ABSTRACT
The chemotherapeutic drug cisplatin causes a number of dose-dependent side effects, including cachexia and testicular damage. Patients receiving a high cumulative dose of cisplatin may develop permanent azoospermia and subsequent infertility. Thus, the development of chemotherapeutic regimens with the optimal postsurvival quality of life (fertility) is of high importance. This study tested the hypothesis that ghrelin administration can prevent or minimize cisplatin-induced testicular damage and cachexia. Ghrelin and its receptor, the growth hormone secretagogue receptor (GHSR-1a), are expressed and function in the testis. Targeted deletion of ghrelin, or its receptor, significantly increases the rate of cell death in the testis, suggesting a protective role. Intraperitoneal administration of vehicle, ghrelin, or cisplatin alone or in combination with ghrelin, in cycles of 9 or 18 days, to adult male C57Bl/6 mice was performed. Body weight was measured daily and testicular and epididymal weight, sperm density and motility, testicular histology, and testicular cell death were analyzed at the time of euthanization. Ghrelin coadministration decreased the severity of cisplatin-induced cachexia and gonadal toxicity. Body, testicular, and epididymal weights significantly increased as testicular cell death decreased with ghrelin coadministration. The widespread damage to the seminiferous epithelium induced by cisplatin administration was less severe in mice simultaneously treated with ghrelin. Furthermore, ghrelin diminished the deleterious effects of cisplatin on testis and body weight homeostasis in wild-type but not Ghsr−/− mice, showing that ghrelin's actions are mediated via GHSR. Ghrelin or more stable GHSR agonists potentially offer a novel therapeutic approach to minimize the testicular damage that occurs after gonadotoxin exposure.
Keywords: cell death, cisplatin, fertility, ghrelin, spermatogenesis
INTRODUCTION
Improvements in the detection and treatment of cancer have increased the survival rate for patients. However, treatment, such as the administration of cytotoxic agents, including the platinum-based drug cisplatin, can cause adverse side effects in a dose-dependent manner [1, 2]. A potentially permanent side effect is damage to the seminiferous epithelium, resulting in impaired spermatogenesis [3–6]. Secondary infertility may result from cisplatin treatment [7]. Indeed, irreversible infertility occurs in men receiving a cumulative dose of more than 600 mg/m2 cisplatin [8]. The effects of chemotherapy on fertility and reproductive function are of particular importance to patients' quality of life after chemotherapy because many cancer survivors wish to become parents. Currently, sperm banking prior to chemotherapy provides one of the few options that allow patients to assure their posttreatment fertility. However, sperm banking is not a viable option for prepubescent cancer patients.
The antineoplastic effects of cisplatin are a product of DNA cross-linking, which interferes with mitotic cell division and ultimately triggers apoptosis [9]. Cisplatin suppresses neovascularization, required for tumor outgrowth, and disrupts mitochondrial enzymatic activity [10, 11]. Widespread damage to seminiferous tubules occurs after cisplatin administration [12]. This includes effects on Sertoli cell morphology and function within 24 h of exposure to cisplatin [13]. Subsequently, extensive apoptosis of the type A spermatogonia leads to permanent spermatogenic deficiency [12, 14]. Spermatogonial stem cell number is also decreased following exposure to cisplatin [15]. Cisplatin administration causes a decrease in LH receptor concentration and steroidogenic enzyme cytochrome P-450scc activity with a corresponding reduction in serum testosterone levels [14]. The fidelity of the tight junctions of the blood-testis barrier is compromised by cisplatin exposure, as indicated by changes in the electrolytes present in the fluid of the seminiferous tubules [13]. In humans as well as in animal models, administration of cisplatin at doses comparable to those used to treat human cancers results in reduced sperm count and motility as well as diminished sperm chromatin quality [16, 17]. Indeed, the scope and duration of these detrimental effects are difficult to predict. For these reasons, semen cryopreservation should always be recommended prior to chemotherapy.
Ghrelin, the endogenous ligand for the growth hormone secretagogue receptor (GHSR-1a), is primarily expressed in the stomach and hypothalamus, although expression also occurs in other cells, including in the Leydig cells of the rat testis and the Leydig and Sertoli cells of the human testis; GHSR-1a is expressed in the Leydig and Sertoli cells of the rat and human testis [18–24]. Acting centrally through the hypothalamus, ghrelin promotes food intake and a positive energy balance [25]. Ghrelin stimulates appetite and adiposity, counteracting cancer-induced cachexia in mouse models [26, 27]. Likewise, appetite and body weight improve in cancer patients with anorexia/cachexia syndrome after ghrelin administration [28]. In addition to its orexigenic properties, ghrelin prevents cell death in numerous tissues [29–33]. Ghrelin administration protects hypothalamic neuronal cells in an oxygen-glucose deprivation model by inhibiting the production of reactive oxygen species, in turn preventing cytochrome c release, and caspase-3 activation [30]. In the testis, ghrelin represses the expression of the proapoptotic factor Bax following hyperthermia [34]. Consequently, ghrelin may act as a powerful antioxidant agent in the testis, and ghrelin administration may prove useful in patients with cachexia [34, 35].
Preliminary studies by our group and work by others shows that targeted deletion of the ghrelin receptor in mouse models significantly increases the rate of testicular apoptosis, suggesting a role for this hormone in testicular function [36]. Because of its antiapoptotic actions in testis as well as in other tissues, we hypothesized that ghrelin could prevent or diminish cisplatin-induced gonadotoxicity in the male. Study of ghrelin- and GHSR-1a-deficient mice allowed us to test whether ghrelin coadministration with cisplatin could prevent or diminish cisplatin-induced spermatogenic damage, impaired sperm parameters, and testicular cell death [37, 38]. Because ghrelin's action to prevent cisplatin induced cachexia and weight-loss was known, this measure provided a positive control to indicate the central action of ghrelin administration. Because exogenous ghrelin administration prevented the testicular injury caused by cisplatin administration in a GHSR-dependent manner, the results demonstrate the importance of ghrelin in the prevention of azoospermia following chemotherapy.
