Abstract
Polysaccharides are attracting the vigil eye of researchers in order to design the green synthesis of silver nanoparticles (Ag NPs) of diverse size, shape, and application. We report an environmentally friendly method to synthesize Ag NPs where no physical reaction conditions were employed. Hydroxypropylcellulose (HPC) was used as a template nanoreactor, stabilizer, and capping agent to obtain Ag NPs. Different concentrations of AgNO3 solutions (50 mmol, 75 mmol, and 100 mmol) were mixed with a concentrated aqueous solution of HPC and the progress of the reaction was monitored by noting color changes of the reaction mixture at different reaction times for up to 24 hours. Characteristic ultraviolet–visible spectroscopy (UV/Vis) absorption bands of Ag NPs were observed in the range of 388–452 nm. The morphology of the Ag NPs was studied by scanning electron microscopy, transmission electron microscopy (TEM), and atomic force microscopy. The TEM images confirmed that the size of the Ag NPs was in the range of 25–55 nm. Powder X-ray diffraction studies showed that the crystal phase of the Ag NPs was face-centered cubic. The as-prepared Ag NPs were found to be stable, and no changes in size and morphology were observed after storage in HPC thin films over 1 year, as indicated by UV/Vis spectra. So, the present work furnishes a green and economical strategy for the synthesis and storage of stable Ag NPs. As-synthesized Ag NPs showed significant antimicrobial activity against different bacterial (Escherichia coli, Staphylococcus epidermidis, S. aureus, Bacillus subtilis, Pseudomonas aeruginosa) and fungal strains (Actinomycetes and Aspergillus niger).
Keywords: green synthesis, nanoreactor, nanobiotechnology, antimicrobial assay, storage, stability
Introduction
Metal nanoparticles (NPs) have versatile applications due to their unique electronic, physicochemical, and optical properties as compared to their bulk counterpart.1–3 Among metal NPs, silver (Ag) NPs hold a place of distinction due to many desirable attributes such as their catalytic activity, electrical conductivity,4,5 anticancer6,7 and antibacterial properties,8,9 as well as their applications in bioimaging10 and biosensors.11 The synthesis of metal NPs has been achieved through different methods, such as electrochemical engineering,12,13 irradiation,14,15 and chemical reduction of Ag salts in solution.16,17
The synthesis of Ag NPs by the chemical reduction method involves toxic solvents,18 dangerous reducing agents like sodium borohydride,19,20 and synthetic/nonbiodegradable stabilizing agents.21,22 Such synthesis strategies may also result in biological hazards and environmental toxicity. Secondly, Ag NPs have a high surface area-to-volume ratio, resulting in their aggregation with the passage of time that limits their potential applications. Therefore, there has been a need to develop synthesis protocols involving nontoxic, biodegradable, and low-cost green chemicals23,24 for the synthesis of Ag NPs.25,26
Polysaccharides are getting attention as an in situ reducing and self-capping agent for the green synthesis of Ag NPs.27–30 Additionally, the film-forming properties of polysaccharides can be exploited for the long-term storage of Ag NPs without agglomeration. Recently, cellulose ether derivatives have been used for the synthesis of Ag NPs. It was recently reported that hydroxyethylcellulose could be used for the green synthesis of Ag NPs. However, the reactions require elevated temperatures around 70°C and the use of strong basic reaction conditions (pH 12).27 Therefore, a need is felt to introduce new cellulose ether derivatives, which may perform reactions under mild conditions. Hence, we aimed to exploit another cellulose ether derivative, ie, hydroxypropylcellulose (HPC), as a reducing agent, in situ stabilizer, and capping agent for the synthesis of Ag NPs. The proposed green and controlled synthesis of stable Ag NPs is achieved without any physical reaction conditions (like temperature or pH) by simply exposing the concentrated aqueous solution of HPC and AgNO3 to sunlight. We also demonstrate the use of HPC thin films as a storage medium for as-synthesized Ag NPs.
Experimental methods
Materials
AgNO3 (99.98%) was purchased from EMD Millipore (Billerica, MA, USA). HPC (MS 3.46, 60% HP moieties) was obtained from Nanjing Yeshun Industry & International Trading Co., Ltd (Jiangsu, People’s Republic of China). Other reagents and analytical grade solvents were obtained from Fluka (Sigma-Aldrich Co., St Louis, MO, USA). All aqueous solutions were prepared in deionized water.
Green synthesis of Ag NPs mediated by HPC
A concentrated solution of HPC was prepared in deionized water. The AgNO3 solution (50 mmol) was then mixed with the HPC solution in equal volume at room temperature and kept under dark conditions. The mixture was subsequently exposed to sunlight and the progress of the reaction was monitored by ultraviolet–visible spectroscopy (UV/Vis) analysis. The same procedure was opted for different concentrations of AgNO3 (75 mmol and 100 mmol) solutions, respectively.
