Abstract
Background
Obesity is associated with increased mortality, and weight loss trials show rapid improvement in many mortality risk factors. Yet, observational studies typically associate weight loss with higher mortality risk. The purpose of this meta-analysis of randomized controlled trials (RCTs) of weight loss was to clarify the effects of intentional weight loss on mortality.
Methods
2,484 abstracts were identified and reviewed in PUBMED, yielding 15 RCTs reporting (1) randomization to weight loss or non-weight loss arms, (2) duration of ≥18 months, and (3) deaths by intervention arm. Weight loss interventions were all lifestyle-based. Relative risks (RR) and 95% confidence intervals (95% CI) were estimated for each trial. For trials reporting at least one death (n = 12), a summary estimate was calculated using the Mantel-Haenszel method. Sensitivity analysis using sparse data methods included remaining trials.
Results
Trials enrolled 17,186 participants (53% female, mean age at randomization = 52 years). Mean body mass indices ranged from 30–46 kg/m2, follow-up times ranged from 18 months to 12.6 years (mean: 27 months), and average weight loss in reported trials was 5.5±4.0 kg. A total of 264 deaths were reported in weight loss groups and 310 in non-weight loss groups. The weight loss groups experienced a 15% lower all-cause mortality risk (RR = 0.85; 95% CI: 0.73–1.00). There was no evidence for heterogeneity of effect (Cochran’s Q = 5.59 (11 d.f.; p = 0.90); I2 = 0). Results were similar in trials with a mean age at randomization ≥55 years (RR = 0.84; 95% CI 0.71–0.99) and a follow-up time of ≥4 years (RR = 0.85; 95% CI 0.72–1.00).
Conclusions
In obese adults, intentional weight loss may be associated with approximately a 15% reduction in all-cause mortality.
Introduction
Advanced age and obesity are risk factors for disability, morbidity, and mortality [1–3]. Weight loss interventions in overweight and obese older adults positively affect several strong risk factors for mortality, including: circulating IL-6 levels [4–6], blood pressure [7,8], fasting plasma glucose [9,10], gait speed [11–13], and cardiorespiratory fitness [12,14,15]. Yet, many observational studies in middle-aged and older adults report an association between weight loss and increased mortality [16–18]. Difficulty reconciling these contradictory findings (the so-called “obesity paradox”), coupled with the strong negative prognostic implication of rapid involuntary weight loss with advanced age, has led to a reluctance to recommend weight loss in older adults [19].
Attempts to refine observational analyses to avoid confounding (i.e. distinguishing between intentional and unintentional weight loss, and restricting populations to those without co-morbid conditions or non-smokers) typically reveal no increase, and perhaps some decrease, in mortality risk with intentional weight loss [20,21]. Indeed, results from the Swedish Obesity Study show a 24% reduction in all-cause 10-year mortality associated with gastric banding compared to matched-obese controls [22]. However, as a non-randomized study it is unclear if selection bias or confounding contributed to the observed mortality advantage. Although results from a randomized controlled trial (RCT) of weight loss would theoretically resolve these issues, such a trial would require a large sample size over a long duration to detect clinically meaningful differences in mortality.
In light of the high prevalence of obesity, its negative impact on health and quality of life, and the discrepancy between the proven risk factor improvements of short-term intentional weight loss and the inverse association of weight loss with increased all-cause mortality frequently seen in observational studies, we conducted a meta-analysis to estimate the effect of interventions which included intentional weight loss on all-cause mortality in overweight and obese adults. We hypothesized that intentional weight loss would be associated with reduced all-cause mortality. Further, as weight loss in older persons is a cause of clinical concern that may lead health care providers to recommend against weight loss for obese, older adults, we sought to examine the effects in a subset of trials with a mean baseline age of at least 55 years.