MATERIALS AND METHODS
Animals
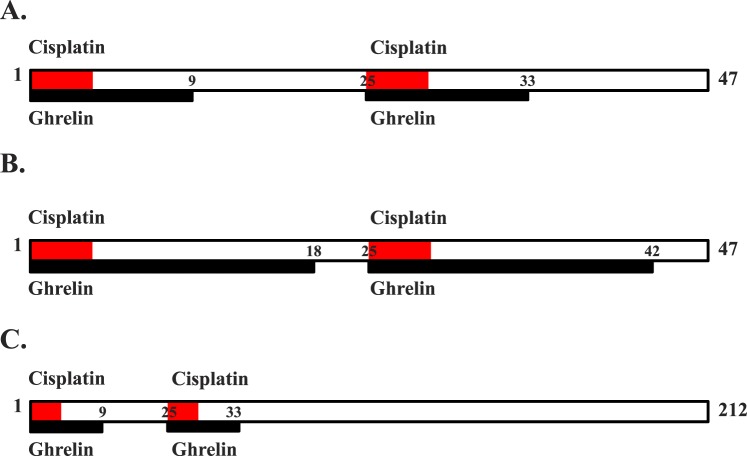
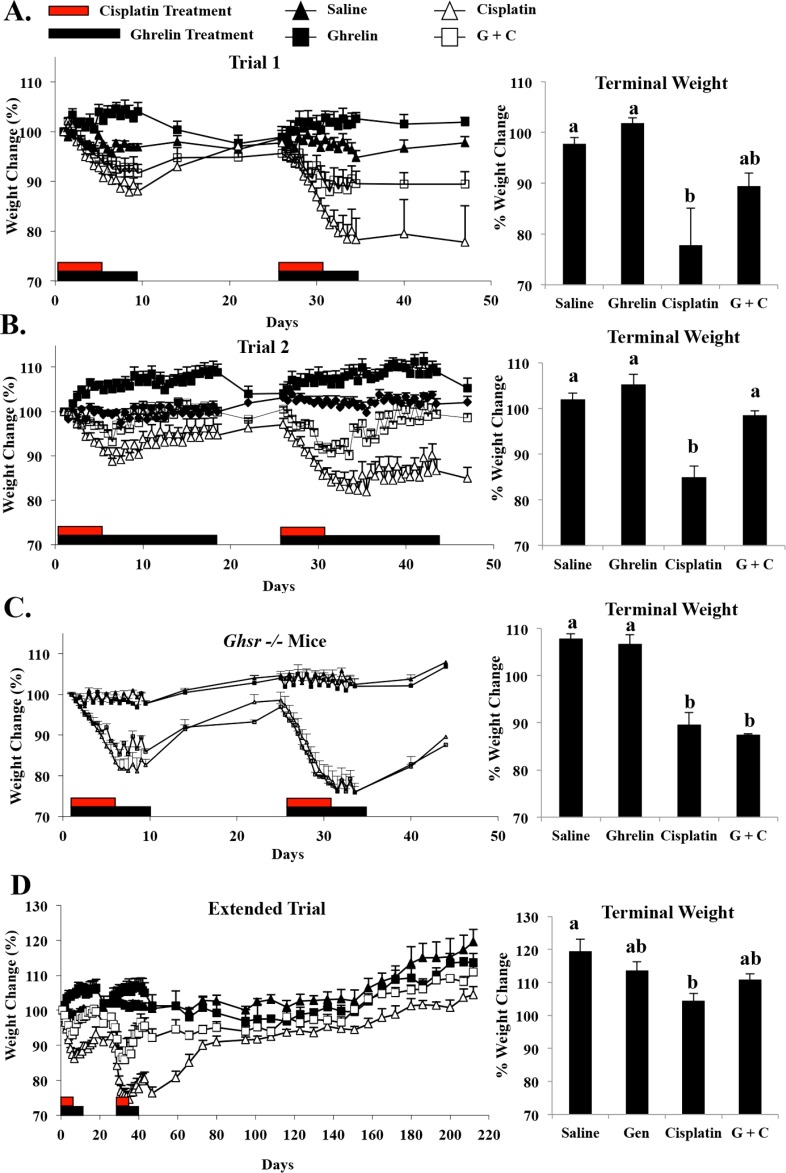
Twelve-week-old male C57/BL/6 mice with a deletion of the growth hormone secretagogue receptor (Ghsr−/−) or carrying wild-type copies of the receptor (Ghsr+/+) [38] were used for all the experiments. Ghsr−/− and Ghsr+/+ mice were backcrossed at least 10 generations to C57/BL/6 mice to create an isogenic line. Mice were maintained on a 14L:10D cycle in the vivarium at Baylor College of Medicine. Food and water were given ad libitum. All the experiments were conducted with the approval of the Institutional Animal Care and Use Committee at Baylor College of Medicine and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male mice (mean weight = 29.03 ± 2.42 g) (Supplemental Fig. S1A; all the Supplemental Data are available online at www.biolreprod.org) were randomized to receive vehicle (0.9% saline), ghrelin (at 16 mg/kg), cisplatin (at 2.5 mg/kg), or ghrelin + cisplatin by intraperitoneal injection (n = 4 mice/treatment). Body weight was measured daily before the morning and evening injections. During and after the treatment cycles of ghrelin and/or cisplatin administration, body weight was plotted as the percent change from baseline. Testis and epididymal weights were measured when the mice were killed. Cisplatin was purchased from (Mckesson, San Francisco, CA), and rodent ghrelin was synthesized at Baylor College of Medicine and checked for purity by high-performance liquid chromatography. For each experimental trial, mice were divided into four groups with four animals per group [39]. Three experimental trials were employed to determine the temporal and dose-dependent effects of ghrelin (Fig. 1). Because of its short half-life, Ghrelin (16 mg/kg) was administered twice daily at 0930 h and 1630 h and was administered over a course of 9 (trial 1) or 18 days (trial 2). For both trials, cisplatin (2.5 mg/kg) was given daily at 1630 h on Days 1–5 and 26–30. This regimen was optimal based on the degree of testicular toxicity [7], the induction of cachexia, and overall mouse survival. The ghrelin- and/or cisplatin-dosing paradigm was repeated in each trial, where animals received a total of 18 days of ghrelin and 10 days of cisplatin (trial 1) or 36 days of ghrelin and 10 days of cisplatin (trial 2). Saline was administered as the vehicle control using the same time course as cisplatin. Animals treated with ghrelin + cisplatin received cisplatin as stated and ghrelin as in trial 1 or 2 (Fig. 1). Trials 1 and 2 mice were killed on Day 47. In the extended trial, mice were killed on Day 212.
FIG. 1.
Schematic of cisplatin and ghrelin administration. A) In trial 1, cisplatin was administered on Days 1–5 and Days 25–33. Concurrent ghrelin injection occurred from Days 1–9 and 25–33 (n = 4 mice/treatment). B) In trial 2, the dosing regimen of ghrelin was extended to an 18-day cycle with ghrelin administered on Days 1–18 and 25–42 and cisplatin was administered on Days 1–5 and 25–29, as in trial 1. Saline for all the trials was given on Days 1–5 and 25–29, as in trial 1 (n = 4 mice/treatment). C) In the extended trial, cisplatin and ghrelin were administered as in trial 1 but the endpoint of this trial was Day 212 (n = 4 mice/treatment).
Sperm Assays
Epididymides were dissected, the caudal region minced in Modified BWW Medium (Irvine Scientific, Santa Ana, CA), and incubated for 30 min at 37°C. For total sperm counts, sperm were immobilized by dilution with water and counted in a hemocytometer. Live sperm were spread onto a slide and classified as motile or immotile. The results were expressed as percent motile sperm.
Histology and Immunohistochemistry
Testes were collected and fixed overnight in Bouin fixative, dehydrated in 70% ethanol, and embedded in paraffin. Tissue was sectioned at 7 μm thickness, mounted on charged slides, and stained by hematoxylin/eosin. The cellular localization of GATA-4, previously shown to be a Sertoli and Leydig cell nuclear antigen, was analyzed using a goat polyclonal primary antibody for GATA-4 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) [40, 41]. Tissue sections were deparaffinized, rehydrated, blocked with 10% normal rabbit serum, and incubated overnight with GATA-4 (1:200 of 200 μg/ml). After the slides were washed with 0.1% PBS-Tween, sections were incubated in biotinylated rabbit anti-goat antibody (1:200 dilution; Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Stain was visualized by incubating with Vectastain ABC for 45 min followed by 3,3′-diaminobenzidine (Vector Laboratories) for 5 min and counterstaining with hematoxylin. Negative controls were performed in the absence of GATA-4 primary antibody.