Film formation of HPC loaded with Ag NPs
The formation of Ag NPs embedded in HPC thin films was carried out by drying a mixture of HPC and AgNO3 (50 mmol, 75 mmol, and 100 mmol) after being exposed to diffused sunlight for 12 hours. The reaction mixture was then dried to thin films under air and dark conditions in a Petri dish. The resultant thin dry films were characterized using UV/Vis spectrophotometry, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and power X-ray diffraction (PXRD).
UV/Vis spectrophotometry
The progress of the reduction of Ag+ under diffused sunlight (ultraviolet [UV] irradiation) was monitored by recording the UV/Vis spectra of the reaction mixtures in a wavelength range of 800–200 cm−1 on the UV/Vis spectrophotometer, UV-1700 PharmaSpec (Shimadzu Corporation, Kyoto, Japan).
FT-IR spectroscopy
Fourier transform infrared (FT-IR) spectra of pure HPC and HPC loaded with Ag NPs were recorded on an IR Prestige-21 (Shimadzu Corporation) spectrometer using the KBr pellet technique. The pellets were dried under vacuum before analysis.
SEM
The size and morphology of the Ag NPs were observed using SEM. Microtomes were sliced from HPC thin films loaded with Ag NPs and analyzed on SEM with carbon stubs (carbon adhesive Leit-Tabs No. G 3347) (PLANO GmbH, Wetzlar, Germany).
TEM
Freshly prepared Ag NP solutions were centrifuged and the isolated Ag NPs were studied by TEM on a Philips 420 instrument with an acceleration voltage of 120 kV. The 1-year stored Ag NP-embedded HPC thin films were dissolved in deionized water and the Ag NPs were isolated by centrifugation before the TEM measurements. The samples were prepared by drop casting on a carbon-coated Cu TEM grid.
AFM
An HPC thin film loaded with Ag NPs was dissolved in deionized water. The sample was dropped on a freshly cleaved mica sheet and dried overnight. The morphology of the Ag NPs was observed using an AFM, Multimode, Nano-scope IIIa (Veeco Instruments, Inc., Plainview, NY, USA) in tapping mode.
PXRD
The crystal structure of the Ag NPs was determined on an X’Pert PRO MPD (PANalytical, Almelo, the Netherlands) diffractometer (over a range of 5°–100°, 2θ), equipped with monochromatic X-rays.
Antimicrobial activity of Ag NPs
The bacterial strains Escherichia coli (American Type Culture Collection [ATCC] 25922), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), and S. epidermidis (ATCC 12228), as well as fungal strains (Actinomycetes and Aspergillus niger) were used to carry out the antimicrobial assay of pristine Ag NPs. Bacterial strains were grown over Mueller–Hinton agar media (Oxoid Ltd., Hampshire, UK), while fungal growth was performed over Sabouraud dextrose agar (Hardy Diagnostics, Santa Maria, CA, USA). Inoculums (microbial culture in their respective media, 10 mL) were inoculated for 24 hours at 37°C for bacteria and for 30–37 hours at 27°C–30°C for fungal strains. Fungal strain culture (7 days old) was washed and suspended in normal saline solution, filtered through aseptic glass wool, and incubated at 28°C. To accelerate the growth of the microbial strains, the tubes were shaken periodically. The turbidity of the inoculums was adjusted by 0.5 McFarland Standards.
The antimicrobial assay of Ag NPs was carried out by the widely used disc (6 mm discs of Whatman number 1 filter paper) diffusion method. Mueller–Hinton agar plates for bacterial strains were used, upon which the inoculum (0.1 mL) was spread uniformly. Ag NP-loaded discs were carefully placed on the surface of the culture medium. The culture plates were incubated for 24 hours at 37°C. A pure dimethyl sulfoxide-loaded (15–20 mL) disc was used as the negative control. All of the assays were performed in triplicate and the mean values were reported.
Results and discussion
Synthesis of Ag NPs
The preparation of Ag NPs by the UV irradiation method produces hydrated electrons in the reaction system, which can be used to reduce Ag ions into zero-valent metallic Ag NPs. Hence, the use of additional toxic and environmentally dangerous reducing agents can be circumvented.19,27 Here, we have synthesized Ag NPs under diffused sunlight using HPC as an in situ capping and reducing agent.
AgNO3 solution containing the Ag+ was mixed with HPC solution. The Ag ion reacted with HPC to produce an (Ag[HPC])+ complex. The (Ag[HPC])+ complex was subsequently exposed to sunlight radiation. The Ag+ was reduced by HPC polymer to (Ag[HPC]) as an intermediate precursor. The negative charge on the hydroxyl groups in the HPC backbone capped the positively-charged groups on the surface of the Ag NPs resulting in the colloidal stabilization of (Ag[HPC]).
