Abstract
INTRODUCTION
We previously reported that the prevalence of abdominal aortic aneurysms (AAAs) was higher in patients undergoing scheduled transthoracic echocardiography (TTE) than in patients undergoing abdominal ultrasonography (AUS); however, intergroup patient backgrounds differed significantly in that report.
PURPOSE
We tested the hypothesis that TTE could detect AAA as effectively as AUS.
DESIGN
A propensity score-matching analysis of a cross-sectional study was adopted as the design for this study.
METHODS
We enrolled 7,619 and 15,433 patients scheduled to undergo TTE with additional evaluation of abdominal aorta at the end of the routine study and AUS, respectively, from 2009 to 2010 in our hospital, as reported. A propensity score for profiles of patients who underwent TTE or AUS was developed to adjust for potential confounding bias. Consequently, 4,388 patients in each group were matched for analyses.
RESULTS
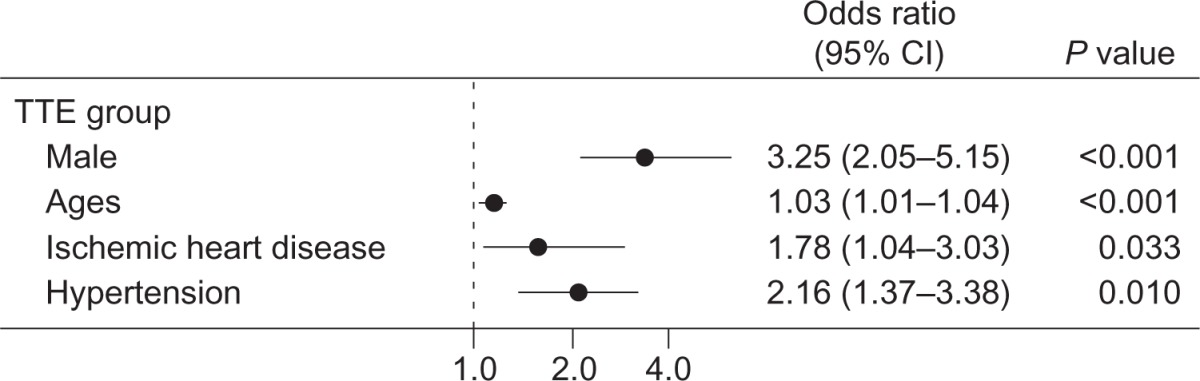
In propensity-matched patients, AAA was detected in 59 patients of the TTE group and in 48 patients of the AUS group; the prevalence of AAA detection did not differ significantly between TTE and AUS groups (P = 0.331). Positive associations were observed between AAA detection and male sex (adjusted odds ratio [OR]: 3.25; 95% confidence interval [CI], 2.05–5.15; P < 0.001), older age (adjusted OR: 1.029; 95% CI: 1.01–1.04; P < 0.001), and the presence of ischemic heart disease (adjusted OR: 1.78; 95% CI: 1.04–3.03; P = 0.033) and hypertension (adjusted OR: 2.16; 95% CI: 1.38–3.37; P = 001).
CONCLUSION
TTE detected AAA with comparable efficacy as AUS in propensity-matched groups who underwent scheduled TTE and AUS.
Keywords: abdominal aortic aneurysms, echocardiography, transthoracic echocardiography
Introduction
An abdominal aortic aneurysm (AAA) is often a clinically silent entity unless it ruptures. Among patients with ruptured AAA, approximately 15% of patients die without intervention, 40% die after repair, and only 40% survive, despite recent advances in treatments.1 The incidence of AAA has decreased gradually in recent years.2 The prevalence of AAA varies from 0.14% to 0.5% in Asian populations3,4 and from 1.0% to 1.7% in Western countries.5–8 Although the mortality rate for ruptured AAA is high, the 30-day mortality rate for elective endovascular repair or surgery on nonruptured AAAs in major randomized trials is only 1.2%–5.8%.9–12 Thus, a crucial clinical goal is to identify AAAs and treat them appropriately before they rupture.
