Abstract
The present study sought novel changes to the hamster testicular transcriptome during modulation of fertility by well-characterized photoperiodic stimuli. Transition from long days (LD, 14 h light/day) to short days (SD, 10 h light/day) triggered testicular regression (61% reduction of testis weight, relative to LD) in SD-sensitive (SD-S) hamsters within 16 weeks. After 22 weeks of SD exposure, a third cohort of hamsters became SD-refractory (SD-R), and exhibited testicular recrudescence (137% testis weight gain, relative to SD-S). Partial interrogation of the testicular transcriptome by annealing-control-primer-modified differential display PCR provided several candidates for regulation of testicular functions. Multiple linear regression modeling indicated the best correlation for aquaporin 11 (Aqp11) with changes in testis weight. Correlations were also strongest for Aqp11 with expression levels of reference cDNAs that control spermatogenesis (Hspa2 and Tnp2), steroidogenesis (Cox2, 3βHsd, and Srebp2), sperm motility (Catsper1, Pgk2, and Tnp2), inflammation (Cox2), and apoptosis (Bax and Bcl2). Moreover, siRNA-mediated knockdown of testicular Aqp11 mRNA and protein reduced Hspa2 and Tnp2 mRNA levels, and it increased 3βHsd mRNA levels. It also reduced mRNA levels for Sept12, which is a testis-specific inducer of spermatogenesis. These results suggest a central role for testicular Aqp11 signaling in the coordinate regulation of crucial components of fertility.
Keywords: photoperiod, RNA interference, short interfering RNA, differential display PCR, mathematical modeling
1. INTRODUCTION
Men are attempting to father children at increasingly older ages, but male fertility gradually declines with older age (Crosnoe and Kim, 2013). Although distal regulators of male reproductive physiology, such as gonadotropins, have been studied extensively, proximal regulators have been incompletely characterized. The understanding of gradual changes in male fertility at the testicular level can be facilitated through the study of testicular physiology in animal models. In particular, male Syrian hamsters provide a model for finding conserved mechanisms that underlie normal reversible reduction of testicular function by photoperiodism (Morales et al., 2007; Pastor et al., 2011; Crosnoe and Kim, 2013) and irreversible reduction of testicular function by aging (Pastor et al., 2011; Morales et al., 2007). By contrast, laboratory rats and mice, show relatively minor changes in testicular function during modulation of the photoperiod (Francisco et al., 2004; Yellon and Tran, 2002).
Time frames and treatment regimens for photoperiodic modulation of reproductive capacity are established in Syrian hamsters (Gaston and Menaker, 1967). Following transfer from long days (LD) consisting of at least 12.5 h of light per day, to short days (SD) consisting of less than 12.5 h of light per day, male hamsters undergo testicular regression, which is characterized by marked reductions in spermatogenesis, steroidogenesis, sperm motility, and testis weight (Butler et al., 2008; Morgan et al., 2003b; Morgan and Shannonhouse, unpublished data; Jin et al., 2002). Moreover, nocturnal secretion of melatonin from the pineal gland is crucial for mammalian photoperiodism (Goldman, 2001). Decreased testosterone secretion begins within three weeks of SD exposure, and atrophy begins within 6 weeks (Urbanski, 1990; Berndtson and Desjardins, 1974; Gaston and Menaker, 1967). After 20 weeks of SD exposure, refractoriness to SD results in spontaneous recrudescence of testicular morphology and function (Butler et al., 2008; Nelson and Zucker, 1987). During SD exposure, chronic elongation of the period of melatonin secretion from the pineal gland (Tamarkin et al., 1976) mediates SD-induced inhibition of hypothalamic gonadotropin-releasing hormone signaling, as well as pituitary gonadotropin and prolactin secretion (Tamarkin et al., 1976; Richardson et al., 1982), which precede testicular atrophy by one to three weeks. Although SD-induced changes at the hypothalamic or pituitary levels of the reproductive axis are documented, mechanisms underlying changes at the testicular level are poorly understood.
