Abstract
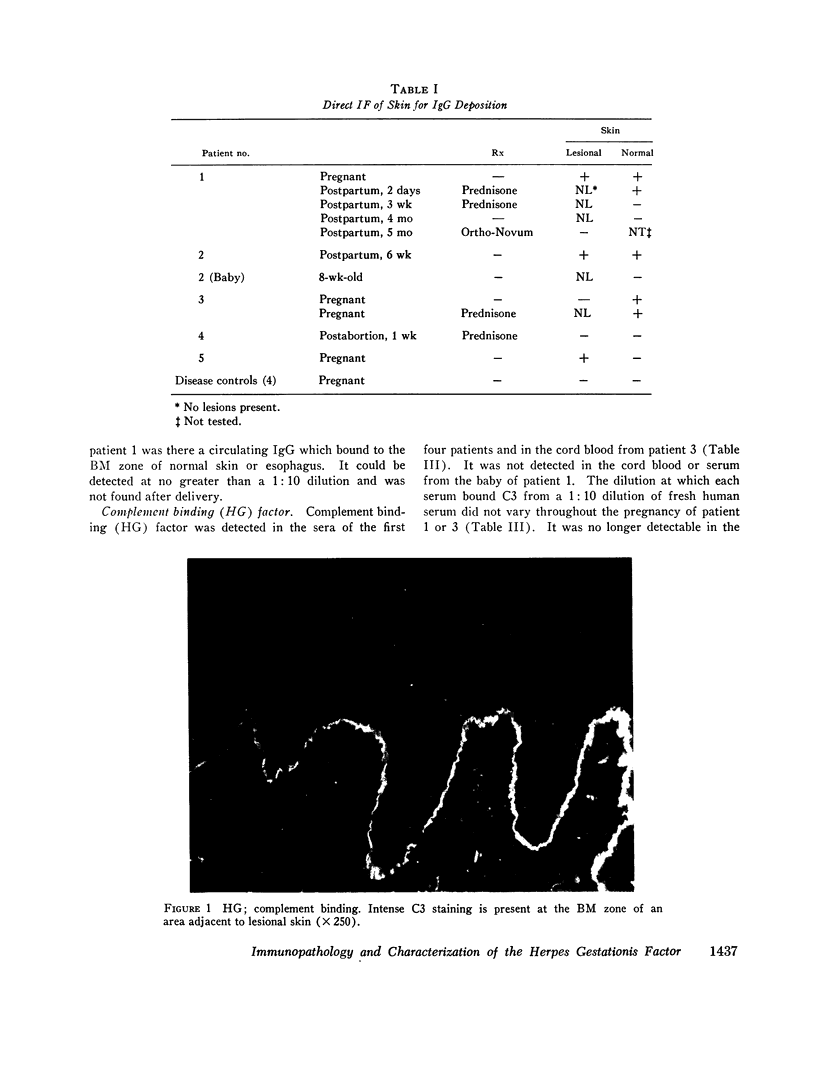
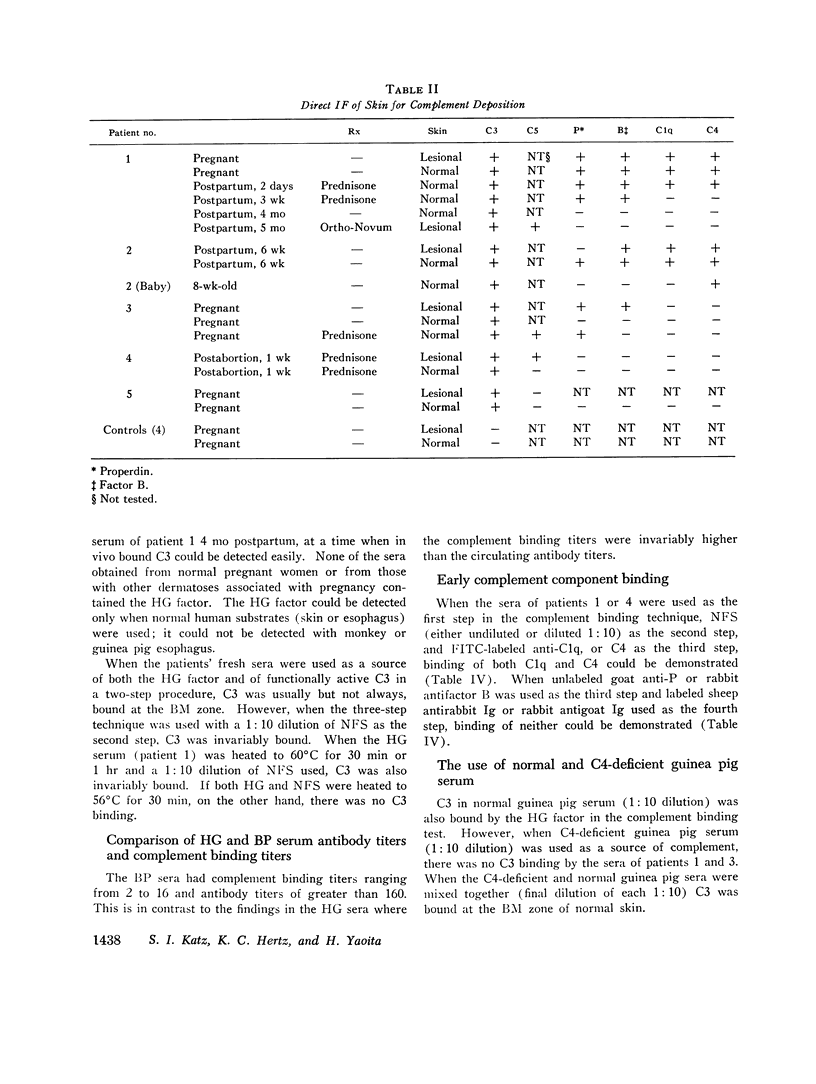
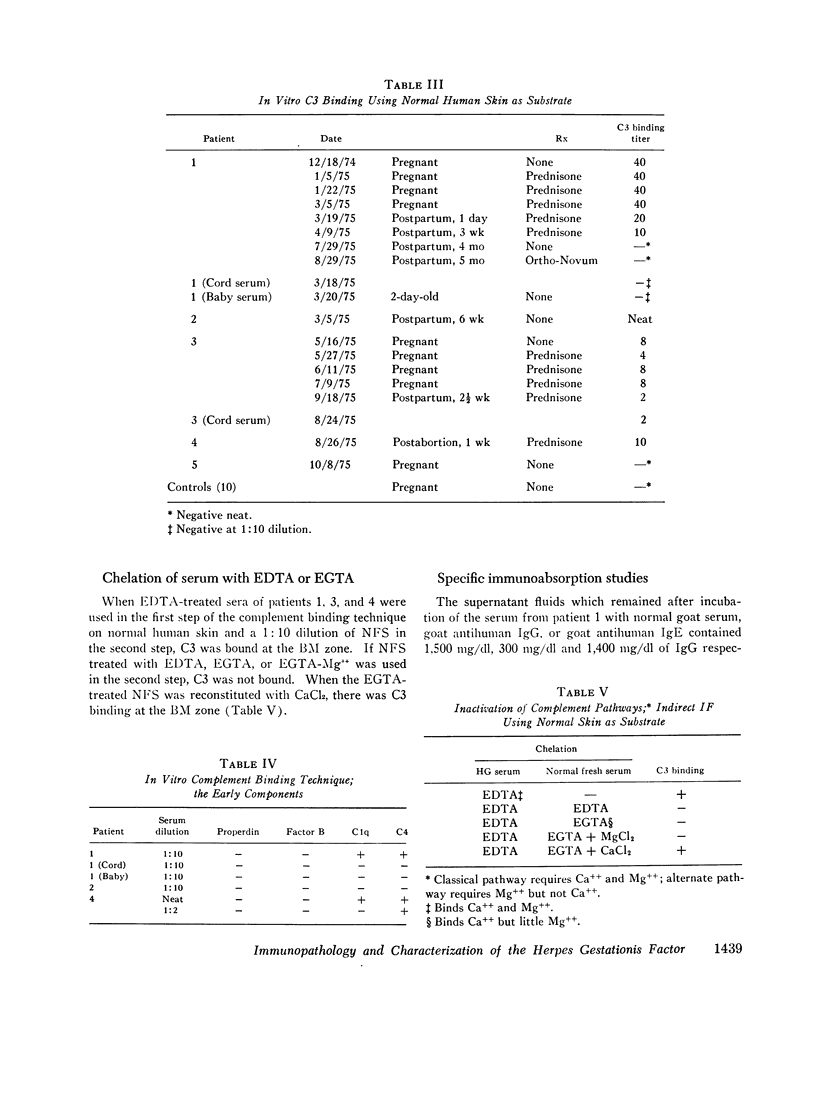
Five patients with herpes gestationis, a blistering disease of pregnancy, were studied immunologically. All had in vivo deposition of C3 in a linear band along the basement membrane zone of lesional and normal-appearing skin, the location of early blister formation. Immunoglobulin deposition was more variable, though four patients had evidence of in vivo bound IgG at the same site. A circulating, complement binding herpes gestationis factor was demonstrated in the sera of four of the patients, its concentration unrelated to the activity of clinical disease. Characterization of this factor by sucrose gradient ultracentrifugation, specific absorption studies, and papain digestion indicates that it is an IgG. Evidence exists for involvement of both the classical and alternate complement pathways in vivo, though in vitro studies implicate the classical pathway as the primary route of complement activation. Three offspring were studied, none with clinical involvement; one showed in vivo deposition of C3 at the basement membrane zone of normal skin and a second showed the herpes gestationis factor in cord blood.