Abdominal examination can discover the presence of AAA; however, the accuracy is low because of the retroperitoneal location of the aorta.13 Abdominal ultrasonography (AUS) is the primary method used for screening, allowing high sensitivity (95%) and specificity (90%).14 Although screening standards in general populations in Western countries are well published,14–18 national screening policies have been adopted only by a few countries.13 In addition, the target populations recommended by such screening guidelines are inconsistent. One-time screening in men aged 65–75 years who have ever smoked and selective screening in men aged 65–75 years who have never smoked are recommended by the US Preventive Services Task Force.19 Men aged 60 years or older with a family history of AAA or men aged 65–75 years who have smoked are recommended to undergo a physical examination and ultrasound screening for AAA, according to the American College of Cardiology/American Heart Association ACC/AHA 2005 practice guidelines.16 In England, the recommendation advises one-time screening in all men aged 65 years and older.20 No benefit was observed for population-based screening in women.21 However, subgroups of women who would benefit from screening for AAA may be identified by nonrandomized studies.22 This indicates the necessity of targeting high-risk patients for cardiovascular disease, which is still the leading cause of death in the US.23 However, even with the aforementioned recommendations, screening is often not performed because patients and physicians may be unaware of the need. Given the low prevalence of AAA in the Asian population, targeted and efficient screening is particularly needed in this population.
Detection of AAA using transthoracic echocardiography (TTE) would involve no additional cost or additional time if the same probe is used with the patients in the supine position. TTE can be used to examine patients for AAA quickly and easily and adds only 2–5 minutes to a cardiac examination.24,25 Routine examination of the abdominal aorta during TTE has been also reported to be useful by single-center studies3,24–30 and one multicenter study.25 However, to include the detection of AAA during TTE as a screening protocol, a comparison of TTE with AUS, one of the standard methods for the detection of AAAs, is necessary.
We have previously reported that the prevalence of AAA in Japanese patients who underwent scheduled TTE (1.8%) was higher than that of AAA in patients who underwent AUS (0.6%).26 However, the background of the patients differed significantly between the TTE and AUS groups, with a greater number of males, a higher mean age, and a larger mean number of comorbidities being observed in the TTE group as compared to the AUS group.
In the present study, we tested the hypothesis that TTE could detect AAA as effectively as AUS. Using propensity score matching, we used the data from a subset of participants for the previous study26 and matched the patients’ background for comparisons.
Methods
Study population
For the current study, we used the data from a subset of participants recruited for the detection of AAA, the methods of which have been previously described.26 Briefly, we included 7,765 patients who underwent scheduled TTE and 15,553 patients who underwent scheduled AUS in the outpatient clinic or while hospitalized from January 2009 to December 2010 at Kitano Hospital (Fig. 1). Exclusion criteria were as follows: patients with a history of AAA (24 patients), patients whose clinical symptoms or signs were thought to be due to AAA for which a TTE was ordered (6 patients), those who underwent prior AAA repair (10 patients), and patients who did not provide consent (16 patients). In AUS, patients with a history of AAA (25 patients), patients whose clinical symptoms or signs were thought to be due to AAA for which an AUS was ordered (14 patients), and those who underwent prior AAA repair (41 patients) were excluded. In AUS, the abdominal aorta is always observed according to the practice guidelines, and consent was obtained from the patients undergoing AUS who were eligible for this study.
Figure 1.
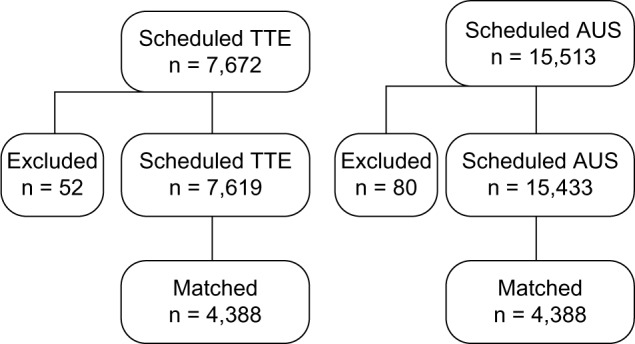
Patient flowchart for this study.