The formation of Ag NPs upon irradiation of the (Ag[HPC])+ intermediate complex was indicated by a change in the color of the reaction mixture. Solutions changed from colorless to reddish-brown with progress of the reaction observed over a period of 6 hours. The photographs showing the color change on exposure to diffused sunlight are shown in Figure 1.
Figure 1.
Photographs of HPC–Ag+ solutions (50 mmol AgNO3) showing color change with a reduction of Ag+ upon exposure to diffused sunlight.
Abbreviations: min, minutes; h, hours; HPC, hydroxypropylcellulose.
Characterization of Ag NPs
UV/Vis spectrophotometry
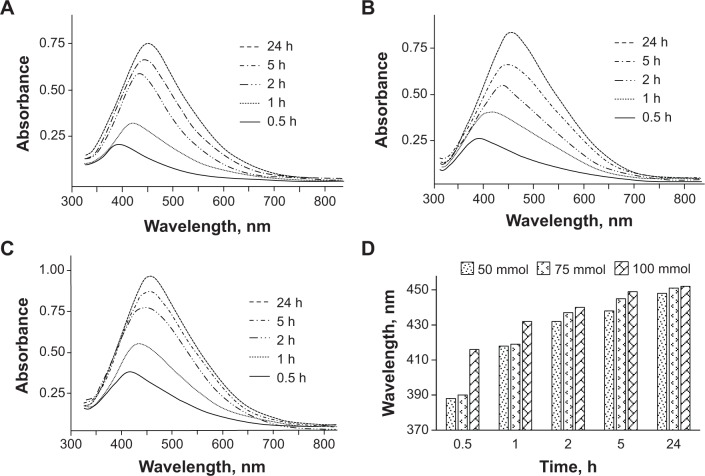
AgNO3 solutions (50 mmol, 75 mmol, and 100 mmol; 2 mL aliquot each) were mixed with concentrated HPC solution (2 mL) and the progress of the reaction was monitored by UV/Vis spectrophotometry. Ag+ was reduced to Ag NPs upon exposure to sunlight. The collective oscillation of conduction electrons in Ag NPs results in the surface plasmon resonance (SPR) phenomenon. This SPR causes a strong absorption by Ag NPs in the visible region.31 Depending upon the particle size and reaction time, the SPR transitions of Ag NPs showed color changes in the reaction mixture from yellowish-brown to reddish-brown. UV/Vis absorption bands were observed at 388 nm, 418 nm, 432 nm, 438 nm, and 448 nm for 50 mmol; 390 nm, 419 nm, 437 nm, 445 nm, and 451 nm for 75 mmol; and 416 nm, 432 nm, 440 nm, 449 nm, and 452 nm for 100 mmol AgNO3 solutions at 0.5 hours, 1 hour, 2 hours, 5 hours, and 24 hours, respectively. The results are in good agreement with the Ag NPs produced by another cellulose ether (ie, hydroxyethylcellulose), where exogenous reducing agents were employed.27 The absorption peaks showed a red shift, which means that the size of the Ag NPs increased with an increase in reaction time. Likewise, an increase in absorption intensity was also noticed with an increase in reaction time from 30 minutes to 24 hours, indicating the continuous reduction of Ag+ by HPC with the passage of time. The results of the UV/Vis analyses are summarized in Figure 2.
Figure 2.
UV/Vis spectra of Ag NPs prepared using HPC.
Notes: (A) 50 mmol, (B) 75 mmol, and (C) 100 mmol solution of AgNO3 at different reaction times, as well as their (D) cumulative graphical representation showing increase in absorption of the Ag NP solutions with an increase in reaction time and concentration.
Abbreviations: h, hours; UV/Vis, ultraviolet–visible spectroscopy; NPs, nanoparticles; HPC, hydroxypropylcellulose.
FT-IR spectroscopy
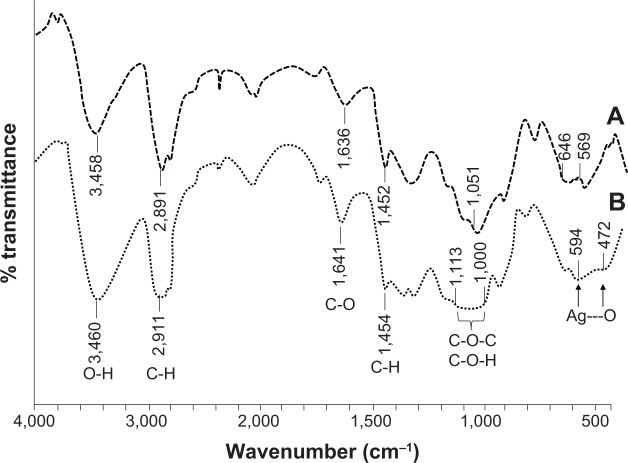
The interactions between hydroxyl groups of HPC and Ag NPs were studied by FT-IR spectroscopy (Figure 3). The peak at 1,051 cm−1 due to C-O-C stretching of HPC was changed to a broad band in the range of 1,000–1,113 cm−1 for HPC–Ag NPs. Ag---O van der Waal interactions showed a band in the range of 472–594 cm−1, which is not observed in the pure HPC spectrum. All other bands were observed at positions that were quite similar to pure HPC. The band in the range of 594–646 cm−1 in HPC was also broadened after the incorporation of Ag NPs. Similar FT-IR spectra were observed for 50 mmol and 100 mmol solutions as well.