Cell Death and Terminal Transferase dUTP Nick End Labeling Assays
After decapsulating the testis, the seminiferous tubules were mechanically minced and cells isolated using a two-step enzymatic digestion as described elsewhere [42]. One-million cells were resuspended in 100 μl of incubation buffer and stained with propidium iodide (PI) and Annexin-V for 15 min at room temperature. Cells were analyzed using a Becton Dickinson FACScanto analytical flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Dead cells were quantified as all Annexin-V-positive cells, including Annexin-V + PI. Terminal transferase dUTP nick end labeling (TUNEL) assays were performed on Bouin-fixed paraffin embedded sections according to the manufacturer's instructions using the Apoptag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore, Billerica, MA). Negative control was performed in the absence of TdT enzyme. Each tissue section was examined for TUNEL-positive cells. The apoptotic index was determined by the ratio of the number of tubules with three or more TUNEL-positive cells relative to the total number of tubules. For each treatment group, at least 200 tubules were scored for TUNEL positivity from at least three experimental animals.
Statistical Analysis
All the data were analyzed using a one-way (treatment) or two-way (treatment × repeated measure, e.g., body weight) ANOVA with the SPSS v.12 statistical software package (SPSS, Chicago, IL).
RESULTS
Ghrelin Administration Diminishes Cisplatin-Induced Gonadotoxicity
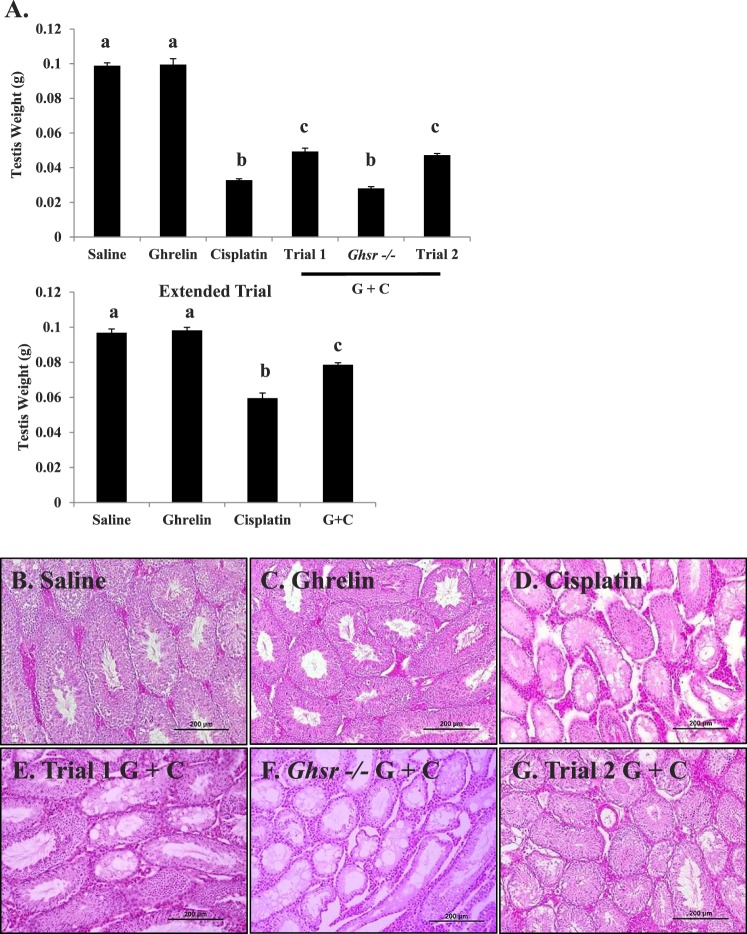
As expected, a decline in testis weight occurred in mice receiving cisplatin but not in untreated wild-type or ghrelin-treated mice (Fig. 2A) [7]. Ghrelin administered in combination with cisplatin partially prevented the testicular weight loss observed in the cisplatin-treated group in both study trials (Fig. 2A). There was no difference in the starting testis weight of Ghsr−/− and wild-type mice (Supplemental Fig. S1B). However, the testicular weights of the Ghsr−/− mice treated with cisplatin or ghrelin + cisplatin were not statistically different from cisplatin-treated C57/BL/6 mice (Supplemental Fig. S2A). To determine if extending the recovery time in cisplatin-treated mice would restore testicular weight, testis were harvested five spermatogenesis cycles (170 days) after the final cisplatin injection (extended trial). The average testicular weight of animals treated with cisplatin in the extended trial remained significantly less compared to saline, ghrelin, and ghrelin + cisplatin treated mice (P < 0.01) (Fig. 2A).
FIG. 2.
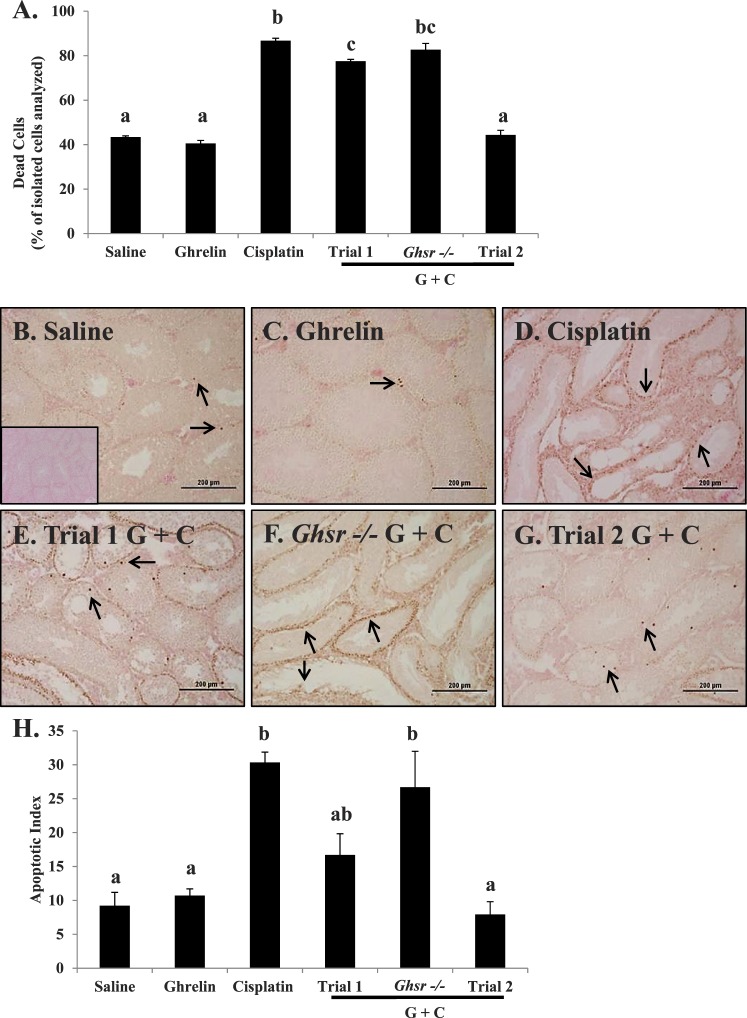
Testicular weight and histology of C57 mice exposed to saline, ghrelin, cisplatin, or ghrelin + cisplatin. A) Mean testicular weight for each treatment group measured on Day 47. Bar graphs show means ± SEM of at least four biological replicates. Letters represent means statistically significant by ANOVA (P < 0.01). Cisplatin-treated mice exhibit significantly lower testis weight (P < 0.001) than trials 1 and 2 ghrelin + cisplatin. Testicular weight of Ghsr−/− mice treated with cisplatin and ghrelin exhibit no statistical difference from mice treated with only cisplatin (treated as in trial 1). Testicular weight from mice treated with saline, ghrelin, cisplatin, and ghrelin + cisplatin and sacrificed 170 days following final cisplatin dose are labeled as extended trial. Testis weight of cisplatin treated mice remains significantly lower than saline, ghrelin, and ghrelin + cisplatin treated animals. Histopathology of C57 mice exposed to saline (B), ghrelin (C), or cisplatin (D): reduced cellularity, numbers of spermatocytes/spermatids, and extensive vacuolization; trial 1 ghrelin + cisplatin (E): reduced cisplatin damage and spermatogenesis present; Ghsr−/− ghrelin + cisplatin (F): damage similar to cisplatin alone; and trial 2 ghrelin + cisplatin (G): minimized cisplatin damage. Sections are stained with hematoxylin and eosin. Histology represents terminal Day 47. Bars = 200 μm.