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bushkell L. L., Jordon R. E., Goltz R. W. Herpes gestationis. New immunologic findings. Arch Dermatol. 1974 Jul;110(1):65–69. [PubMed] [Google Scholar]
- Ellman L., Green I., Judge F., Frank M. M. In vivo studies in C4-deficient guinea pigs. J Exp Med. 1971 Jul 1;134(1):162–175. doi: 10.1084/jem.134.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Jeannet M., Creighton W. D., Carbonara A. Immunological studies of the human placenta. Characterization of immunoglobulins on trophoblastic basement membranes. J Clin Invest. 1974 Nov;54(5):1011–1019. doi: 10.1172/JCI107844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Jablonska S., Chorzelski T. P., Beutner E. H., Maciejowska E., Rzesa G. Immunologic phenomena in herpes gestationis. Arch Dermatol Forsch. 1975 Jul 18;252(4):267–274. doi: 10.1007/BF00560366. [DOI] [PubMed] [Google Scholar]
- Jordon R. E. Complement activation in bullous skin diseases. J Invest Dermatol. 1975 Jul;65(1):162–169. doi: 10.1111/1523-1747.ep12598113. [DOI] [PubMed] [Google Scholar]
- Jordon R. E., Sams W. M., Jr, Beutner E. H. Complement immunofluorescent staining in bullous pemphigoid. J Lab Clin Med. 1969 Oct;74(4):548–556. [PubMed] [Google Scholar]
- Katz S. I., Halprin K. M., Inderbitzin T. M. The use of human skin for the detection of anti-epithelial autoantibodies. A diagnostic and prognostic test. J Invest Dermatol. 1969 Dec;53(6):390–399. doi: 10.1038/jid.1969.166. [DOI] [PubMed] [Google Scholar]
- Kocsis M., Eeg T. L., Husby G., Rajka G. Immunofluorescence studies in herpes gestationis. Acta Derm Venereol. 1975;55(1):25–29. [PubMed] [Google Scholar]
- Provost T. T., Tomasi T. B., Jr Evidence for complement activation via the alternate pathway in skin diseases, I. Herpes gestationis, systemic lupus erythematosus, and bullous pemphigoid. J Clin Invest. 1973 Jul;52(7):1779–1787. doi: 10.1172/JCI107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C., PILLEMER L. The properdin system and immunity. V. The bactericidal activity of the properdin system. J Exp Med. 1956 May 1;103(5):553–575. doi: 10.1084/jem.103.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]