Differential display PCR (DD-PCR) is useful to assess changes in gene expression without prior knowledge of gene sequences (Liang and Pardee, 1992). Thus, it is useful for the hamster model whose genomes have not been sequenced completely. Although it is a potent gene discovery tool, the original DD-PCR approach is plagued by false-positive results (Liang and Pardee, 1992; Dilks et al., 2003). Annealing control primer (ACP) technology, however, has been found to markedly enhance PCR specificity, and it has been used to reduce false-positive results during the use of ACP-DD-PCR (Kim et al., 2004). The objective of the present study was to find novel changes in the testicular transcriptome that correlate with the photoperiodic modulation of reproductive capacity in Syrian hamsters, using ACP-DD-PCR.
2. MATERIALS AND METHODS
2.1. Animals and experimental design
Syrian golden hamsters (Mesocricetus auratus), HsdHan:AURA (Harlan, Indianapolis, IN) or Lak:LVG(SYR)BR (Charles River, Kingston, NY), were purchased at age 8 weeks. Hamsters were group-housed in long days (LD) (14 h light, 10 h dark, lights on at 0600h) at 22 ± 1 °C for 2 weeks. LabDiet 5001 (Purina, Richmond, IN) and water were provided ad libitum. At age 10 weeks, some hamsters were exposed to short days (SD) (10 h light: 14 h dark, lights on at 0800 h) for 16, 16, or 22 weeks, respectively. After euthanasia by decapitation, testes were excised and weighed. Procedures used in this study were approved by Institutional Animal Care and Use Committees.
2.2. RNA extraction
Testis total RNA was extracted using TriZol (Invitrogen, Carlsbad, CA) or a guanidinium isothiocyanate method, as we have described (Morgan, 2012; Morgan et al., 2003a). Samples were homogenized in buffered 4M guanidinium isothiocyanate and sodium lauroyl sarcosine. RNA was precipitated in 3M LiCl and centrifuged. Protein was removed by proteinase K digestion, phenol/chloroform extraction, and ethanol precipitation. DNA was digested with DNase I or extracted with acidic phenol.
2.3. Annealing Control Primer - Differential display PCR
The GeneFishing DEG Kit and instructions (SeeGene, Rockville, MD) were used. Reverse transcription was performed with MMLV reverse transcriptase and dT-ACP1 primer. PCR was performed in duplicate with SeeAmp ACP Master Mix and arbitrary primers. cDNA bands were visualized on 2% agarose gels.
2.4. Reverse transcription PCR
RT reactions were performed with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and either oligo dT12-18 or random hexamers, as we have described (Morgan, 2012; Morgan et al., 2003a). PCR was performed with ReadyMix Taq PCR Mix (Sigma, St. Louis, MO). Absence of genomic DNA contamination was determined by PCR performed on RT reactions with or without RT. PCR co-amplifications were divided into two stages. First, 12-μl pre-amplification reactions were performed with primers for lower abundance cDNA. Second, 10 μl of pre-amplification reaction were combined with 10 μl of reaction mix containing the other primer. Conditions and primer sequences are provided in Table 1.
Table 1.