Spermatogenesis appeared normal in the wild-type vehicle, Ghsr−/−, and ghrelin-treated wild-type mice (Figs. 2B and Supplemental Fig. S1B). In contrast, cisplatin caused extensive germ cell loss and severe damage to the seminiferous epithelium (Fig. 2D) with tubules showing decreased diameter size and vacuolization of the Sertoli cells. Animals receiving ghrelin and cisplatin in combination displayed less seminiferous tubule damage with active spermatogenesis present in many of the tubules (Fig. 2E). When ghrelin was administered over a longer period of time, as in trial 2, spermatogenesis was nearly normal in the testes from the ghrelin + cisplatin treated mice (Fig. 2G). The testicular histology of Ghsr−/− mice treated with ghrelin + cisplatin resembled that in mice treated only with cisplatin (Fig. 2F).
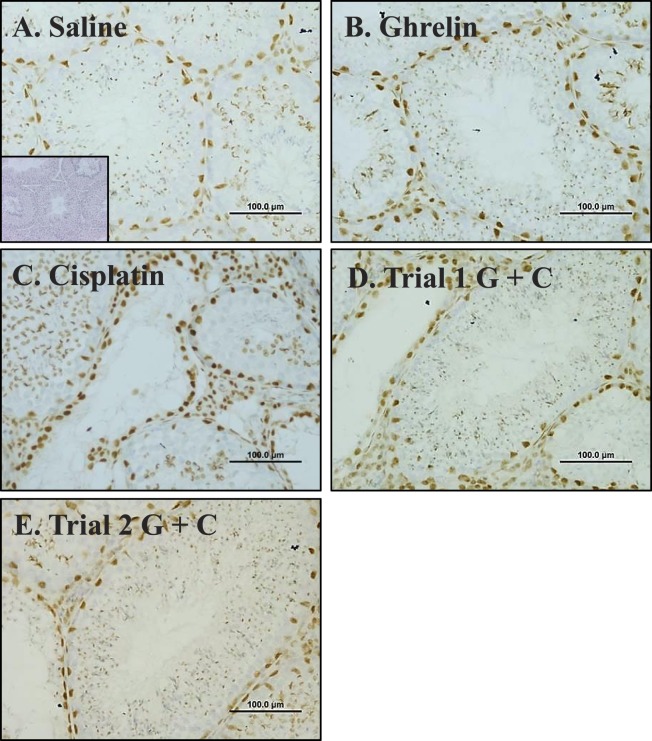
Immunohistochemical localization of GATA-4, a marker protein found in Sertoli and Leydig cell nuclei, was performed on cross-sections of testiscular tissue from the vehicle, ghrelin, cisplatin, and ghrelin + cisplatin (trials 1 and 2) treatment groups (Fig. 3). Sertoli and Leydig cells persisted following cisplatin administration, whereas germ cells (unstained) loss was markedly apparent compared to controls (Fig. 3C).
FIG. 3.
Immunohistochemical localization of GATA4 in seminiferous tubules of mice exposed to saline (A; inset: no primary control), ghrelin (B), cisplatin (C), trial 1 ghrelin + cisplatin (D), or trial 2 ghrelin + cisplatin (E). Incubation with primary antibody to GATA4 and counterstained with hematoxylin revealed similar number of Sertoli and Leydig cells in vehicle (A), ghrelin (B), trial 1 (D), and trial 2 (E). Due to reduction in other cell types present, cisplatin-treated animals (C) appear to have increased relative numbers of Sertoli cells. Bars = 100 μm.
Ghrelin Administration Partially Prevented Cisplatin-Induced Damage to Epididymis and Sperm Parameters
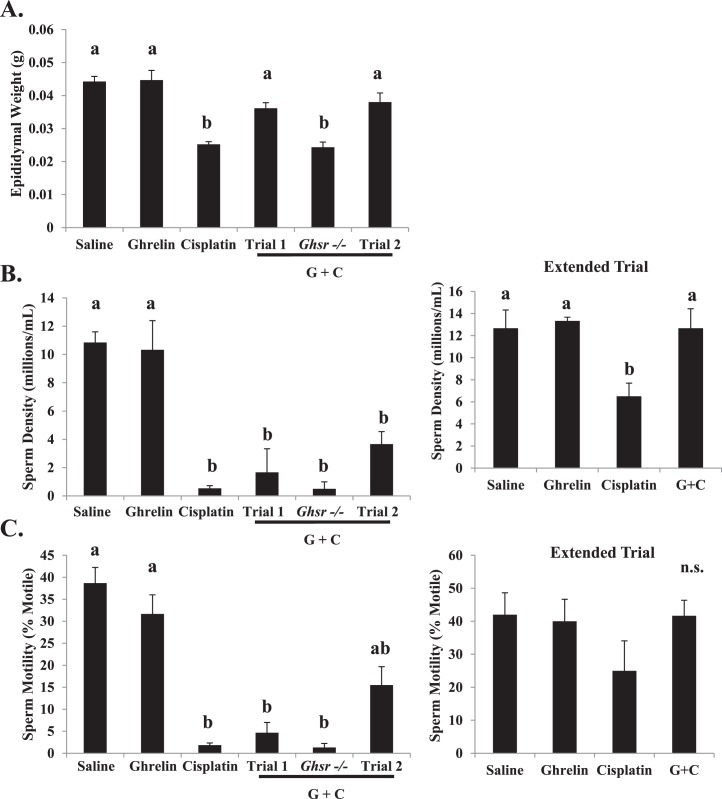
Cisplatin treatment induced significant epididymal weight loss when compared to vehicle and ghrelin-treated control mice (Fig. 4A). Epididymal weights increased to levels comparable to vehicle in animals treated with cisplatin together with ghrelin (trials 1 and 2) compared to mice treated with cisplatin alone. Again, ghrelin administration did not prevent the detrimental effect caused by cisplatin in the Ghsr−/− mice. The significant reduction in epididymal weight observed in these mice indicates that GHS-R1a is required for ghrelin's protective actions on spermatogenesis.
FIG. 4.