Technical methods of TTE
As reported previously, the indication of TTE or AUS in each patient depended on the physician ordering the examination, in accordance with practice guidelines. At the end of the routine TTE examination, the abdominal aorta was visualized with the patient in the supine position using the two-dimensional mode with color Doppler. First, a longitudinal image of the abdominal aorta was obtained; then, the sector-type transducer was rotated 90° clockwise to scan the central line of the abdominal aorta, which was traced distally from the subxiphoid window to the level of the bifurcation of the common femoral arteries, as reported previously by us26 and others.3,29 The definition of AAA was an abdominal aorta measuring >30 mm in diameter or a 50% increase in diameter as compared to a normal aorta.
Data collection
TTE and AUS data were recorded in an ultrasonographic database. We collected comorbidities using the electronic medical record database. Comorbidities included ischemic heart disease, cerebrovascular diseases, hypertension, diabetes mellitus, and hyperlipidemia. Comorbidities were regarded to be present when the diagnoses were recorded in the hospital charts. The research protocol was approved by the Institutional Review Board of Kitano Hospital according to the ethical guidelines of the 1975 Declaration of Helsinki.
Generation of propensity score
In order to compare the TTE and AUS groups, we developed a propensity score for the profiles of patients who underwent TTE or AUS for a potential confounding bias. Specifically, a multivariate logistic regression model was fitted with TTE or AUS as a dependent variable, which included age, sex, numbers of comorbidities, and the presence of each comorbidity. The model was assessed by the Hosmer–Lemeshow test (P = 1.00), and the results indicated matching goodness of fit. The area under the curve was 0.817 (95% confidence interval [CI]: 0.812–0.822). Consequently, 4,388 patients in each group were matched for analyses.
Statistics
Continuous variables were expressed as the mean ± standard deviation. Categorical variables were expressed as a number or as a percentage (%). In comparisons of the baseline characteristics of the study population, the chi-square test was used for categorical variables and the Mann–Whitney test for continuous variables when appropriate. When there were two crossed factors, differences in continuous variables between the TTE and AUS groups were analyzed with a two-way factorial ANOVA followed by post-hoc comparisons with the Bonferroni test; differences in categorical variables were analyzed using the Cochran–Mantel–Haenszel test. After propensity score matching, differences in continuous variables between the TTE and AUS groups were assessed using the paired t-test, while differences in categorical variables were analyzed using the Cochran–Mantel–Haenszel test. Odds ratios (ORs) were calculated using multivariate logistic regression after adjustment for propensity scores and all covariables. In all tests, values of P < 0.05 were considered statistically significant.
Results
Characteristics of the study population and comparison with the propensity-matched population
A total of 7,619 and 15,433 patients, all Asian, were enrolled during scheduled TTE and AUS, respectively. Demographic data for the study populations are summarized in Table 1, as previously reported.26 In the propensity-matched patients, no differences were noted for age (P = 1.00), the prevalence of males (P = 1.00), the number of comorbidities (P = 1.00), and the presence of each comorbidity (P = 1.00) between the TTE and AUS groups. AAA was detected in 59 patients in the TTE group and 48 patients in the AUS group (Table 1), and the prevalence of the detection of AAA did not differ between the TTE and AUS groups (P = 0.331).
Table 1.
Patient characteristics before and after the propensity matching.
| TTE | AUS | P VALUE | TTE | AUS | P VALUE | |
|---|---|---|---|---|---|---|
| Total number | (n = 7,619) | (n = 15,433) | (n = 4,388) | (n = 4,388) | ||
| Age (mean, SD) | 66.1 (15.1) | 52.9 (17.5) | <0.001 | 63.8 (14.4) | 63.8 (14.4) | 1.000 |
| Male (%) | 52.4 | 41.1 | <0.001 | 50 | 50 | 1.000 |
| Number of comorbidities (mean, SD) | 1.74 (2.08) | 0.09 (1.08) | <0.001 | 0.63 (1.18) | 0.63 (1.18) | 1.000 |
| Comorbidities (%) | ||||||
| Ischemic heart diseases (%) | 30.9 | 10.5 | <0.001 | 7.6 | 7.6 | 1.000 |
| Cerebrovascular diseases (%) | 25.3 | 9.1 | <0.001 | 3.5 | 3.5 | 1.000 |
| Hypertension (%) | 54.6 | 21.0 | <0.001 | 32.6 | 32.6 | 1.000 |
| Diabetes mellitus (%) | 28.5 | 19.0 | <0.001 | 13.2 | 13.2 | 1.000 |
| Hyperlipidemia (%) | 27.8 | 13.4 | <0.001 | 5.8 | 5.8 | 1.000 |
| Number of AAA detected (%) | 136 (1.78) | 96 (0.62) | <0.001 | 59 (1.34) | 48 (1.09) | 0.331 |
Factors associated with AAA in patients who underwent TTE
After adjusting for the covariables and propensity scores, positive associations were observed between AAA detection and male sex (adjusted OR, 3.25; 95% CI, 2.05–5.15; P < 0.001), older age (adjusted OR, 1.03; 95% CI, 1.01–1.04; P < 0.001), and the presence of ischemic heart disease (adjusted OR, 1.78; 95% CI, 1.04–3.03; P = 0.033) and hypertension (adjusted OR, 2.16; 95% CI, 1.37–3.38; P = 0.010, Table 2).