PCR Pre-amplification Conditions
| cDNA | Primers | Ta (°C) | Preamp Cycles |
|---|---|---|---|
| A. Experimental cDNA Clones | |||
| Aqp11 | Fwd: ACTGGCTTGCTCCTTCTCTAGGT | 59.5 | 2 Gapdh |
| Rev: TTGAAACAGTGACGAGTCCAGTGC | 1 Actb | ||
| At7l1B | Fwd: ATCCCGTTAACAGCACCACCTCT | 59.5 | 1 Gapdh |
| Rev: AAAGGAACGAAGGAACTCACAGCGTC | 4 Actb | ||
| Corolc | Fwd: ACAGGGAYCTCAAGGTNGTCAAGAAGA | 59.5 | 8 Actb |
| Rev: AAGAGTGGATCTGGGAGCAGTTCA | |||
| Dusp3 | Fwd: GTGCARGATCTCAACGACCTGCT | 59.5 | 5 Gapdh |
| Rev: CATAAGCTCCCTAAGGGTGGAGTGA | |||
| Jlsl | No primers | N/A | |
| Niam | Fwd: ATGCARCTATGAGCCTTGAAGCCTT | 59.5 | 2 Gapdh |
| Rev: AAGCTTATCCCAGGGTACACTGTCA | |||
| Undl | N/A | N/A | |
| Und2 | N/A | N/A | N/A |
| Zfp639 | Fwd: TGTGCAGTTCTCCTCAAGCAGTGA | 59.5 | 3 Gapdh |
| Rev: TTCCCAAACCTCATCAGGCAGTCA | |||
| B. Reference cDNA Clones | |||
| Bax | Fwd: GCTGCAGAGGATGATTGCTA | 55.0 | 7 Actb |
| Rev: GATGGTGAGTGAGGCAGTGA | |||
| Bcl-2 | Fwd: CCTTCCAGCTTGAGAGCAAC | 56.0 | 8 Actb |
| Rev: CAGATGCCGGTTCAGGTACT | |||
| Catsper1 | Fwd: ATGGTCATGGCAGTGCTGGACTT | 59.0 | 8 Actb |
| Rev: CCAGGTTGAGGAAGATGAAGTACTGGAT | |||
| Cox1 | Fwd: CAACTCCATCTTTGGGGAGA | 54.0 | 12 Actb |
| Rev: AACATACGGGCAGGTCTTTG | |||
| Cox2 | Fwd: CAACTCCCTTGGGTGTGAAAGGAA | 59.0 | 15 Actb |
| Rev: TGAGTGTCATTGACTGTGGGAGGA | |||
| 3P-Hsd | Fwd: CCTGAAAAATGGTGGCACTT | 54.0 | 10 Actb |
| Rev: TGTGACCAAGTATCGGGTGA | |||
| Hspa2 | Fwd: CCATGGTCCTCACTAAGATGAAGGAGAT | 59.0 | 3 Actb |
| Rev: GTTGGGCCCAATGTCCTTCTTGT | |||
| Pgk2 | Fwd: GCTATCCTTGGTGGAGCCAAAGT | 59.0 | 3 Actb |
| Rev: GTTCCTTTAGCAAAGGCATCCCATTCA | |||
| Sept12 | Fwd: TGGTGAACACGCTGTTCAAGTCCAA | 59.0 | 2 Actb |
| Rev: CTCAGGATAGGGTCCCAGCACTT | |||
| Tnp2 | Fwd: AGACCTTTGAAGGGAAAGTGAGCAAGA | 59.0 | 1 Actb |
| Rev: ATTGCTGCAGTGACATGTTCCTGT | |||
| C. Housekeeping cDNA Clones | |||
| Actb | Fwd: TCGTACCACAGGCATTGTGATGGA | N/A | N/A |
| Rev: ACTCCTGCTTGCTGATCCACATCT | |||
| Gapdh (1) | Fwd: TCACCATCTTCCAGGAGCGAGAYCC | N/A | N/A |
| Rev: CCTGCTTCACCACCTTCTTGATGT | |||
| Gapdh (2) | Fwd: TCCTGCACCACCAYCTGCTTAG | N/A | N/A |
| Rev: CCTGCTTCACCACCTTCTTGATGT | |||
Preamp cycles: # of PCR cycles performed on target before adding primers for internal standard. Gapdh and β-actin were pre-amplified before adding Aqp11 primers, due to aquaporin 11 abundance in testis.
2.5. cDNA cloning and sequencing
Differentially represented bands from the ACP-DDPCR screening were excised from agarose gel and they, along with reference cDNAs generated by RT-PCR, were cloned using the TOPO-TA Cloning Kit (Invitrogen) according to the manufacturer's instructions. Plasmid containing the screened and reference cDNAs were prepared using the Wizard SV System (Promega, Madison, WI), and sequenced at the Gene Technology Laboratory facility (Texas A&M University). Information on rat orthologs of candidate hamster cDNA clones are provided in Table 2.