Figure 3.
FT-IR (KBr) spectra of pure HPC and HPC films loaded with Ag NPs prepared from a 75 mmol AgNO3 solution.
Notes: (A) Pure HPC; (B) HPC films loaded with Ag NPs.
Abbreviations: FT-IR, Fourier transform infrared; HPC, hydroxypropylcellulose; NPs, nanoparticles.
SEM of films
Microtomes sliced from the HPC thin films embedded with Ag NPs were analyzed by SEM to see the distribution of Ag NP-embedded thin films. The uniform dispersion of Ag NPs in HPC thin films was therefore witnessed by SEM images. The results are shown in Figure 4.
Figure 4.
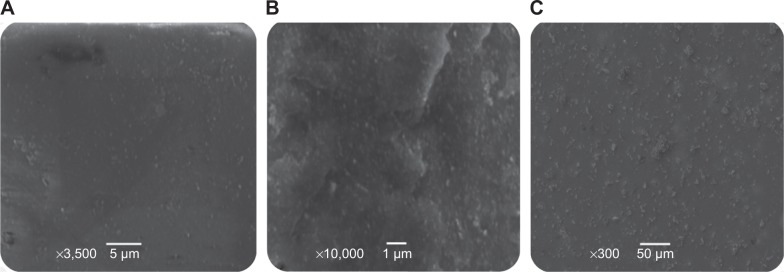
SEM images of Ag NPs embedded in HPC thin films.
Notes: (A) 50 mmol, (B) 75 mmol, and (C) 100 mmol AgNO3 solutions showing the distribution of Ag NPs.
Abbreviations: SEM, scanning electron microscopy; NPs, nanoparticles; HPC, hydroxypropylcellulose.
TEM of isolated Ag NPs
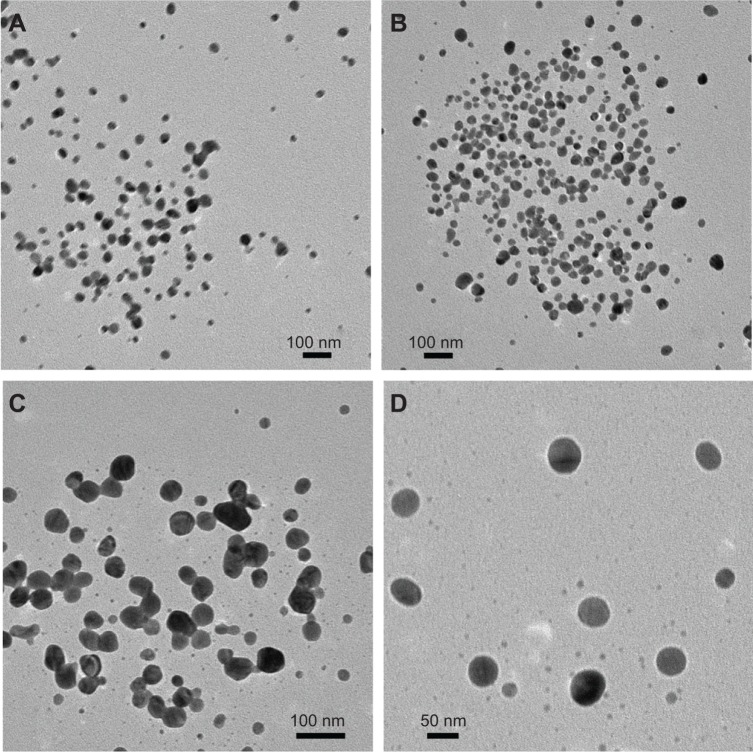
HPC thin films embedded with Ag NPs were dissolved in distilled water, and isolated Ag NPs were separated using centrifugation. The isolated Ag NPs were analyzed by TEM to assess the size distribution and morphology of the Ag NPs. The TEM images confirmed the formation of highly spherical Ag NPs with a size distribution in the range of 25–45 nm, 25–50 nm, and 25–55 nm for 50 mmol, 75 mmol, and 100 mmol AgNO3 solutions, respectively (Figure 5). TEM images of the Ag NPs isolated from the 1-year stored sample (100 mmol) were also recorded. It was noted that Ag NPs retained their size and morphology after 1 year of storage, which further confirmed the useful storage properties of HPC thin films.