Table 2.
Odds ratio of the presence of AAA in the TTE group adjusted by covariables and propensity scores. The bullets indicate odds ratio and bars indicate 95% confidence interval (CI) of odds ratio.
|
Discussion
We observed that TTE detected AAA comparably with AUS in propensity-matched groups containing participants who underwent scheduled TTE and AUS. Considering that patients who underwent scheduled TTE had multiple comorbidities related to atherosclerosis and a higher prevalence of AAA than those who underwent scheduled AUS, we consider that routine examination for AAA during clinical scheduled TTE might be clinically useful in patients undergoing scheduled TTE for any reason.
Propensity score matching of the two study populations
A clear selection bias existed in patients who underwent TTE and AUS, as previously reported25; this is shown in Table 1 before matching, although patients came from the same community. Within the same population, because AUS is believed to be acceptable as the standard diagnostic test with a high sensitivity and specificity for AAA, its diagnostic accuracy was considered to be high. To our knowledge, no study has assessed the same population for AAA by TTE and a standard diagnostic method. Further, we generated propensity scores, which included age, sex, numbers of comorbidities, and the presence of each comorbidity. After the propensity matching, the presence of AAA detected was compared between the groups. TTE detected AAA comparably with AUS in this matched group; however, the confounding factors that were not recorded and adjusted in the present study may have influenced the observed prevalence of AAA in both the groups.
Comparison of TTE with AUS
Because the sector-type probe used in screening for AAA during TTE has fewer ultrasonic transducer lines than the convex-type probe typically used in AUS, the diagnostic quality of the image might be poorer as compared to the image obtained by AUS, especially in the case of obese patients. However, using TTE, a reliable image is obtained 75%–97% of the time in echocardiography laboratories.3,24,27 The accurate sensitivity and specificity of TTE could not be determined in the present study, for which sequential measurements in the same patient would be necessary, which is the future perspective and beyond the scope of the present study.
Risk factors and their clinical relevance
In our study, the risk factors associated with AAA were male sex, older age, and the presence of ischemic heart disease and hypertension. This is consistent with a meta-analysis for the prevalence of AAA; however, the reports on the associated risk factors are limited (12 studies among 56 studies analyzed),31 and only one study3 has been reported among the Asian population. Routine screening for AAA during clinical TTE provided a low yield (1.8% in the unmatched TTE group) in our study, probably due to a low prevalence (0.5%) of AAA in the general Asian population. Moreover, the genetic background may differ according to the ethnicity due to the presence of polymorphisms related to hypertension.32–34 Other risk factors for cardiovascular disease, such as microRNAs, should also be evaluated.35,36 There is good evidence of the important disadvantages of screening in the general population and early treatment, including an increased number of surgeries with an associated increase in clinically significant morbidity and mortality and short-term psychological adverse effects37; however, multiple comorbidities related to atherosclerosis were observed in patients who underwent scheduled TTE. Hence, additional examination of the abdominal aorta during scheduled TTE might be an efficient method for assessing patients at high risk for AAA.3,24–26,29
Limitations
This study has certain limitations. We recognize that the confounding factors that were not recorded or adjusted in the present study may have influenced the observed prevalence of AAA in both the groups. Further, because data were collected from an electronic medical database, information regarding smoking38 and family history was lacking. Furthermore, data for blood chemistry and blood pressure were also lacking.