Comparison of epididymal weights and sperm parameters in all the treatment groups at terminal Day 47. Bar graphs show means ± SEM of at least four biological replicates. Letters represent means statistically significant by ANOVA. A) Epididymal weight is significantly lower (P < 0.01) in mice treated with cisplatin and Ghsr−/− mice treated with ghrelin + cisplatin. Ghrelin + cisplatin coadministration significantly increases (P < 0.01) epididymal weight. B) Sperm density significantly decreases (P < 0.001) with cisplatin exposure. In the extended trial, sperm density recovers only in the ghrelin + cisplatin treated group. Sperm density remains significantly lower in the cisplatin treated group of the extended trial. C) Sperm motility significantly decreases (P < 0.001) with cisplatin exposure. Motility values for mice treated with ghrelin + cisplatin in trial 2 are higher than cisplatin, trial 1 ghrelin + cisplatin, and Ghsr−/− ghrelin + cisplatin treated mice. Sperm motility is not significantly different between groups in the extended trial. Values represent means ± SEM; n.s. = groups not significantly different.
Epididymal sperm count and motility were compared between treatment groups and genotypes (Figs. 4B, Supplemental Figs. S1C, and S2B). Cisplatin administration significantly decreased sperm concentration and motility when compared with the concentration in vehicle- and ghrelin-treated groups. In trial 1 (with a shorter ghrelin treatment), epididymal sperm counts were not statistically different from those in mice treated with cisplatin alone. In contrast, longer treatment with ghrelin (trial 2) reduced the cisplatin-induced decline in sperm motility. Coadministration of ghrelin with cisplatin to Ghsr−/− mice did not prevent the damage induced by cisplatin to sperm count or motility (trial 1 or 2). In the extended trial, sperm density remained significantly lower and motility remained numerically lower 170 days following the final cisplatin dose. Epididymal sperm counts in animals receiving ghrelin + cisplatin returned to near normal levels during the extended recovery period.
Cisplatin-Induced Testicular Cell Death Is Diminished by Coadministration of Ghrelin
Considering the substantial evidence that ghrelin is able to prevent damage-induced cell death in several tissue-types (Supplemental Fig. S1D), cell death was measured by flow cytometry and TUNEL staining in testis of mice treated with vehicle, ghrelin, cisplatin, and ghrelin + cisplatin (trials 1 and 2) [29–33]. Expression of Annexin-V was markedly increased in testicular cells from cisplatin-treated mice as evident by flow cytometry (Figs. 5A and Supplemental Fig. S2C). Coadministration of ghrelin significantly prevented increased Annexin-V levels, concomitant with the decrease in testicular apoptosis. In trial 2, ghrelin administration effectively prevented cisplatin-increased Annexin-V expression and cell death in the testis. In the absence of receptor, ghrelin was ineffective as a protective agent in Ghsr−/− mice treated with cisplatin. DNA damage, measured using a TUNEL assay (Fig. 5B), showed evidence of apoptotic nuclei in the seminiferous tubules of all the treatment groups. Coadministration of ghrelin with cisplatin significantly attenuated the increase in TUNEL-positive cells in trial 1 and to a greater extent in trial 2 (Fig. 5, E and G), whereas the testes of Ghsr−/− mice treated with ghrelin + cisplatin displayed extensive TUNEL-positive cells similar to those observed in mice treated with cisplatin alone (Fig. 5F). Quantification of the apoptotic index agreed with cell death analysis by flow cytometry (Fig. 5H).
FIG. 5.
Cisplatin-induced testicular cell death diminished by ghrelin coadministration. A) Samples were analyzed by flow cytometry from testicular single-cell suspension on Day 47. All the isolated cells were stained with PI and Annexin-V. Cells positive for Annexin V were classified as dead, and cells negative for PI and Annexin-V were classified as live. Cells are graphed as percent dead cells of those isolated cells analyzed. Cisplatin induced significant testicular cell death (P < 0.01). Mice treated with ghrelin + cisplatin (trials 1 and 2) display significantly less (P < 0.01) dead cells. Values represent means ± SEM. DNA strand breaks, determined by TUNEL assay and counterstained with eosin, in mice exposed to saline (B; inset: no TdT enzyme control), ghrelin (C), cisplatin (D), trial 1 ghrelin + cisplatin (E): reduced cisplatin damage and spermatogenesis present; Ghsr−/− (F): damage similar to cisplatin alone; and trial 2 ghrelin + cisplatin (G): minimized cisplatin damage. Arrows highlight TUNEL-positive cells in each treatment group. Bars = 200 μm. H) Quantification of the apoptotic index was determined for all treatment groups. Cisplatin induced significant increases to the apoptotic index (P < 0.01). Ghsr−/− mice also exhibited a significantly higher apoptotic index (P < 0.01). Values represent mean ± SEM representing at least three animals per treatment group. Letters represent means statistically significant by ANOVA (P < 0.01).
Ghrelin Administration Ameliorates the Weight Loss Caused by Cisplatin Exposure
Coadministration of ghrelin can prevent cisplatin-induced cachexia, so the percentage of body weight change in animals tested in trials 1 and 2 and in the extended recovery trial was evaluated [35]. Vehicle-treated control animals exhibited relatively stable body weight (defined as the percentage of the starting weight) in across both trials. Wild-type ghrelin-treated mice gained a relatively small amount of weight, which typically normalized following ghrelin withdrawal (Fig. 6, A and B). The mice receiving the 25 mg/kg cumulative dose of cisplatin lost a significant amount of weight. One cycle of cisplatin treatment resulted in a loss of 12% ± 1% body weight during the first 5 days. After the second treatment cycle, mice receiving cisplatin alone lost an average 20% ± 4.3% of their starting weight. Subsequent to the final dose of cisplatin, treated mice regained lost body weight, although their weights remained significantly lower than the vehicle-treated wild-type control mice (Fig. 6A–D). Animals treated with ghrelin + cisplatin demonstrated better recovery of their body weight than cisplatin-treated mice. The longer period of ghrelin coadministration (trial 2) resulted in less total body weight loss (Fig. 6B). The final weight of the ghrelin + cisplatin treated mice in this group was not statistically different from saline controls. This indicates that, as previously reported, ghrelin markedly attenuated cisplatin-induced cachexia in a time- and dose-dependent manner [35]. The body weights of the Ghsr−/− mice treated with both ghrelin and cisplatin were similar to those treated with cisplatin alone, suggesting that the receptor is necessary for the anticachectic effects of ghrelin (Fig. 6C). The cisplatin-treated mice allowed to recover for an extended period remained statistically smaller than saline-treated mice (Fig. 6D). While animals in the ghrelin + cisplatin treatment group lost weight initially, ghrelin coadministration facilitated body weight recovery to values similar to ghrelin-only treated mice.
FIG. 6.
Body weight change per group. A) Body weights of ghrelin-treated mice increase concurrent with dosing and return to predose level following discontinuation of ghrelin treatment. Body weights of cisplatin-treated mice are significantly reduced with exposure, and as cumulative dose increases, body weight decreases. Trial 1 ghrelin + cisplatin is able to protect against cisplatin-induced body weight loss, shown clearly during the second period of injections. B) Increasing the time regimen of ghrelin cotreatment in trial 2 ghrelin + cisplatin further protected against cisplatin-induced weight loss, where mice recovered to levels similar to saline treatment after 47 days. C) Body weights of Ghsr−/− mice treated with cisplatin and ghrelin exhibit no statistical difference from cisplatin-alone-treated mice. D) Body weights of mice allowed to recover for 170 following final cisplatin dose demonstrate improved recovery. However, cisplatin-treated mice remain statistically smaller than saline-treated mice. Values represent mean ± SEM. Letters represent means statistically significant by ANOVA (P < 0.01). Black bars on graphs represent ghrelin dosing. Red bars represent cisplatin dosing.