Figure 5.
TEM images of isolated Ag NPs from 50 mmol, 75 mmol, 100 mmol, and 100 mmol (1-year stored sample) AgNO3 solutions reduced by HPC.
Notes: (A) 50 mmol, (B) 75 mmol, (C) 100 mmol, and (D) 100 mmol (1-year stored sample).
Abbreviations: TEM, transmission electron microscopy; NPs, nanoparticles; HPC, hydroxypropylcellulose.
PXRD
The crystal phase of the as-synthesized Ag NPs isolated by centrifugation was confirmed by PXRD analyses of 50 mmol, 75 mmol, and 100 mmol solutions (Figure 6) over the range of 5°–100°, 2θ. The crystal phase of face-centered cubic Ag NPs was inferred from the diffraction peaks centered at (111), (200), (220), and (311) in all samples. The X-ray diffraction of the isolated Ag NPs after storing the Ag NPs in HPC thin films for 1 year was also recorded. The results revealed that there was also no change in the diffraction pattern of the stored sample. Therefore, it is inferred that the HPC thin films can be utilized for the long-term storage of Ag NPs.
Figure 6.
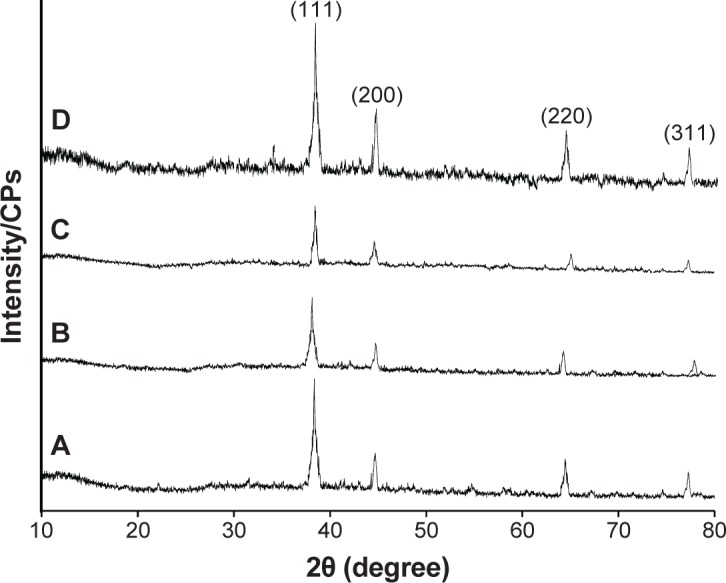
PXRD spectra of isolated Ag NPs.
Notes: (A) 50 mmol, (B) 75 mmol, and (C) 100 mmol and (D) 50 mmol (recorded after a 1-year storage period).
Abbreviations: CPs, counts per second; PXRD, power X-ray diffraction; NPs, nanoparticles.
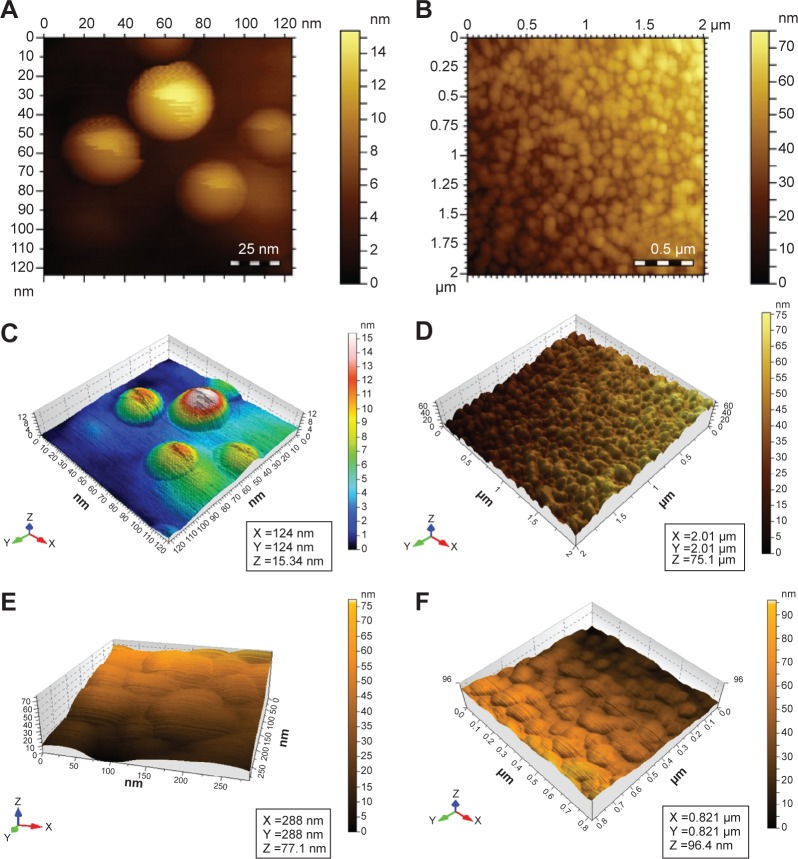
AFM
Well-dispersed Ag NPs showed a spherical surface morphology in the AFM images (Figure 7). The capping of Ag NPs by HPC prevented their aggregation. Thus, it was concluded that HPC can be used as stabilizer and storage medium for Ag NPs.