Second, this study was not a screening study for the general population in Japan, but an observational study for a specific population in our hospital who underwent scheduled TTE and AUS. Further, we generated a propensity-matched score in order to compare the prevalence of AAA detected in this population. Generalization of this method for screening would require further validation of the sensitivity and specificity of TTE for identifying AAA in the general population.
Third, the validity of the additional examination of abdominal aorta during clinically scheduled TTE should be tested by assessing its effects on morbidity and mortality, socioeconomic effects, and short-term psychological issues. In terms of the feasibility of the procedure,25 patients routinely expose the anterior chest and abdominal area for TTE. Sonographers can screen the abdominal aorta from the subxiphoid window to the level of the bifurcation of the common femoral arteries quickly after examining the heart with a single probe without additionally asking patients to expose the abdominal area. Although data related to time were not collected in the present study, Aboyans et al.25 reported a median delay of 1.7 minutes.
Conclusion
TTE detected AAA with efficacy comparable to that of AUS in propensity-matched groups who underwent scheduled TTE and AUS. This preliminary result suggested that additional examination of the abdominal aorta during scheduled TTE would be an efficient technique for detecting AAA in patients scheduled to undergo TTE, although further studies are required for the accurate assessment of the sensitivity and specificity of TTE in the general population as well as for validating its effects on morbidity, mortality, and other issues in specific populations undergoing clinically scheduled TTE.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: This study was supported by funds from the AstraZeneca Research Grant and the Tazuke Kofukai Medical Institute. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the study: TK, SM, MI. Analyzed the data: TK, SI, TI, YB, JF, EN, TI, TH, KU, RN. Contributed to the writing of the manuscript: TK. Made critical revisions and approved final version: TK, MI. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–23. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 2.Mell MW, Callcut RA, Bech F, et al. Predictors of emergency department death for patients presenting with ruptured abdominal aortic aneurysms. J Vasc Surg. 2012;56:651–5. doi: 10.1016/j.jvs.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Oh SH, Chang SA, Jang SY, et al. Routine screening for abdominal aortic aneurysm during clinical transthoracic echocardiography in a Korean population. Echocardiography. 2010;27:1182–7. doi: 10.1111/j.1540-8175.2010.01223.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng SW, Ting AC, Tsang SH. Epidemiology and outcome of aortic aneurysms in Hong Kong. World J Surg. 2003;27:241–5. doi: 10.1007/s00268-002-6470-x. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: validation cohort and final results. Arch Intern Med. 2000;160:1425–30. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 6.Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg. 2001;34:122–6. doi: 10.1067/mva.2001.115275. [DOI] [PubMed] [Google Scholar]
- 7.Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA, Scott RA. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg. 2000;87:195–200. doi: 10.1046/j.1365-2168.2000.01353.x. [DOI] [PubMed] [Google Scholar]
- 8.Teufelsbauer H, Prusa AM, Wolff K, et al. Endovascular stent grafting versus open surgical operation in patients with infrarenal aortic aneurysms: a propensity score-adjusted analysis. Circulation. 2002;106:782–7. doi: 10.1161/01.cir.0000028603.73287.7d. [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Mendoza J, Pinto Miranda VA, Quevedo HC, Hebert K. Trends in abdominal aortic aneurysm prevalence and mortality in non-European countries. Int J Cardiol. 2013;170:e38–40. doi: 10.1016/j.ijcard.2013.10.074. [DOI] [PubMed] [Google Scholar]