Table 2.
Rat Orthologs of Candidate Hamster cDNA Clones
| Hamster | Rat | Size (bp) | Accession # |
|---|---|---|---|
| cDNA1 | Aquaporin-11 (Aqp11) | 476 | NM_173105 |
| cDNA2 | Ataxin-7-like protein 1 (At7l1) | 414 | XM_234056 |
| cDNA3 | Coronin actin-binding protein 1c | 233 | NM_001109327 |
| cDNA4 | Dual specificity phosphatase (Dusp3) | 135 | NM_028207 |
| cDNA5 | Nuclear interactor of Arf and Mdm2 (Niam) | 151 | NM 001009344 |
| cDNA6 | Unique Sequence (Jls1) | 218 | N/A |
| cDNA7 | Undetermined 1 (Und1) | N/A | N/A |
| cDNA8 | Undetermined 2 (Und2) | N/A | N/A |
| cDNA9 | Zinc finger protein-639 (Zfp639) | 752 | NM_001080907 |
Dusp3 and Niam were identified by relaxing BLAST search parameters, and their amino acid sequences were not deduced, as their clones fell outside the coding regions. Two attempts to clone Und1 and Und2 cDNA were unsuccessful.
2.6. RNA interference
Aqp11 template cDNA (204 bp) was prepared by ligating T7 RNA Polymerase promoters onto amplicon termini with T4 DNA Ligase. The polymerase promoter sequence is: 5’- TAATACGACTCACTATAGGGAGAY-3’. Complementary sense and antisense RNAs (cRNAs) were transcribed in vitro using T7 RNA Polymerase, as we have described (Morgan, 2012; Morgan et al., 2003a). These cRNAs were incubated in hybridization buffer (20 mM HEPES at pH 7.9 and 0.1 M NaCl) in a thermal cycler at 50 °C, 94 °C, and stepping down 2 °C per cycle to reach 60 °C. Aqp11 dsRNA was purified from agarose gel and digested with ShortCut RNase III into a heterogeneous mix of short interfering RNAs of 18–25 bp (siRNA), according to manufacturer's instructions. Hamsters at ages 16-18 weeks, under light ether anesthesia (Jana et al., 2002), received intra-testicular injections: unilateral, Aqp11 siRNA (1 μg); and contralateral, vehicle (0.9% saline). After 72 h, subjects were sacrificed by CO2 asphyxiation, decapitated, and testes were excised and stored at −80 °C. Testes were cut with RNase-free razor blades into 3-mm sections in an acrylic matrix (Stoelting, Wood Dale, IL) on dry ice. Samples were punched from frozen sections with a 1-mm micropunch for RNA extraction. The siRNA reagents were purchased from New England Biolabs (Ipswich, MA).
2.7. Western blotting
Lysates were prepared by homogenizing frozen testis samples in lysis buffer (50 mM HEPES, pH 7.4, 1% Triton X-100, 50 mM sodium pyrophosphate, 0.1 M sodium fluoride, 10 mM EDTA, 10 mM sodium orthovanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mM benzamidine, and 2 mM PMSF). AQP11 (1:500 dilution) was analyzed by Western blot, as we have described (Wu et al., 2005), using AQP11 antiserum Alpha Diagnostics (San Antonio, TX).
2.8. Statistical analyses
Either repeated measures one-way ANOVA and Student-Newman-Keuls posthoc test or Student's t-test was used to determine statistical differences between means (± SEM). Paired Student's t-test was used to determine statistical differences by Western analysis. If the normality test failed, Kruskal-Wallis One Way ANOVA on Ranks was performed. Linear and multiple linear regressions were used to determine the relationship between testis weight and gene expression. Statistical analyses were performed using InStat 3.0 (GraphPad, San Diego, CA). Multiple linear regression with a forward variable selection algorithm was performed using SAS-Enterprise Guide 4.3 with 0.05 significance level for entry (SAS Institute Inc., Cary, NC).