Figure 7.
AFM images of Ag NPs embedded in HPC thin films confirming the symmetrical and spherical geometry of the Ag NPs.
Notes: (A and C) HPC–Ag NPs 50 mmol; (B and D) HPC–Ag NPs 75 mmol; (E and F) HPC–Ag NPs 100 mmol AgNO3.
Abbreviations: AFM, atomic force microscopy; NPs, nanoparticles; HPC, hydroxypropylcellulose.
Storage of HPC–Ag NPs in thin films
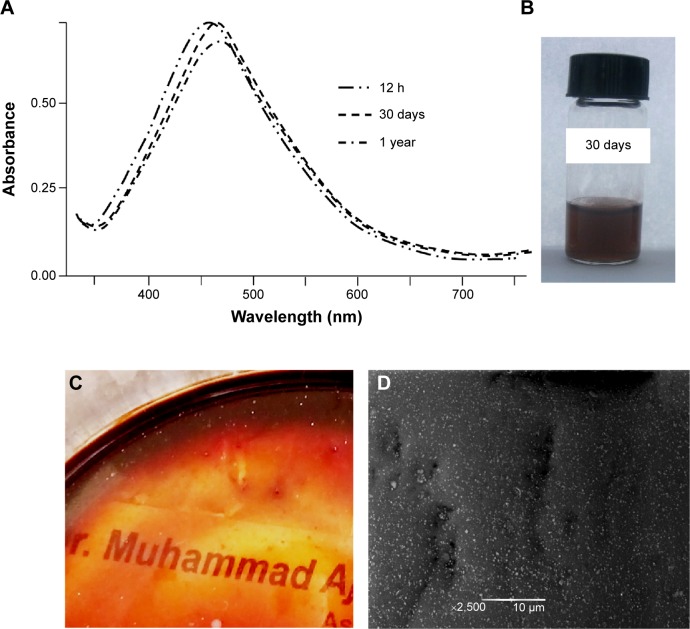
HPC was also evaluated as a storage medium for Ag NPs in solution, as well as in thin films. For this purpose, the AgNO3 solution (100 mmol) was reduced by HPC for 12 hours under sunlight. The UV/Vis absorption spectra were recorded before and after 30 days’ storage of the samples under dark, and comparable absorption maxima were obtained for the sample. A similar sample was dried and stored under dark conditions after UV/Vis irradiation for 12 hours. The resultant see-through thin films were stored for 1 year and UV analysis was recorded after dissolving the films in deionized water. No significant changes in the absorption intensity and shape of the UV/Vis spectra indicated that there was no sign of agglomeration of the Ag NPs during storage on the thin films. The SEM analyses of the 1-year stored Ag NP-embedded HPC thin films have further confirmed the hypothesis, and results symmetrical to the SEM images of the freshly prepared thin films (see Figure 4) were obtained. All of the storage results are depicted in Figure 8.
Figure 8.
UV/Vis spectra of a 100 mmol AgNO3 solution in HPC, a vial indicating the color of the solution, see-through Ag NP-embedded HPC thin film, and SEM image of thin films taken after 1 year of storage under dark conditions.
Notes: (A) UV/Vis spectra of a 100 mmol AgNO3 solution in HPC recorded after 12 h, 30 days, and 1 year of storage. (B) Vial indicating the color of the solution. (C) See-through Ag NP-embedded HPC thin film. (D) SEM image of thin films taken after 1 year of storage under dark conditions.
Abbreviations: h, hours; UV/Vis, ultraviolet–visible spectroscopy; HPC, hydroxypropylcellulose; NP, nanoparticle; SEM, scanning electron microscopy.