- 10.Greenhalgh RM, Brown LC, Kwong GP, et al. EVAR trial participants Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomized controlled trial. Lancet. 2004;364:843–8. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 11.Prinssen M, Verhoeven EL, Buth J, et al. Dutch Randomized Endovascular Aneurysm Management (DREAM)Trial Group A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–18. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 12.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–74. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 13.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101–8. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 14.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203–11. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Davis M, Harris M, Earnshaw JJ. Implementation of the National Health Service Abdominal Aortic Aneurysm Screening Program in England. J Vasc Surg. 2013;57:1440–5. doi: 10.1016/j.jvs.2012.10.114. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Trans-Atlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 17.Chaikof EL, Brewster DC, Dalman RL, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009;50:880–96. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Takagi H, Niwa M, Mizuno Y, Goto SN, Umemoto T. All-Literature Investigation of Cardiovascular Evidence Group. The last judgment upon abdominal aortic aneurysm screening. Int J Cardiol. 2013;167:2331–2. doi: 10.1016/j.ijcard.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 19.US Preventive Services Taskforce . Screening for Abdominal Aortic Aneurysm. Rockville, MD: U.S. Preventive Task Force; 2014. [Google Scholar]
- 20.NHS . NHS Abdominal Aneurysm Screening Programme. London: National Health Service; 2013. [Google Scholar]
- 21.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–70. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 22.Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–48. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 23.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. JCvD. 2013;1:1–2. [Google Scholar]
- 24.Spittell PC, Ehrsam JE, Anderson L, Seward JB. Screening for abdominal aortic aneurysm during transthoracic echocardiography in a hypertensive patient population. J Am Soc Echocardiogr. 1997;10:722–7. doi: 10.1016/s0894-7317(97)70115-9. [DOI] [PubMed] [Google Scholar]
- 25.Aboyans V, Bataille V, Bliscaux P, et al. Investigators of the E2T3A study. Effectiveness of screening for abdominal aortic aneurysm during echocardiography. Am J Cardiol. 2014;114:1100–4. doi: 10.1016/j.amjcard.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Kato T, Ishida S, Miyamoto S, et al. Detection of abdominal aortic aneurysm during transthoracic echocardiography. IJC Heart & Vessels. 2014;4:223–5. [Google Scholar]
- 27.Eisenberg MJ, Geraci SJ, Schiller NB. Screening for abdominal aortic aneurysms during transthoracic echocardiography. Am Heart J. 1995;130:109–15. doi: 10.1016/0002-8703(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz KV, Rashkow AM, Akella MS. Detection of abdominal aortic aneurysm during routine echocardiography. Echocardiography. 1996;13:71–4. doi: 10.1111/j.1540-8175.1996.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 29.Bekkers SC, Habets JH, Cheriex EC, et al. Abdominal aortic aneurysm screening during transthoracic echocardiography in an unselected population. J Am Soc Echocardiogr. 2005;18:389. doi: 10.1016/j.echo.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Roshanali F, Mandegar MH, Yousefnia MA, Mohammadi A, Baharvand B. Abdominal aorta screening during transthoracic echocardiography. Echocardiography. 2007;24:685–8. doi: 10.1111/j.1540-8175.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population – a meta-analysis. PLoS One. 2013;8:e81260. doi: 10.1371/journal.pone.0081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 Gene Deletion Induces Hypertension. J Am Heart Assoc. 2012;1:e001081. doi: 10.1161/JAHA.112.001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanni F, Santulli G, Izzo R, et al. The Pl(A1/A2) polymorphism of glycoprotein IIIa and cerebrovascular events in hypertension: increased risk of ischemic stroke in high-risk patients. J Hypertens. 2007;25:551–6. doi: 10.1097/HJH.0b013e328013cd67. [DOI] [PubMed] [Google Scholar]
- 34.Santulli G, Trimarco B, Iaccarino G. G-protein-coupled receptor kinase 2 and hypertension: molecular insights and pathophysiological mechanisms. High Blood Press Cardiovasc Prev. 2013;20:5–12. doi: 10.1007/s40292-013-0001-8. [DOI] [PubMed] [Google Scholar]
- 35.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015;213:60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 36.Charan Reddy KV. Regulatory noncoding RNAs in cardiovascular disease: shedding light on ‘Dark Matter’. JCvD. 2015;3:301–7. [Google Scholar]
- 37.U.S. Preventive Services Task Force Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142:198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]
- 38.Sode BF, Nordestgaard BG, Grønbæk M, Dahl M. Tobacco smoking and aortic aneurysm: two population-based studies. Int J Cardiol. 2013;167:2271–7. doi: 10.1016/j.ijcard.2012.06.003. [DOI] [PubMed] [Google Scholar]