3. RESULTS
3.1. Putative Photoperiodic Regulation of Testicular cDNA Expression
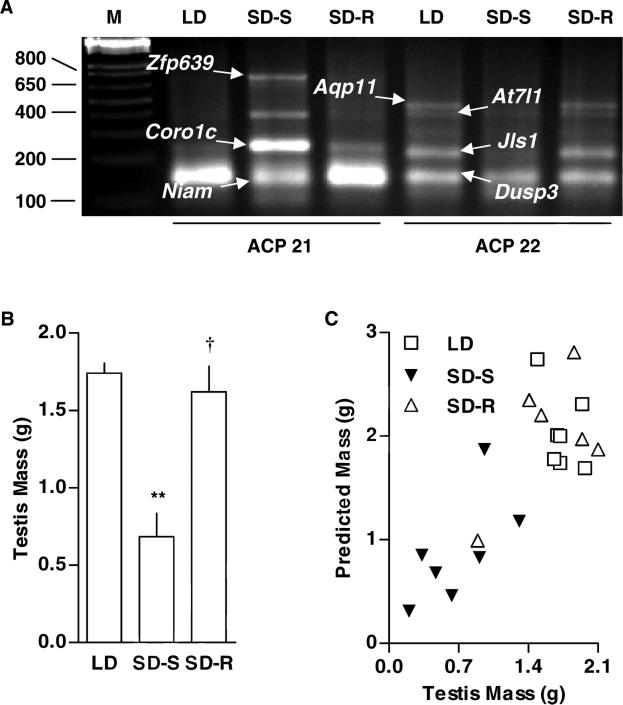
Hamsters were exposed to 16 weeks of long days (LD), 16 weeks of short days during the photosensitive period (SD-S), or 22 weeks of short days into the photorefractory period (SD-R). After screening duplicate cDNA samples prepared from the testis of hamsters sequentially subjected to LD, SD-S, or SD-R with two annealing control primers (ACPs) that were chosen randomly, nine candidate bands were selected for cloning and sequencing because they were differentially represented on agarose gels (Fig. 1A). Each ACP consists of a: (a) 3′-end with an annealing nucleotide sequence complementary to a site on template cDNA; (b) 5′-end with an arbitrary sequence; and (c) central regulator that is comprised of at least one universal base that regulates the annealing portion in association with annealing temperature. Information on the hamster candidate and reference cDNA clones are provided in Tables 3 and 4, respectively. Seven of the nine cDNA fragments were cloned successfully. Upon sequencing and BLAST searching the NCBI GenBank database, six candidates were determined to contain aquaporin-11 (Aqp11), ataxin-7-like protein 1 (At7l1), coronin actin-binding protein-1c (Coro1c), dual specificity phosphatase-3 (Dusp3), nuclear interactor of Arf and Mdm2 (Niam), and zinc finger protein-639 (Zfp639). A seventh band containing a sequence with an undetermined identity (Jls1) was AT-rich, and therefore provided only poor targets for PCR primers. The two remaining cDNA candidates were ~400 bp and less than 100 bp, respectively, and repeated attempts to clone them were unsuccessful.
Figure 1. Photoperiodic regulation of testicular expression and functionality.
(A) Differential display PCR was used to assess changes in testicular transcript levels of hamsters exposed to long days (LD: 14 h light and 10 h dark for 16 weeks, n = 7), short days during the photosensitive period (SD-S: 10 h light and 14 h dark for 16 weeks, n = 7), or short days during the photorefractory period (SD-R: 10 h light and 14 h dark for 22 weeks, n = 6).Differentially represented cDNAs were extracted from the agarose gel, cloned into the TOPO-TA cloning vector, and sequenced. Among the genes whose roles in testicular function have not been established, we identified aquaporin-11 (Aqp11), ataxin-7-like protein-1 (At7l1), coronin actin-binding protein-1c, dual specificity phosphatase-3 (Dusp3), nuclear interactor of Arf and Mdm2 (Niam), a cDNA of undetermined identity (Jls1), and zinc finger protein-639 (Zfp639). (B) Relative to LD exposure, SD-S exposure decreased testis weight by 61%. Relative to SD-S exposure, SD-R exposure increased testis weight by 140%. Means (± SEM) are shown. For LD vs SD-S comparison, **p < 0.01. For SD-S vs SD-R comparison, †p < 0.01. (C) Multivariate regression analysis, across the three photoperiodic treatments, revealed that Aqp11 gene expression had the highest correlation with testis weight.