Antimicrobial activity of Ag NPs
The Ag NPs showed significant antimicrobial activity against the most commonly tested strains of bacteria and fungi (ie, S. epidermidis, S. aureus, P. aeruginosa, B. subtilis, E. coli, Actinomycetes, and A. niger). The zones of inhibition (radial diameter) for the aforementioned strains were found to be 26 nm, 29 nm, 33 nm, 33 nm, 20 nm, 22 nm, and 21 mm, respectively, using an Ag NP solution with a 50 mmol concentration. Similarly, 75 mmol and 100 mmol solutions of Ag NPs were tested against the aforementioned strains, and the results are cumulatively shown in Figure 9. In simultaneously performed experiments for controls (HPC solution and sterile distilled water-filled cavities), there was no antimicrobial activity, whereas AgNO3 solution (0.01 M) was found to be active against the mentioned strains. Antimicrobial assay was carried out in triplicate and the mean values have been reported. As a typical example, the antimicrobial activity of Ag NPs (50 mmol) against P. aeruginosa is shown in Figure 9, clearly indicating the zone of inhibition. However, polymer HPC and distilled water did not show any activity. The Ag NPs prepared by said novel and green method are potentially active against a variety of microbes; therefore, such pristine Ag NPs can be used as effective therapeutic tools.
Figure 9.
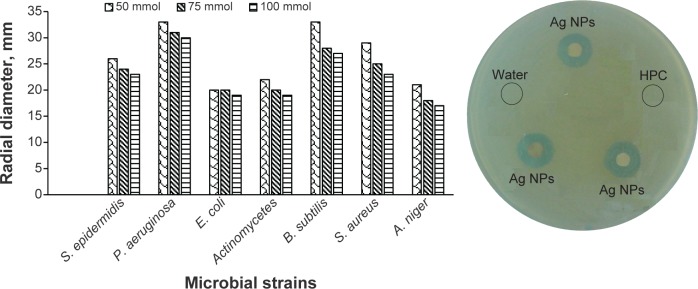
Inhibitory zone of Ag NPs (50 mmol, 75 mmol, and 100 mmol) versus different bacterial and fungal strains.
Abbreviations: S. epidermidis, Staphylococcus epidermidis; P. aeruginosa, Pseudomonas aeruginosa; E. coli, Escherichia coli; B. subtilis, Bacillus subtilis; S. aureus, Staphylococcus aureus; A. niger, Aspergillus niger; NPs, nanoparticles; HPC, hydroxypropylcellulose.
Conclusion
The present study reports a green and low-cost method for the synthesis of Ag NPs based on HPC. The role of HPC in the formation and stabilization of Ag NPs has been investigated. AgNO3 solutions were reduced to Ag NPs through a nontoxic and green reducing agent (ie, HPC). The stability of the Ag NPs after 1 year of storage has been achieved using HPC. The green synthesis of Ag NPs can be exploited as bioactive materials in biopolymer composites. The Ag NP–biopolymer composites have hybrid properties of both polymer and Ag NPs. Such hybrid materials could find potential applications in catalysis, nanobiotechnology, and therapeutics.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sun YP, Riggs JE, Rollins HW, Guduru R. Strong optical limiting of silver-containing nanocrystalline particles in stable suspensions. J Phys Chem B. 1999;103(1):77–82. [Google Scholar]
- 2.Kamat PV. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J Phys Chem B. 2002;106:7729–7744. [Google Scholar]
- 3.Oliveira MM, Ugarte D, Zanchet D, Zarbin AJ. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J Colloid Interface Sci. 2005;292(2):429–435. doi: 10.1016/j.jcis.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 4.Shipway AN, Katz E, Willner I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. Chemphyschem. 2000;1(1):18–52. doi: 10.1002/1439-7641(20000804)1:1<18::AID-CPHC18>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Hayward RC, Saville DA, Aksay IA. Electrophoretic assembly of colloidal crystals with optically tunable micropatterns. Nature. 2000;404(6773):56–59. doi: 10.1038/35003530. [DOI] [PubMed] [Google Scholar]
- 6.Mfouo-Tynga I, El-Hussein A, Abdel-Harith M, Abrahamse H. Photodynamic ability of silver nanoparticles in inducing cytotoxic effects in breast and lung cancer cell lines. Int J Nanomedicine. 2014;9(1):3771–3780. [Google Scholar]
- 7.Zhao Q, Duan R, Yuan J, Quan Y, Yang H, Xi M. A reusable localized surface plasmon resonance biosensor for quantitative detection of serum squamous cell carcinoma antigen in cervical cancer patients based on silver nanoparticles array. Int J Nanomedicine. 2014;9:1097–1104. doi: 10.2147/IJN.S58499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M, Khan ST, Khan M, et al. Antibacterial properties of silver nanoparticles synthesized using Pulicaria glutinosa plant extract as a green bioreductant. Int J Nanomedicine. 2014;9:3551–3565. doi: 10.2147/IJN.S61983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo Y, Hwang J, Kim J, Jeong Y, Hwang MP, Choi J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int J Nanomedicine. 