Materials and Methods
Study selection and data extraction
We sought to identify all published RCTs of intentional weight loss that reported mortality data either as an endpoint or as an adverse event, including study designs where participants were randomized to weight loss or non-weight loss, or weight loss plus a co-intervention (e.g. weight loss plus exercise) or the weight stable co-intervention (i.e. exercise alone). A comprehensive literature search was conducted using the PUBMED database (National Library of Medicine, Bethesda, MD) inclusively through December 7, 2013 on RCTs using the medical subject headings: weight loss, humans, and adult. References within identified papers were also examined for potential inclusion. Articles retrieved using this search string were then limited to trials including weight loss and non-weight loss arms, a trial duration (weight loss and maintenance phase) ≥18 months, and mortality data by intervention group.
Data were extracted in duplicate by two of the authors (SBK and KMB) and included intervention duration and length of follow up, number of participating subjects, population characteristics (age, gender, baseline BMI and health status), intervention arm descriptions, initial weight loss, and number of deaths reported. Manuscript authors were contacted for clarification when necessary.
Statistical methods
Relative risks (RR) and 95% confidence intervals (95% CI) were estimated for each trial. Three trials reported no deaths in one of the intervention arms, thus the estimate of the RR was undefined for those trials. For the 12 trials with at least one death in each intervention group, the Mantel-Haenszel estimator of the common RR was estimated from the stratified 2x2 tables relating the intervention to mortality, with trial being the stratifying factor; 95% CIs on the common estimate were calculated using the variance estimate of Greenland and Robins (1985) [23]. Cochrane’s Q statistics and Higgins I2 were used to evaluate heterogeneity of the RR across trials [24]. In addition, following the recommendations of Bradburn et al. (2007) [25], sensitivity analyses were performed using the reciprocal of the number of participants in the other intervention arm as the continuity correction [26], and re-estimating the RR with the Mantel-Haenszel estimator, thus including all 15 trials when obtaining a common estimate. Lastly, three distinct sub-analyses were performed in which trials limited to those reporting: (1) relatively older participants (≥55 years of age at baseline), (2) longer follow-up periods (≥4 years), and (3) at least five kg weight loss in the weight loss intervention arm.
Results
Study selection and publication bias
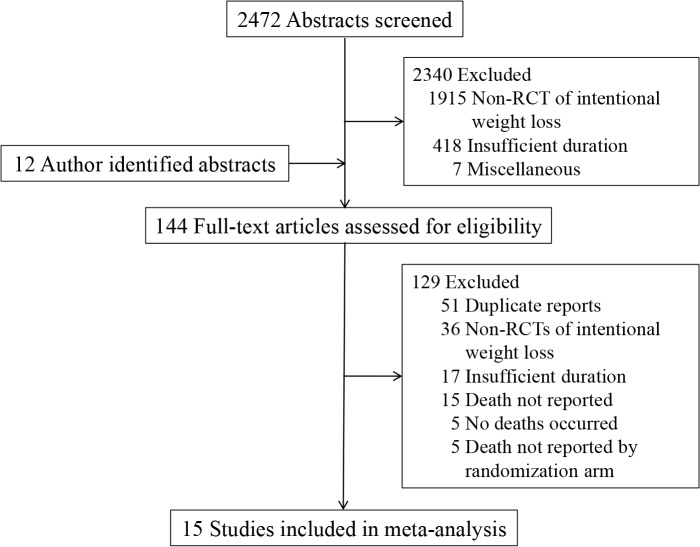
Fig. 1 shows the study selection diagram. Our search string generated 2,472 abstracts which were initially screened for potential inclusion, of which 2,340 did not meet inclusion criteria: 1,915 were non-RCTs of intentional weight loss or compared participants receiving varied degrees of caloric restriction; 418 were of insufficient duration; and, seven were judged to be out of scope for miscellaneous reasons. Twelve abstracts came to the attention of study authors by other means (e.g. references within identified papers), yielding a total of 144 full-text articles which were independently assessed (by SBK and KMB) for eligibility. Fifty-one articles were duplicate reports, 26 were non-RCTs of intentional weight loss or compared participants receiving varied degrees of caloric restriction, 17 were of insufficient duration, 15 did not report mortality data, five reported that no deaths occurred, and in five trials death was reported, but not by randomization arm (when indicated, authors were contacted to attempt to retrieve missing information). Thus, this meta-analysis consists of data from 15 RCTs of intentional weight loss [15,27–40].