Table 3.
ACP-DD-PCR Analysis of Candidate Hamster cDNA Clones
| cDNA | Size (bp) | Change | Accession # |
|---|---|---|---|
| Aquaporin-11 (Aqp11) | 393 | ↓in SD-S | GQ254789.1 |
| Ataxin-7-like protein-1 (At7l1) | 331 | ↓in SD-S | GQ254792.1 |
| Coronin actin-binding protein-1c (Coro1c) | 152 | ↑in SD-S | GQ254791.1 |
| Dual specificity phosphatase-3 (Dusp3) | 640 | ↓in SD-S | GU170206.1 |
| Unknown Sequence (Jls1) | 218 | ↓in SD-S | TBD |
| Nuclear interactor of Arf and Mdm2 (Niam) | 323 | ↓in SD-S | GU170205.1 |
| Undetermined 1 (Und1) | N/A | N/A | N/A |
| Undetermined 1 (Und1) | N/A | N/A | N/A |
| Zinc Finger protein-639 (Zfp639) | 639 | ↑in SD-S | GQ254790.1 |
Testicular mRNA levels were assessed by differential display PCR for hamsters (n = 2?) exposed to 16 weeks of long days (LD, 14L:10D), 16 weeks of short days during photosensitivity (SD-S, 10L:14D), or 22 weeks of SD during photorefractoriness (SD-R, 10L:14D).
Table 4.
Hamster Reference cDNA Clones
| cDNA | Known Functions | Results |
|---|---|---|
| Aquaporin-11 (Aqp11) | Renal function | ↓in SD-S, vs LD & SD-R |
| Ataxin-7-like protein-1 (At7l1) | SAGA histone acetylation | ↓in SD-S, vs LD & SD-R |
| Coronin actin-binding protein-1c (Coro1c) | Wound healing, cytoskeleton in cellular projections | No detectable change |
| Dual specificity phosphatase-3 (Dusp3) | Jak/Stat pathway, cell cycle control | ↓in SD-S, vs LD & SD-R |
| Unknown Sequence (Jls1) | N/A | N/A |
| Nuclear interactor of Arf & Mdm2 (Niam) | Cell cycle, chromosome stability | ↓in SD-S, vs LD & SD-R |
| Undetermined 1 (Und1) | N/A | N/A |
| Undetermined 1 (Und1) | N/A | N/A |
| Zinc Finger protein-639 (Zfp639) | CRE binding | No detectable change |
| Bcl2-associated protein (Bax) | Pro-apoptosis | ↓in SD-R, vs SD-S |
| B-cell lymphoma-2 (Bcl2) | Anti-apoptosis | ↓in SD-R, vs SD-S |
| Cation channel sperm-associated protein-1 (Catsper1) | Sperm motility, spermatid-specific | ↓in SD-S, vs LD & SD-R |
| Cyclooxygenase-2 (Cox2) | Prostaglandin synthesis, inflammation, steroidogenesis | ↓in SD-S & SD-R, vs LD |
| Heat-shock-related protein-2 (Hspa2) | Spermatid-specific spermatogenesis | ↓in SD-S & SD-R, vs LD |
| 3Beta-hydroxysteroid dehydoxylase (3β-Hsd) | Steroidogenesis | ↓in SD-S & SD-R, vs LD |
| Phosphoglycerate kinase 2 (Pgk2) | Spermatid-specific motility, spermatogenesis | ↓in SD-S, vs LD & SD-R |
| Sterol regulatory element binding protein-2 (Srepb2) | Steroidogenesis | ↓in SD-S, vs LD & SD-R |
| Transition protein-2 (Tnp2) | Spermatid-specific motility, DNA condensation, spermatogenesis | ↓in SD-S, vs LD & SD-R |
Testis mRNA levels for hamsters (n = 5-7) exposed to 16 weeks of long days (LD, 14L:10D), 16 weeks of short days during photosensitivity (SD-S, 10L:14D), or 22 weeks of SD during photorefractoriness (SD-R, 10L:14D) were assessed by RT-PCR.