DISCUSSION
Utilization of the chemotherapeutic cisplatin improves the likelihood of long-term survival for cancer patients, but its use is associated with significant side effects. While several agents expected to diminish cisplatin side effects show promise [35, 43–46], there are no approved treatments that prevent or diminish these serious complications. Two of the most serious side effects induced by cisplatin administration are evident in the mouse models used in this study: cachexia and male infertility. Cotreatment of cisplatin with ghrelin diminishes cisplatin-induced weight loss and testicular damage in a dose-dependent manner. Ghrelin and cisplatin cotreatment in Ghsr−/− mice demonstrated that the GHS-R1a signal transduction pathway is required for ghrelin-mediated protection of weight loss and testicular damage. Moreover, cisplatin exposure results in persistent testicular damage and reduced body weight. When ghrelin and cisplatin are employed together, the long-term damage is diminished. Thus, the protective properties of ghrelin may adequately reduce the detrimental effects of cisplatin utilizing an optimized dosing regimen.
After cisplatin exposure, both humans and experimental animals display delayed gastric emptying, early satiety, anorexia, nausea, and vomiting. Collectively, these side effects are described as cancer-associated dyspepsia syndrome (CADS) [7, 47]. Administration of exogenous ghrelin reduces the symptoms of cisplatin-induced CADS in a murine model [35]. The difference in the cisplatin and ghrelin + cisplatin treated groups for the maintenance of body weight and overall recovery is more pronounced when the time period of ghrelin administration is doubled. These results in the mouse are similar to those previously reported in rats [35]. Extending the recovery period demonstrates the enduring cachexic effects of cisplatin. Moreover, Ghsr−/− mice did not respond to ghrelin treatment, confirming that the action of ghrelin is mediated through the GHSR receptor.
Long-term damage to spermatogenesis occurs in mice following cisplatin administration of doses analogous to those given to cancer patients. Low doses of cisplatin damage the highly proliferative spermatogonia [48], while higher doses can lead to irreversible loss [49]. Extending the dose of cisplatin induces spermatogenic cell death [48], perhaps reflecting damage to both the germ and somatic cells of the testis. Cultured Sertoli cells treated with cisplatin exhibit a dose-dependent decrease in androgen-binding protein, lactate, and estradiol secretion [50, 51]. Disruption of the tight junctions and abnormal Sertoli cell secretory function are observed in vivo as well [13]. Cisplatin exposure can alter sperm DNA integrity, a measure that seems to correlate with fertility and may negatively affect progeny outcome [17]. Indeed, paternal treatment with cisplatin in rats results in impaired reproductive function in male offspring [6]. Our observations in mice suggest that a patient's chemotherapeutic regimen may negatively affect testicular function and the potential to father offspring in the future. Our studies suggest ghrelin may be protective.
Ghrelin protects against apoptosis in several cell types, including: hypothalamic neuronal cells [30], lung alveolar cells [33, 52], cardiomyocytes [31, 53], pancreatic beta-cells [54], endothelial cells [55], and spermatocytes and spermatogonia in the rat [34]. Studies in these cell types indicate that ghrelin inhibits apoptosis through multiple mechanisms. Inhibition of the mitochondrial pathway of apoptosis was found in hypothalamic neuronal cells, cardiomyocytes, pancreatic beta-cells, and spermatocytes and spermatogonia. Activation of extracellular signal-regulated kinase-1/2 (ERK1/2) plays a role in the antiapoptotic actions of ghrelin in hypothalamic neuronal cells and endothelial cells, while inhibition of c-June N-terminal kinase (JNK) mediates ghrelin's actions in lung alveolar cells and pancreatic beta-cells. In response to DNA damage from ionizing radiation, ghrelin produces a protective effect in the mouse testis that involved modulation of the p53 signaling pathway [36]. Furthermore, ghrelin possesses anti-inflammatory properties, which may counteract the proinflammatory response induced by cisplatin [56]. Ghrelin inhibits proinflammatory cytokine production [57, 58] as well as NF-κB activation, a critical inflammatory signaling molecule [59]. Although it is not yet clear how ghrelin and its receptor are mediating the apoptotic response to cisplatin, ghrelin may be acting as a survival factor through the inhibition of these apoptotic pathways and the preservation of mitochondrial integrity. Because of the ability of ghrelin to directly protect cells against apoptosis, it is likely that the prevention of cisplatin-induced toxicity in the testis is a testis-specific effect rather than a benefit of reduced cachexia. This is further supported by the incidence of permanent cisplatin-induced infertility in patients receiving high cumulative doses, even when these patients regain their appetite and weight [5].
Ghrelin administration does not appear to produce long-term adverse effects or interfere with the efficacy of cisplatin in cancer-causing cells, although additional studies are required. There is no agreement concerning the direct actions of ghrelin on tumor growth. Some in vitro studies suggest a decrease [60, 61] while others suggest an increase in cell growth [62, 63]. However, there are no published in vivo studies that examine tumor growth following ghrelin treatment. There are several studies in both animal models and human patients that have determined both the safety and efficacy of ghrelin in cancer-induced cachexia (reviewed in [64]). In healthy, human volunteers, intravenous ghrelin administration was safe, tolerable, and tended to increase hunger sensation in a dose-dependent manner [65]. In a cancer-induced cachexia animal model, ghrelin treatment increased food intake, body weight, and whole body fat, with no effect on tumor growth [27]. A phase I trial of ghrelin administration in patients with advanced cancer and anorexia/cachexia syndrome revealed no dose-limiting toxicity or increase in tumor-growth [66]. In fact, administration of anamorelin, a novel ghrelin mimetic, in patients with cancer-related cachexia results in significant improvement in metabolic effects and patient-reported symptoms [67]. Phase I trials have also verified the safety and tolerability of RM-131, a potent ghrelin receptor agonist [68]. These studies suggest ghrelin and ghrelin receptor agonists are well-tolerated and relieve CADS-associated symptoms in both animal models and patients, although further research is advised [65, 67–69].
In conclusion, cotreatment of adult mice with cisplatin and ghrelin prevented the severity of cachexia and testicular damage commonly induced by cisplatin. The Ghsr−/− mice provides direct evidence that the hormone-receptor signaling pathway is essential for ghrelin's protective role. Therefore, ghrelin agonists may show promise for the treatment of cachexia and gonadal toxicity in cancer patients.
ACKNOWLEDGMENT
We thank Charlotte Tiner, Emily Rutledge, and Aysegul Sahin for their technical assistance and Yuxiang Sun for providing the Ghsr−/− mice.
Footnotes
Supported in part by a grant from the National Institute of Kidney and Digestive Disease, National Institutes of Health (NIH), T32 DK007763-06 to D.J.L. (trainee: S.D.W.), the Department of Veterans Affairs (MREP, SCNCDA, and MERIT BX000507 to J.M.G.), NIH AG019230 (R.G.S.), and NIH HD060870 and AG040583 (J.M.G.). Presented in part at the Endocrine Society's 90th Annual Meeting, June 15–18, 2008, San Francisco, California.
These authors contributed equally to this work.