Fig 1. Study Selection Flowchart.
Flowchart for the selection of eligible studies.
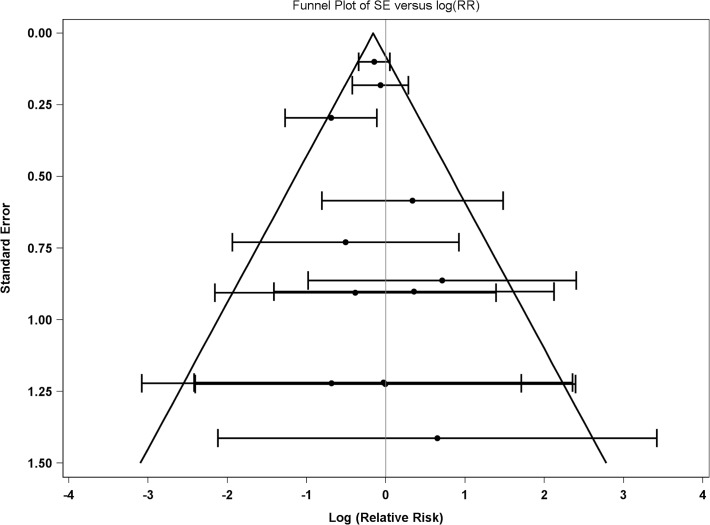
Of the 15 included studies, three did not have any deaths in one of the intervention arms [32,34,35]. The remaining 12 studies were used for estimating the common relative risk using the Mantel-Haenszel approach. To assess potential publication bias, a funnel plot of the data was produced (see Fig. 2). The pattern does not indicate that the smaller trials were more likely to observe a result that differs from the overall result indicating a lower likelihood of publication bias.
Fig 2. Funnel Plot.
Funnel plot of the point estimate and 95% confidence interval of 12 randomized clinical trials of intentional weight loss.
Study and participant characteristics
A summary of study details is presented in Table 1. The 15 eligible RCTs included a total of 17,186 participants (53% female) with an average age of 52 years at baseline. A total of 574 deaths were reported: 264 among those assigned to weight loss and 310 among non-weight loss comparison groups. Included trials were conducted over about 25 years, with the earliest trial published in 1987 [27]. The health status of the study-specific target populations varied and included: hypertension [27–30,36], osteoarthritis [33], pre-diabetes/diabetes [31,39], and overweight/obesity [15,32,34,35,37,38,40]. In all cases, reported mean baseline body mass index (BMI) classified participants as obese (range: 30–46 kg/m2), with an average BMI among trials of 35 kg/m2 (BMI was not reported in four trials [27–30]).
Table 1. Study characteristics of randomized controlled trials of weight loss interventions.