*p < 0.050
**p < 0.010
Relative to LD, SD-S apparently reduced Aqp11 mRNA levels 77%, and SD-R increased them 293%. SD-S and SD-R apparently decreased and increased At7l1 mRNA levels 63% and 113%, respectively (Fig. 1A). SD-S and SD-R apparently decreased and increased Dusp3 mRNA levels 23% and 18%, respectively (Fig. 1A). SD-S and SD-R apparently decreased and increased and Niam mRNA levels 37% and 2%, respectively (Fig. 1A). SD-S and SD-R apparently decreased Jls1 mRNA levels by 68% and 202%, respectively (Fig. 1A). By contrast, Coro1c and Zfp639 mRNA levels appeared to go in the opposite directions of those of the other cDNAs (Fig. 1A). SD-S and SD-R apparently increased and decreased Coro1c mRNA levels by 277% and 72%, respectively (Fig. 1A). Zfp639 mRNA was barely detectable in LD and SD-R and poorly quantifiable, but apparently changes in SD-S and SD-R samples were well in excess of 100% (Fig. 1A).
As expected, relative to LD, SD-S reduced testis weight 61%, and relative to SD-S, SD-R increased testis weight 137% (Fig. 1B). Linear regression analysis of the differential representation of mRNAs, determined after agarose gel electrophoresis, revealed the highest correlation for Aqp11 with testis weight (r2=0.68, p < 0.01) across all three photoperiodic treatment groups (Fig. 1C). The correlations for cDNAs, ranking from highest to lowest, were Dusp3 (r2=0.52, p < 0.01), Niam (r2=0.45, p < 0.01), Zfp639 (r2=0.34, p < 0.05) and At7l1 (r2=0.14, p=0.170), and Coro1c (r2=0.06, p=0.910).
3.2. Verification of Photoperiodic Regulation of Testicular cDNA Expression
Candidate mRNA levels were assessed by relative semi-quantitative RT-PCR using beta-actin (Actb) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as an internal standard. Linear regression analyses for Aqp11 and Dusp3, normalized to Actb or Gapdh, yielded similar results (Aqp11, r2=0.75 and Dusp3, r2=0.97). The internal standard was, therefore, selected to provide the best agarose gel resolution of amplicons for targets that were co-amplified with internal standards. Robust differences were found in expression of cDNAs isolated by ACP-DDPCR using 5 LD, 5 SD-S, and 4 SD-R samples, but the differences were smaller when reference mRNAs levels were assessed. Sample sizes were, therefore, increased to 7 LD, 7 SD-S and 6 SD-R in subsequent analyses. Tables 5A and 5B contain one-way ANOVA of candidate and reference mRNA levels, respectively, from the three treatment groups. Results of linear regression analyses of testis weight and candidate mRNA levels from these treatment groups are described in Table 6.
Table 5.