REFERENCES
- Porter GA, Bennett WM. Nephrotoxic acute renal failure due to common drugs. Am J Physiol. 1981;241:F1–F8. doi: 10.1152/ajprenal.1981.241.1.F1. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia FM. Toxic effects of cis-dichlorodiammineplatinum(II) in man. Cancer Treat Rep. 1979;63:1527–1531. [PubMed] [Google Scholar]
- Petersen PM, Hansen SW. The course of long-term toxicity in patients treated with cisplatin-based chemotherapy for non-seminomatous germ-cell cancer. Ann Oncol. 1999;10:1475–1483. doi: 10.1023/a:1008322909836. [DOI] [PubMed] [Google Scholar]
- Strumberg D, Brugge S, Korn MW, Koeppen S, Ranft J, Scheiber G, Reiners C, Mockel C, Seeber S, Scheulen ME. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13:229–236. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kamidono S, Fujisawa M. Fertility after high-dose chemotherapy for testicular cancer. Urology. 2004;63:137–140. doi: 10.1016/j.urology.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Favareto AP, de Toledo FC. Kempinas W de G. Paternal treatment with cisplatin impairs reproduction of adult male offspring in rats. Reprod Toxicol. 2011;32:425–433. doi: 10.1016/j.reprotox.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Sawhney P, Giammona CJ, Meistrich ML, Richburg JH. Cisplatin-induced long-term failure of spermatogenesis in adult C57/Bl/6J mice. J Androl. 2005;26:136–145. [PubMed] [Google Scholar]
- Pont J, Albrecht W. Fertility after chemotherapy for testicular germ cell cancer. Fertil Steril. 1997;68:1–5. doi: 10.1016/s0015-0282(97)81465-3. [DOI] [PubMed] [Google Scholar]
- Poklar N, Pilch DS, Lippard SJ, Redding EA, Dunham SU, Breslauer KJ. Influence of cisplatin intrastrand crosslinking on the conformation, thermal stability, and energetics of a 20-mer DNA duplex. Proc Natl Acad Sci U S A. 1996;93:7606–7611. doi: 10.1073/pnas.93.15.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa A, Saura R, Matsubara T, Mizuno K. A mechanism of cisplatin action: antineoplastic effect through inhibition of neovascularization. Kobe J Med Sci. 1997;43:109–120. [PubMed] [Google Scholar]
- Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–1127. doi: 10.1016/0006-291x(89)92225-0. [DOI] [PubMed] [Google Scholar]
- Seaman F, Sawhney P, Giammona CJ, Richburg JH. Cisplatin-induced pulse of germ cell apoptosis precedes long-term elevated apoptotic rates in C57/BL/6 mouse testis. Apoptosis. 2003;8:101–108. doi: 10.1023/a:1021734604913. [DOI] [PubMed] [Google Scholar]
- Pogach LM, Lee Y, Gould S, Giglio W, Meyenhofer M, Huang HF. Characterization of cis-platinum-induced Sertoli cell dysfunction in rodents. Toxicol Appl Pharmacol. 1989;98:350–361. doi: 10.1016/0041-008x(89)90239-1. [DOI] [PubMed] [Google Scholar]
- Maines MD, Sluss PM. Iscan M. cis-platinum-mediated decrease in serum testosterone is associated with depression of luteinizing hormone receptors and cytochrome P-450scc in rat testis. Endocrinology. 1990;126:2398–2406. doi: 10.1210/endo-126-5-2398. [DOI] [PubMed] [Google Scholar]
- Marcon L, Zhang X, Hales BF, Robaire B, Nagano MC. Effects of chemotherapeutic agents for testicular cancer on rat spermatogonial stem/progenitor cells. J Androl. 2011;32:432–443. doi: 10.2164/jandrol.110.011601. [DOI] [PubMed] [Google Scholar]
- Stephenson WT, Poirier SM, Rubin L, Einhorn LH. Evaluation of reproductive capacity in germ cell tumor patients following treatment with cisplatin, etoposide, and bleomycin. J Clin Oncol. 1995;13:2278–2280. doi: 10.1200/JCO.1995.13.9.2278. [DOI] [PubMed] [Google Scholar]
- Delbes G, Hales BF, Robaire B. Effects of the chemotherapy cocktail used to treat testicular cancer on sperm chromatin integrity. J Androl. 2007;28:241–249. doi: 10.2164/jandrol.106.001487. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–122. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, Pinilla L, Casanueva FF, Dieguez C, Aguilar E. Novel expression and functional role of ghrelin in rat testis. Endocrinology. 2002;143:717–725. doi: 10.1210/endo.143.2.8646. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C, Pinilla L, Paniagua R, Nistal M, Casanueva FF, Aguilar E, Dieguez C, et al. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab. 2004;89:400–409. doi: 10.1210/jc.2003-031375. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt MJ, Jr, Fisher MH, Nargund RP, Patchett AA. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- Barreiro ML, Suominen JS, Gaytan F, Pinilla L, Chopin LK, Casanueva FF, Dieguez C, Aguilar E, Toppari J, Tena-Sempere M. Developmental, stage-specific, and hormonally regulated expression of growth hormone secretagogue receptor messenger RNA in rat testis. Biol Reprod. 2003;68:1631–1640. doi: 10.1095/biolreprod.102.008862. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, Osuye K, Kangawa K, Matsukura S, Nakazato M. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;301:275–279. doi: 10.1016/s0006-291x(02)03028-0. [DOI] [PubMed] [Google Scholar]
- Wang W, Andersson M, Iresjo BM, Lonnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006;28:1393–1400. [PubMed] [Google Scholar]
- Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- Zhao H, Liu G, Wang Q, Ding L, Cai H, Jiang H, Xin Z. Effect of ghrelin on human endothelial cells apoptosis induced by high glucose. Biochem Biophys Res Commun. 2007;362:677–681. doi: 10.1016/j.bbrc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Chung H, Kim E, Lee DH, Seo S, Ju S, Lee D, Kim H, Park S. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148:148–159. doi: 10.1210/en.2006-0991. [DOI] [PubMed] [Google Scholar]
- Xu XB, Pang JJ, Cao JM, Ni C, Xu RK, Peng XZ, Yu XX, Guo S, Chen MC, Chen C. GH-releasing peptides improve cardiac dysfunction and cachexia and suppress stress-related hormones and cardiomyocyte apoptosis in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H1643–H1651. doi: 10.1152/ajpheart.01042.2004. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Huang C, Meng B, Tang T, Shi Q, Yang H. Acute effect of ghrelin on ischemia/reperfusion injury in the rat spinal cord. Int J Mol Sci. 2012;13:9864–9876. doi: 10.3390/ijms13089864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazu Y, Yanagi S, Miyoshi K, Tsubouchi H, Yamashita S, Matsumoto N, Ashitani J, Kangawa K, Nakazato M. Ghrelin ameliorates bleomycin-induced acute lung injury by protecting alveolar epithelial cells and suppressing lung inflammation. Eur J Pharmacol. 2011;672:153–158. doi: 10.1016/j.ejphar.2011.09.183. [DOI] [PubMed] [Google Scholar]