| Study and Author (year) | Target Population | Intervention Arm Descriptions | Average Age (years) | Baseline Body Mass Index (kg/m2) | Women (%) | Length of Intervention / Maintenance*(months) | Length of Follow-Up (months) | N (# of Deaths) |
|---|---|---|---|---|---|---|---|---|
| HCP; Stamler (1987) [27] | Hypertensive | Weight loss w/ Na+ and ETOH restriction | 57 | —- | 35 | 48 | 48 | 97 (3) |
| Control w/o pharmacotherapy | 55 | 38 | 92 (2) | |||||
| TOHP I (1992) [29] | Hypertensive | Weight loss | 43 | —- | 27 | 18 | 18 | 308 (1) |
| Control | 43 | 37 | 1158 (1) | |||||
| TAIM; Davis (1993) [28] | Overweight/obese hypertensive | Weight loss w and w/o pharmacotherapy | 48 | —- | 46 | 30 | 54 | 291 (4) |
| Usual diet w/ and w/o pharmacotherapy | 296 (2) | |||||||
| TOHP II (1997) [30] | Overweight/obese hypertensive | Weight loss w/ and w/o Na+ restriction | 44 | —- | 34 | 6/30 | 36 | 1192 (7) |
| No weight loss w/ and w/o Na+ restriction | 33 | 1190 (5) | ||||||
| DPP; Knowler (2002) [31] | Pre-diabetic | Dietary weight loss and exercise | 51 | 34 | 68 | 34 | 34 | 1079 (3) |
| Placebo tablets | 69 | 1082 (5) | ||||||
| Johnson (2008) [32] | Overweight/obese | Multiple behavioral change weight loss | 45 | 31 | 46 | 9 | 24 | 628 (0) |
| Control | 45 | 31 | 49 | 649 (3) | ||||
| ADAPT; Shea (2010) [33] | Osteoarthritic | Dietary weight loss w/ and w/o exercise | 69 | 34 | 72 | 6/12 | 96 | 159 (15) |
| Exercise and attention control | 71 | 159 (30) | ||||||
| LOSS; Ryan (2010) [34] | Morbidly obese | Diet, behavior, medication therapy | 47 | 46 | 84 | 24 | 24 | 200 (1) |
| Usual care | 47 | 47 | 84 | 190 (0) | ||||
| ORBIT; Fitzgibbon (2010) [35] | Obese | Culturally proficient weight loss | 46 | 39 | 100 | 6/12 | 18 | 107 (1) |
| Control | 46 | 39 | 100 | 106 (0) | ||||
| TONE; Shea (2011) [36] | Hypertensive | Weight loss w/ and w/o Na+ restriction | 66 | 31 | 47 | 8/22 | 152 | 294 (49) |
| Na+ restriction or attention control | 57 | 291 (52) | ||||||
| WOMAN; Gabriel (2011) [37] | Overweight/Obese | Weight loss and exercise | 57 | 31 | 100 | 36 | 48 | 253 (1) |
| Health education control | 57 | 31 | 100 | 255 (2) | ||||
| CLIP; Rejeski (2011) [15] | Overweight/obese w/CVD | Weight loss and exercise | 67 | 33 | 68 | 6/12 | 18 | 98 (1) |
| Exercise or successful aging | 67 | 33 | 66 | 190 (2) | ||||
| ALIFE@Work; van Wier (2011) [38] | Overweight | Phone/internet delivered weight loss | 43 | 30 | 33 | 6 | 24 | 926 (2) |
| Control | 43 | 30 | 33 | 460 (1) | ||||
| Look AHEAD; Wing (2013) [39] | Type 2 diabetic | Dietary weight loss and exercise | 59 | 36 | 59 | 12/103 | 115 | 2570 (174) |
| Attention control | 60 | 2575 (202) | ||||||
| ACHIEVE; Daumit (2013) [40] | Overweight/obesew/mental illness | Dietary weight loss and exercise | 47 | 36 | 51 | 18 | 18 | 144 (2) |
| Control | 44 | 37 | 49 | 147 (3) |
*Intervention duration refers to the total time period in which weight loss was advocated (not including weight loss maintenance).
Weight loss interventions were all lifestyle-based, with an average duration of 27 months (range: six-96 months). Only three trials considered mortality as an endpoint [33,36,39]; other trials reported death as an adverse event. A weight loss goal of 5–10% of baseline weight was specified in nine trials [15,27,28,30,31,33,35,36,39]. For trials that reported average initial weight loss (n = 6), the average initial weight loss in the weight loss and non-weight loss arms was 5.5 kg (range: -1.8 to -13.1 kg) and 0.2 kg (range: -1.1 to +0.2), respectively [29,33–36,40].