2014;9:4621–4629. doi: 10.2147/IJN.S69561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Ding Y, Tian B, Wang ZL, Zhuang X. Luminescent and Raman active silver nanoparticles with polycrystalline structure. J Am Chem Soc. 2008;130(32):10472–10473. doi: 10.1021/ja803302p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang K, Ma H. Electrochemical DNA biosensor based on silver nanoparticles/poly(3-(3-pyridyl) acrylic acid)/carbon nanotubes modified electrode. Anal Biochem. 2009;387(1):13–19. doi: 10.1016/j.ab.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 12.Eustis S, el-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35(3):209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 13.Dosescu D, Nistor CL, Purcar C, et al. Formation and dissolution of silver nanoparticles. Journal of Optoelectronics and Advanced Materials. 2009;1:44–48. [Google Scholar]
- 14.Tsuji T, Thang DH, Okazaki Y, Nakanishi M, Tsuboi Y, Tsuji M. Preparation of silver nanoparticles by laser ablation in polyvinylpyrrolidone solutions. Appl Surf Sci. 2008;254(16):5224–5230. [Google Scholar]
- 15.Shameli K, Ahmad MB, Yunus WM, Ibrahim NA, Gharayebi Y, Sedaghat S. Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int J Nanomedicine. 2010;5:1067–1077. doi: 10.2147/IJN.S15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oves M, Khan MS, Zaidi A, et al. Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia. PLoS One. 2013;8(3):e59140. doi: 10.1371/journal.pone.0059140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shameli K, Ahmad MB, Yunus WM, et al. Green synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV irradiation method and evaluation of antibacterial activity. Int J Nanomedicine. 2010;5:875–887. doi: 10.2147/IJN.S13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastroiza-Santos I, Liz-Marzan LM. Formation of PVP-protected metal nanoparticles in DMF. Langmuir. 2002;18(7):2888–2894. [Google Scholar]
- 19.Chen M, Wang LY, Han JT, Zhang JY, Li ZY, Qian DJ. Preparation and study of polyacryamide-stabilized silver nanoparticles through a one-pot process. J Phys Chem B. 2006;110(23):11224–11231. doi: 10.1021/jp061134n. [DOI] [PubMed] [Google Scholar]
- 20.Kuo PL, Chen WF. Formation of silver nanoparticles under structured amino groups in pseudo-dendritic poly(allylamine) derivatives. J Phys Chem B. 2003;107:11267–11272. [Google Scholar]
- 21.Zou X, Bao H, Guo H, et al. Mercaptoethane sulfonate protected, water-soluble gold and silver nanoparticles: syntheses, characterization and their building multilayer films with polyaniline via ion-dipole interactions. J Colloid Interface Sci. 2006;295(2):401–408. doi: 10.1016/j.jcis.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 22.Popa M, Pradell T, Crespo D, Calderón-Moreno JM. Stable silver colloidal dispersions using short-chain polyethylene glycol. Colloids Surf A Physicochem Eng Asp. 2007;303(3):184–190. [Google Scholar]
- 23.Vimala K, Samba Sivudu K, Murali Mohan Y, Sreedhar B, Mohana Raju K. Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly(acrylamide) and carbohydrates: a rational methodology for antibacterial application. Carbohydr Polym. 2009;75(3):463–471. [Google Scholar]
- 24.Murphy CJ. Sustainability as an emerging design criterion in nanopar-ticle synthesis and applications. J Mater Chem. 2008;18:2173–2176. [Google Scholar]
- 25.Prozorova GF, Pozdnyakov AS, Kuznetsova NP, et al. Green synthesis of water-soluble nontoxic polymeric nanocomposites containing silver nanoparticles. Int J Nanomedicine. 2014;9:1883–1889. doi: 10.2147/IJN.S57865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muzzarelli RAA. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr Polym. 2009;77(1):1–9. [Google Scholar]
- 27.El-Sheikh MA, El-Rafie SM, Abdel-Halim ES, El-Rafie MH. Green synthesis of hydroxyethyl cellulose-stabilized silver nanoparticles. Journal of Polymers. 2013:650837. [Google Scholar]
- 28.Abdel-Halim ES, Al-Deyab SS. Utilization of hydroxypropyl cellulose for green and efficient synthesis of silver nanoparticles. Carbohydr Polym. 2011;86(4):1615–1622. [Google Scholar]
- 29.Hebeish AA, El-Rafie MH, Abdel-Mohdy FA, Abdel-Halim ES, Emam HE. Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohydr Polym. 2010;82(3):933–941. [Google Scholar]
- 30.Angelova T, Rangelova N, Yuryev R, Georgieva N, Müller R. Antibacterial activity of SiO2/hydroxypropyl cellulose hybrid materials containing silver nanoparticles. Mater Sci Eng C Mater Biol Appl. 2012;32(5):1241–1246. [Google Scholar]
- 31.Mock JJ, Barbic M, Smith DR, Schultz DA, Schultz S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys. 2002;116:6755–6759. [Google Scholar]