- Kheradmand A, Dezfoulian O, Alirezaei M. Ghrelin regulates Bax and PCNA but not Bcl-2 expressions following scrotal hyperthermia in the rat. Tissue Cell. 2012;44:308–315. doi: 10.1016/j.tice.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–460. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zeng Y, Zhao J, Zhu CJ, Hou WG, Upregulation Zhang S. and nuclear translocation of testicular ghrelin protects differentiating spermatogonia from ionizing radiation injury. Cell Death Dis. 2014;5:e1248. doi: 10.1038/cddis.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThidarMyint H, Yoshida H, Ito T, Kuwayama H. Dose-dependent response of plasma ghrelin and growth hormone concentrations to bovine ghrelin in Holstein heifers. J Endocrinol. 2006;189:655–664. doi: 10.1677/joe.1.06746. [DOI] [PubMed] [Google Scholar]
- Bagheri-Fam S, Argentaro A, Svingen T, Combes AN, Sinclair AH, Koopman P, Harley VR. Defective survival of proliferating Sertoli cells and androgen receptor function in a mouse model of the ATR-X syndrome. Hum Mol Genet. 2011;20:2213–2224. doi: 10.1093/hmg/ddr109. [DOI] [PubMed] [Google Scholar]
- McClusky LM, Patrick S, Barnhoorn IE, van Dyk JC, de Jager C, Bornman MS. Immunohistochemical study of nuclear changes associated with male germ cell death and spermiogenesis. J Mol Histol. 2009;40:287–299. doi: 10.1007/s10735-009-9240-3. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- Amin A, Hamza AA, Kambal A, Daoud S. Herbal extracts counteract cisplatin-mediated cell death in rat testis. Asian J Androl. 2008;10:291–297. doi: 10.1111/j.1745-7262.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- Vawda AI. Effect of testosterone on cisplatin-induced testicular damage. Arch Androl. 1994;32:53–57. doi: 10.3109/01485019408987767. [DOI] [PubMed] [Google Scholar]
- Cakil B, Basar FS, Atmaca S, Cengel SK, Tekat A, Tanyeri Y. The protective effect of Ginkgo biloba extract against experimental cisplatin ototoxicity: animal research using distortion product otoacoustic emissions. J Laryngol Otol. 2012;126:1097–1101. doi: 10.1017/S0022215112002046. [DOI] [PubMed] [Google Scholar]
- Longo F, Mansueto G, Lapadula V, Stumbo L, Del Bene G, Adua D, De Filippis L, Bonizzoni E, Quadrini S. Combination of aprepitant, palonosetron and dexamethasone as antiemetic prophylaxis in lung cancer patients receiving multiple cycles of cisplatin-based chemotherapy. Int J Clin Pract. 2012;66:753–757. doi: 10.1111/j.1742-1241.2012.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K, Walsh D, Sheehan F. Cancer and chemotherapy-related upper gastrointestinal symptoms: the role of abnormal gastric motor function and its evaluation in cancer patients. Support Care Cancer. 2002;10:455–461. doi: 10.1007/s00520-002-0340-9. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Finch M, da Cunha MF, Hacker U, Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42:122–131. [PubMed] [Google Scholar]
- Schrader M, Muller M, Straub B, Miller K. Testicular sperm extraction in azoospermic patients with gonadal germ cell tumors prior to chemotherapy—a new therapy option. Asian J Androl. 2002;4:9–15. [PubMed] [Google Scholar]
- Huang HF, Pogach LM, Nathan E, Giglio W. Acute and chronic effects of cisplatinum upon testicular function in the rat. J Androl. 1990;11:436–445. [PubMed] [Google Scholar]
- Favareto AP, Fernandez CD, da Silva DA, Anselmo-Franci JA. Kempinas W de G. Persistent impairment of testicular histology and sperm motility in adult rats treated with cisplatin at peri-puberty. Basic Clin Pharmacol Toxicol. 2011;109:85–96. doi: 10.1111/j.1742-7843.2011.00688.x. [DOI] [PubMed] [Google Scholar]
- Li B, Zeng M, He W, Huang X, Luo L, Zhang H, Deng DY. Ghrelin protects alveolar macrophages against lipopolysaccharide-induced apoptosis through growth hormone secretagogue receptor 1a-dependent c-Jun N-terminal kinase and Wnt/beta-catenin signaling and suppresses lung inflammation. Endocrinology. 2015;1:203–217. doi: 10.1210/en.2014-1539. [DOI] [PubMed] [Google Scholar]
- Yang C, Wang Y, Liu H, Li N, Sun Y, Liu Z, Yang P. Ghrelin protects H9c2 cardiomyocytes from angiotensin II-induced apoptosis through the endoplasmic reticulum stress pathway. J Cardiovasc Pharmacol. 2012;59:465–471. doi: 10.1097/FJC.0b013e31824a7b60. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang D, Zhao H, Chen Y, Liu Y, Cao C, Han L, Liu G. Ghrelin inhibits cell apoptosis induced by lipotoxicity in pancreatic beta-cell line. Regul Pept. 2010;161:43–50. doi: 10.1016/j.regpep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Sodhi A. Increased release of interleukin-1 and tumour necrosis factor by interleukin-2-induced lymphokine-activated killer cells in the presence of cisplatin and FK-565 Immunol Cell Biol 1992. 70 (Pt 1): 15 24 [DOI] [PubMed] [Google Scholar]
- Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:E486–E492. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- Mungan NA, Eminferzane S, Mungan AG, Yesilli C, Seckiner I, Can M, Ayoglu F, Akduman B. Diagnostic value of serum ghrelin levels in prostate cancer. Urol Int. 2008;80:245–248. doi: 10.1159/000127334. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Allia E, Marrocco T, Ghe C, Ghigo E, Muccioli G, Papotti M. Ghrelin and cortistatin in lung cancer: expression of peptides and related receptors in human primary tumors and in vitro effect on the H345 small cell carcinoma cell line. J Endocrinol Invest. 2006;29:781–790. doi: 10.1007/BF03347371. [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Waseem T, Ito H, Robinson MK, Zinner MJ, Ashley SW, Whang EE. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem Biophys Res Commun. 2003;309:464–468. doi: 10.1016/j.bbrc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Yeh AH, Jeffery PL, Duncan RP, Herington AC, Chopin LK. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogen-activated protein kinase in prostate cancer. Clin Cancer Res. 2005;11:8295–8303. doi: 10.1158/1078-0432.CCR-05-0443. [DOI] [PubMed] [Google Scholar]
- Akamizu T, Kangawa K. The physiological significance and potential clinical applications of ghrelin. Eur J Intern Med. 2012;23:197–202. doi: 10.1016/j.ejim.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, Tada H, Miura K, Shimizu A, Fukushima M, Yokode M, Tanaka K, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol. 2004;150:447–455. doi: 10.1530/eje.0.1500447. [DOI] [PubMed] [Google Scholar]
- Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, Kaufmann K, Holst B, Brandle M, von Moos R, Demmer R, Cerny T. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21:129–137. doi: 10.1007/s00520-012-1500-1. [DOI] [PubMed] [Google Scholar]
- Shin A, Camilleri M, Busciglio I, Burton D, Stoner E, Noonan P, Gottesdiener K, Smith SA, Vella A, Zinsmeister AR. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palus S, Schur R, Akashi YJ, Bockmeyer B, Datta R, Halem H, Dong J, Culler MD, Adams V, Anker SD, Springer J. Ghrelin and its analogues, BIM-28131 and BIM-28125, improve body weight and regulate the expression of MuRF-1 and MAFbx in a rat heart failure model. PLoS One. 2011;6:e26865. doi: 10.1371/journal.pone.0026865. [DOI] [PMC free article] [PubMed] [Google Scholar]