-regulated in breast cancer. J Biol Chem. 1999;274:15633–15645. doi: 10.1074/jbc.274.22.15633. [DOI] [PubMed] [Google Scholar]
- Catchpole S, Spencer-Dene B, Hall D, Santangelo S, Rosewell I, Guenatri M, Beatson R, Scibetta AG, Burchell JM, Taylor-Papadimitriou J. PLU-1/JARID1B/KDM5B is required for embryonic survival and contributes to cell proliferation in the mammary gland and in ER+ breast cancer cells. Int J Oncol. 2011;38:1267–1277. doi: 10.3892/ijo.2011.956. [DOI] [PubMed] [Google Scholar]
- Kidder BL, Hu G, Yu ZX, Liu C, Zhao K. Extended self-renewal and accelerated reprogramming in the absence of Kdm5b. Mol Cell Biol. 2013;33:4793–4810. doi: 10.1128/MCB.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MR, Cao J, Liu Z, Huh SJ, Polyak K, Yan Q. Histone demethylase jumonji AT-rich interactive domain 1B (JARID1B) controls mammary gland development by regulating key developmental and lineage specification genes. J Biol Chem. 2014;289:17620–17633. doi: 10.1074/jbc.M114.570853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod. 2002;66:112–119. doi: 10.1095/biolreprod66.1.112. [DOI] [PubMed] [Google Scholar]
- Lai L, Prather RS. A method for producing cloned pigs by using somatic cells as donors. Methods Mol Biol. 2004;254:149–164. doi: 10.1385/1-59259-741-6:149. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma W, Li YH, Hou Y, Li SW, Meng XQ, Sun XF, Sun QY, Wang WH. Intra-oocyte localization of MAD2 and its relationship with kinetochores, microtubules, and chromosomes in rat oocytes during meiosis. Biol Reprod. 2004;71:740–748. doi: 10.1095/biolreprod.104.028282. [DOI] [PubMed] [Google Scholar]
- Rasmussen PB, Staller P. The KDM5 family of histone demethylases as targets in oncology drug discovery. Epigenomics. 2014;6:277–286. doi: 10.2217/epi.14.14. [DOI] [PubMed] [Google Scholar]
- Benevolenskaya EV. Histone H3K4 demethylases are essential in development and differentiation. Biochem Cell Biol. 2007;85:435–443. doi: 10.1139/O07-057. [DOI] [PubMed] [Google Scholar]
- Zou MR, Cao J, Liu Z, Huh SJ, Polyak K, Yan Q. Histone demethylase jumonji AT-rich interactive domain 1B (JARID1B) controls mammary gland development by regulating key developmental and lineage specification genes. J Biol Chem. 2014;289:17620–17633. doi: 10.1074/jbc.M114.570853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Li Q, Lian S, Dai Z, Xiang Q, Dai X. BGDB: a database of bivalent genes Database (Oxford) 2013. 2013:bat057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX. and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- Cao R, Wang H, He J, Erdjument-Bromage H, Tempst P, Zhang Y. Role of hPHF1 in H3K27 methylation and Hox gene silencing. Mol Cell Biol. 2008;28:1862–1872. doi: 10.1128/MCB.01589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gong Y, Yue J, Qiang B, Yuan J, Peng X. Cooperation between EZH2, NSPc1-mediated histone H2A ubiquitination and Dnmt1 in HOX gene silencing. Nucleic Acids Res. 2008;36:3590–3599. doi: 10.1093/nar/gkn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- Hong S, Cho YW, Yu LR, Yu H, Veenstra TD, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci U S A. 2007;104:18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigut T, Wysocka J. H3K27 demethylases, at long last. Cell. 2007;131:29–32. doi: 10.1016/j.cell.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007;318:447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, Iwase S, Alpatov R, Issaeva I, Canaani E, Roberts TM, Chang HY, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–694. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Zhao XD, Han X, Chew JL, Liu J, Chiu KP, Choo A, Orlov YL, Sung WK, Shahab A, Kuznetsov VA, Bourque G, Oh S, et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Sui X, Price C, Li Z, Chen J. Crosstalk between DNA and histones: Tet's new role in embryonic stem cells. Curr Genomics. 2012;13:603–608. doi: 10.2174/138920212803759730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Hamm J, Whitworth K, Spate L, Park KW, Murphy CN, Prather RS. Dynamics of TET family expression in porcine preimplantation embryos is related to zygotic genome activation and required for the maintenance of NANOG. Dev Biol. 2014;386:86–95. doi: 10.1016/j.ydbio.2013.11.024. [DOI] [PubMed] [Google Scholar]