Weight loss and mortality
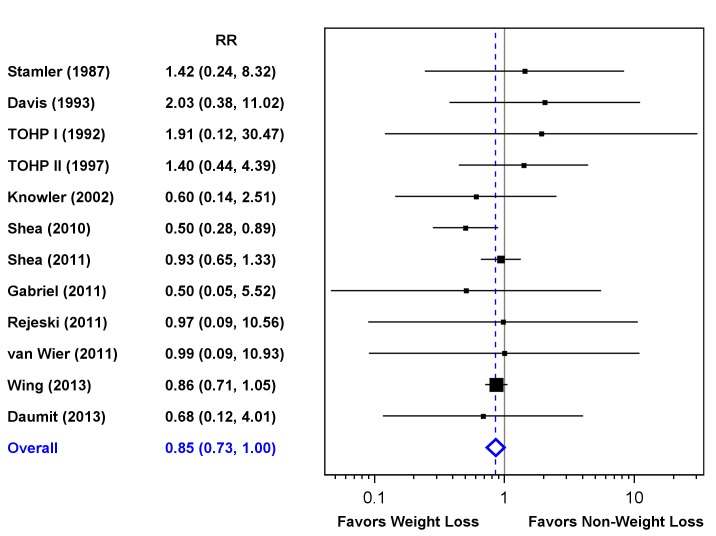
Fig. 3 shows the point estimates, 95% CIs, and summary RRs for mortality for the 12 trials that reported deaths in each arm (death n = 569). Across all trials, there was a 15% reduction in all-cause mortality for participants randomized to weight loss (RR = 0.85; 95% CI: 0.73–1.00). Cochran’s Q was 5.59 (11 d.f.; p = 0.90), with an associated I2 = 0, indicating no evidence of heterogeneity of the RR among the individual trials. Inclusion of the three trials with sparse data, after applying continuity corrections, resulted in an identical estimate (RR = 0.85; 95% CI 0.73–1.00). Six of the 15 trials had point estimates favoring weight loss, and ADAPT [33] showed a significant benefit for weight loss. Only three trials (ADAPT [33], TONE [36], Look AHEAD [39]) contributed more than 30 deaths to the analysis, with Look AHEAD contributing 65.5% of the total deaths. Total mortality was lower in the weight loss arms in each of these trials. The summary estimate omitting Look AHEAD data was 0.83 (95% CI 0.64–1.08).
Fig 3. Forest Plot.
Forest plot showing individual and pooled relative risks of all-cause mortality with 95% confidence intervals across 12 randomized clinical trials of weight loss interventions. Three trials did not report deaths in one intervention arm and are not included in this figure.
Sub-analyses: modifying effects of age, follow up duration, and magnitude of weight loss
Six trials had a mean age at randomization ≥55 years [15,27,33,36,37,39]. The summary estimate for these trials was 0.84 (95% CI 0.71–0.99). Six trials reported follow-up times ≥4 years [27,28,33,36,37,39]; the summary estimate for these trials was 0.85 (95% CI 0.72–1.00). Not all trials reported the degree of weight loss achieved. In the six trials in which an average weight loss of at least five kg was reported for the weight loss intervention arm [15,29,31,36,37,39], the summary estimate was 0.88 (95% CI: 0.74–1.04).
Discussion
In this meta-analysis of 15 RCTs of intentional weight loss in obese adults, the risk of all-cause mortality was 15% lower for individuals randomized to weight loss, compared to non-weight loss, groups. Results did not materially differ when examining only the trials of relatively older participants, trials with longer follow-up periods, or those reporting at least five kilograms of weight loss.
Although we present novel summary data on the effects of intentional weight loss on mortality risk from RCTs, our findings are comparable to a 2009 meta-analysis of the effect of lifestyle-based weight loss on all-cause mortality risk from prospective studies [20]. In this analysis of 26 studies, authors concluded that unintentional, but not intentional, weight loss increases risk of mortality. Akin to our results, authors also report intentional weight loss reduces all-cause mortality risk by approximately 15% in individuals with obesity-related risk factors; however, this finding did not extend to overweight (but not obese) or otherwise healthy individuals. Despite the overall finding, it is worth noting that several of the observational studies included in this meta-analysis found self-reported intentional weight loss to be associated with increased risk of mortality [20]. It is possible that the attribution of intentionality is unreliable, or that in some persons unintentional and intentional weight loss occur simultaneously. RCT data presented here circumvent potential confounding by self-report in observational studies; importantly, we found an upper boundary of the 95% CI for the association between randomization to weight loss and mortality of 1.00, thus providing evidence that a mortality excess may not exist in obese adults who lose weight intentionally.
Undertaking of this meta-analysis was partly motivated by the desire to resolve uncertainty regarding the long-term safety of weight loss for older adults. In addition to mortality, theoretical long-term safety concerns relate to the loss of muscle and bone mass that occur with weight loss [41], which might predispose older adults to impaired physical function and increased fracture risk. The mortality point estimate for the six trials with a mean age ≥55 years at baseline did not differ from the overall estimate; however, only three trials specifically limited their target population to older adults (i.e. mean baseline age of >65 years). Of these, ADAPT [33] showed a statistically significant benefit of weight loss, TONE [36] tended to favor weight loss, and CLIP [15] showed no effect. While these results are reassuring for geriatricians contemplating the recommendation of weight loss to their obese patients, more long-term data is needed to better understand the net benefits and risks of intentional weight loss in this population.
The most straightforward mechanism by which weight loss might reduce mortality in overweight and obese older adults is through the improvement of risk factors that either predict mortality on their own, or contribute to overall mortality through obesity-related disease (e.g. stroke and heart disease). The data needed to assess mediation by these factors was not available. There are clear benefits of weight loss for the reduction of strong mortality risk factors in older adults including increased peak VO2 [12] and walking speed [5,13,15], and reduced circulating IL-6 [5,6], blood pressure [8], and glucose levels [10]. Uncertainty exists, however, over what length of time the effects of weight loss on mortality might manifest themselves. Results from the Swedish Obesity Study [22]and the Look AHEAD trial [39] suggest mortality benefit only appears after four to five years of follow-up. However, data from the 18-month ADAPT trial showed apparent benefit over the entire course of the post-trial follow-up [33]. We excluded studies lasting fewer than 18 months because deaths occurring within a short-time after randomization are more likely due to pathological processes active at randomization rather than the intervention itself. Only three trials (HCP [27], WOMAN [37], and Look AHEAD [39]) were designed with an intervention length greater than 36 months, making it difficult to reach a conclusion with respect to intervention duration; however, restricting the meta-analysis to the six studies with at least four years of follow-up time gave similar results as the overall analysis. Lastly, several studies show that weight gain, especially in persons who are already obese, is a strong risk factor for mortality [42–44]. Thus, the protective effect of weight loss on mortality may relate to interrupting this trajectory.
This meta-analysis has several limitations. First, as with all meta-analyses, our results depend on the quality and consistency of data presented in the source documents. Data presentation styles were inconsistent and affected by changing reporting practices over time. For example, BMI was unreported in four of the earliest trials and weight loss targets/end of trial weights were reported as absolute amounts or percentages, or were unreported in several trials. Second, we were not able to include five trials which did not consider (or report) deaths by intervention arm. These trials tended to be smaller, in relatively younger populations, and of short-duration; thus, the impact of these missing trials on the overall effect measure is likely to be small. Third, our inclusion criteria were heterogeneous with trials targeting persons with hypertension, diabetes or osteoarthritis. Data are too sparse to conclude that the benefits of weight loss relate to any specific baseline condition. No cause of death information was identified, so we cannot comment on whether the observed mortality benefit is due to the reduction of specific causes. Fourth, in many trials, persons in the non-weight loss arms received active interventions, including in some cases pharmacotherapy or exercise training. It is possible that these interventions may have had an effect on mortality in the comparison groups and whether this would tend to magnify or diminish the group mortality differences is unclear. Additionally, the vast majority of weight loss arms coupled caloric restriction with an additional therapy (i.e. sodium restriction, exercise training). Thus, it is reasonable to speculate whether the observed mortality benefit of “weight loss” is attributable to weight loss alone. Although limited data exist to answer this question, results from the ADAPT study [33], where participants were randomized to weight loss and long-term exercise, alone or in combination, attribute the observed mortality benefit to weight loss (rather than exercise). Fifth, the degree to which different health behaviors adopted during the active intervention phase may have been maintained after the end of the trial is unclear, and there are no data on the extent to which weight-related mortality risk factors may have changed after the conclusion of any of the trials. Lastly, because our study selection criteria deliberately excluded trials in which randomized groups received differing degrees of weight loss, we cannot comment on a weight loss/mortality dose-response. Although, when trials were restricted to those reporting the greatest weight reductions (i.e. >5 kg), results differed little from the overall effect estimate.
Conclusion
In conclusion, this meta-analysis of 15 randomized controlled trials of weight loss in obese and overweight adults shows a 15% reduction in all-cause mortality in those randomized to weight loss. The magnitude of this benefit is on par with the reductions in all-cause mortality risk seen with treating hypertension or reducing total serum cholesterol by 1 mmol/L [45,46]. Most of the relevant literature in this area pertains to middle-aged adults. Given the increasing prevalence of obesity in older adults and its impact on physical function and chronic disease, additional evidence from well-conducted trials in older adults is needed to clarify the long-term safety of intentional weight loss in this population [47].
Supporting Information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Wake Forest Older Americans Independence Center (P30 AG021332) and by R01 AG033087. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000; 160: 898–904. [DOI] [PubMed] [Google Scholar]
- 2. Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010; 11: 671–685. 10.1111/j.1467-789X.2009.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc. 2009; 109: 1886–1895. 10.1016/j.jada.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 4. Volpato S, Guralnik JM, Ferrucci L, Balfour J, Chaves P, Fried LP, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation. 2001; 103: 947–953. [DOI] [PubMed] [Google Scholar]
- 5. Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013; 310: 1263–1273. 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beavers KM, Ambrosius WT, Nicklas BJ, Rejeski WJ. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J Am Geriatr Soc. 2013; 61: 1089–1094. 10.1111/jgs.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National High Blood Pressure Education Program Working Group report on primary prevention of hypertension. Arch Intern Med. 1993; 153: 186–208. [PubMed] [Google Scholar]
- 8. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998; 279: 839–846. [DOI] [PubMed] [Google Scholar]
- 9. Bano G. Glucose homeostasis, obesity and diabetes. Best Pract Res Clin Obstet Gynaecol. 2013; 27: 715–726. 10.1016/j.bpobgyn.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 10. Espeland MA, Rejeski WJ, West DS, Bray GA, Clark JM, Peters AL, et al. Intensive weight loss intervention in older individuals: results from the Action for Health in Diabetes Type 2 diabetes mellitus trial. J Am Geriatr Soc. 2013; 61: 912–922. 10.1111/jgs.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011; 305: 50–58. 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011; 364: 1218–1229. 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004; 50: 1501–1510. [DOI] [PubMed] [Google Scholar]
- 14. Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2004; 24: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rejeski WJ, Brubaker PH, Goff DC Jr, Bearon LB, McClelland JW, Perri MG, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011; 171: 880–886. 10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorensen TI. Weight loss causes increased mortality: pros. Obes Rev. 2003; 4: 3–7. [DOI] [PubMed] [Google Scholar]
- 17. Sorensen TI, Rissanen A, Korkeila M, Kaprio J. Intention to lose weight, weight changes, and 18-y mortality in overweight individuals without co-morbidities. PLoS Med. 2005; 2: e171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knudtson MD, Klein BE, Klein R, Shankar A. Associations with weight loss and subsequent mortality risk. Ann Epidemiol. 2005; 15: 483–491. [DOI] [PubMed] [Google Scholar]
- 19. Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008; 9: 302–312. 10.1016/j.jamda.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 20. Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009; 22: 93–108. 10.1017/S0954422409990035 [DOI] [PubMed] [Google Scholar]
- 21. Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007; 8: 503–513. [DOI] [PubMed] [Google Scholar]
- 22. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007; 357: 741–752. [DOI] [PubMed] [Google Scholar]
- 23. Greenland S, Robins JM. Estimators of the Mantel-Haenszel Variance Consistent in Both Sparse Data and Large-Strata Limiting Models. Biometrics. 2985; 42: 311–323. [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 25. Bradburn MJ, Deeks JJ, Berlin JA, Localio AR. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Statistics in Medicine. 2007; 26: 53–77. [DOI] [PubMed] [Google Scholar]
- 26. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine. 2004; 23: 1351–1375. [DOI] [PubMed] [Google Scholar]
- 27. Stamler R, Stamler J, Grimm R, Gosch FC, Elmer P, Dyer A, et al. Nutritional therapy for high blood pressure. Final report of a four-year randomized controlled trial—the Hypertension Control Program. JAMA. 1987; 257: 1484–1491. [DOI] [PubMed] [Google Scholar]
- 28. Davis BR, Blaufox MD, Oberman A, Wassertheil-Smoller S, Zimbaldi N, Cutler JA, et al. Reduction in long-term antihypertensive medication requirements. Effects of weight reduction by dietary intervention in overweight persons with mild hypertension. Arch Intern Med. 1993; 153: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 29. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992; 267: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 30. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997; 157: 657–667. [PubMed] [Google Scholar]
- 31. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson SS, Paiva AL, Cummins CO, Johnson JL, Dyment SJ, Wright JA, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med. 2008; 46: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shea MK, Houston DK, Nicklas BJ, Messier SP, Davis CC, Miller ME, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci. 2010; 65: 519–525. 10.1093/gerona/glp217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryan DH, Johnson WD, Myers VH, Prather TL, McGlone MM, Rood J, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med. 2010; 170: 146–154. 10.1001/archinternmed.2009.508 [DOI] [PubMed] [Google Scholar]
- 35. Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity reduction black intervention trial (ORBIT): 18-month results. Obesity (SilverSpring). 2010; 18: 2317–2325. 10.1038/oby.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shea MK, Nicklas BJ, Houston DK, Miller ME, Davis CC, Kitzman DW, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr. 2011; 94: 839–846. 10.3945/ajcn.110.006379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gabriel KK, Conroy MB, Schmid KK, Storti KL, High RR, Underwood DA, et al. The impact of weight and fat mass loss and increased physical activity on physical function in overweight, postmenopausal women: results from the Women on the Move Through Activity and Nutrition study. Menopause. 2011; 18: 759–765. 10.1097/gme.0b013e31820acdcc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Wier MF, Dekkers JC, Hendriksen IJ, Heymans MW, Ariens GA, Pronk NP, et al. Effectiveness of phone and e-mail lifestyle counseling for long term weight control among overweight employees. J Occup Environ Med. 2011; 53: 680–686. 10.1097/JOM.0b013e31821f2bbb [DOI] [PubMed] [Google Scholar]
- 39. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013; 369: 145–154. 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013; 368: 1594–1602. 10.1056/NEJMoa1214530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond). 2007; 31: 743–750. [DOI] [PubMed] [Google Scholar]
- 42. Zheng H, Tumin D, Qian Z. Obesity and mortality risk: new findings from body mass index trajectories. Am J Epidemiol. 2013; 178: 1591–1599. 10.1093/aje/kwt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klenk J, Rapp K, Ulmer H, Concin H, Nagel G.Changes of body mass index in relation to mortality: results of a cohort of 42,099 adults. PLoS One. 2014; 9: e84817 10.1371/journal.pone.0084817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Somes GW, Kritchevsky SB, Shorr RI, Pahor M, Applegate WB. Body mass index, weight change, and death in older adults: the systolic hypertension in the elderly program. Am J Epidemiol. 2002; 156: 132–138. [DOI] [PubMed] [Google Scholar]
- 45. Gould AL, Davies GM, Alemao E, Yin DD, Cook JR. Cholesterol reduction yields clinical benefits: meta-analysis including recent trials. Clin Ther. 2007; 29: 778–794. [DOI] [PubMed] [Google Scholar]
- 46. Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005; 165: 1410–1419. [DOI] [PubMed] [Google Scholar]
- 47. Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, et al. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society Published by The Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014; 22 Suppl 2: S5–S39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.