Photoperiodic Regulation of Testicular mRNA Levels
| A. cDNA Clones from ACP-DD-PCR Screen | ||||||
|---|---|---|---|---|---|---|
| cDNA | LD | SD-S | SD-R | Samples | F-statistic | p-value |
| Aqp11 | 0.53 ± 0.05 | 0.25 ± 0.05** | 0.45 ± 0.05† | n = 6-7 | F2,17 = 8.64 | 0.003 |
| At7l1 | 1.46 ± 0.29 | 0.61 ± 0.11** | 1.28 ± 0.23†† | n = 5 | F2,12 = 4.04 | 0.046 |
| Coro1c | 1.13 ± 0.10 | 1.11 ± 0.17 | 0.99 ± 0.13 | n = 5 | F2,12 = 0.31 | 0.740 |
| Dusp3 | 1.92 ± 0.18 | 0.71 ± 0.19** | 1.25 ± 0.10 | n = 6-7 | F2,17 = 13.38 | 0.001 |
| Jls1 | N/D | N/D | N/D | n = 5 | N/D | N/D |
| Niam | 1.04 ± 0.07 | 0.66 ± 0.12** | 1.01 ± 0.10† | n = 6-7 | F2,17 = 4.69 | 0.024 |
| Und1 | N/D | N/D | N/D | n = 5 | N/D | N/D |
| Und2 | N/D | N/D | N/D | n = 5 | N/D | N/D |
| Zfp639 | 0.51 ± 0.05 | 0.51 ± 0.05 | 0.55 ± 0.08 | n = 5 | F2,12 = 0.14 | 0.871 |
| B. Reference cDNA Clones | ||||||
|---|---|---|---|---|---|---|
| cDNA | LD | SD-S | SD-R | Samples | F-statistic | p-value |
| Bax | 1.42 ± 0.15 | 1.66 ± 0.09 | 1.15 ± 0.12† | n = 5 | F2,12 = 4.34 | 0.038 |
| Bcl2 | 0.82 ± 0.20 | 0.89 ± 0.07 | 0.61 ± 0.13† | n = 5 | F2,12 = 1.03 | 0.386 |
| Catsper1 | 1.26 ± 0.17 | 0.42 ± 0.15* | 1.58 ± 0.27†† | n = 6-7 | F2,17 = 9.33 | 0.002 |
| Cox2 | 0.41 ± 0.12 | 0.67 ± 0.15 | 0.63 ± 0.08 | n = 5 | F2,12 = 1.36 | 0.294 |
| 3βHsd | 0.59 ± 0.06 | 0.24 ± 0.05** | 0.36 ± 0.05* | n = 5 | F2,12 = 11.04 | 0.002 |
| Hspa2 | 0.70 ± 0.09 | 0.44 ± 0.07 | 0.43 ± 0.14 | n = 6-7 | F2,17 = 2.39 | 0.122 |
| Pgk2 | 1.14 ± 0.13 | 0.43 ±0.16** | 0.99 ± 0.07†† | n = 6-7 | F2,17 = 8.54 | 0.003 |
| Srepb2 | 0.61 ± 0.07 | 0.35 ± 0.06* | 0.64 ± 0.06† | n = 6-7 | F2,17 = 6.29 | 0.009 |
| Tnp2 | 0.52 ± 0.11 | 0.17 ± 0.10* | 0.50 ± 0.07† | n = 6-7 | F2,17 = 4.19 | 0.033 |
Testis mRNA levels were assessed by RT-PCR for hamsters (n = 5-7) exposed to 16 weeks of long days (LD, 14L:10D), 16 weeks of short days during photosensitivity (SD-S, 10L:14D), or 22 weeks of SD during photorefractoriness (SD-R, 10L:14D). (A) cDNA clones screened by ACP-DD-PCR and normalized after Actb co-amplification. ANOVA for mRNA expression levels between groups (F-statistic) are shown. (B) Reference cDNA normalized after Actb co-amplification. Means (± SEM) are shown. For SD-S or SD-R vs LD
p < 0.050
p < 0.010.
For SD-S vs SD-R comparisons
p < 0.050
p < 0.010. N/D, not determined. Degrees of freedom (associated with F-statistics) represent treatments between groups (i.e., 2) and residuals within groups (i.e., 12 or 17).
Table 6.
Relationship Between Testis Mass and mRNA Levels
| cDNA | All, r2 | All, p | LD, r2 | LD, p | SD-S, r2 | SD-S, p | SD-R, r2 | SD-R, p |
|---|---|---|---|---|---|---|---|---|
| Aqp11 | 0.68** | 0.001 | 0.010 | 0.976 | 0.640** | 0.003 | 0.544 | 0.094 |
| At7l1 | 0.14 | 0.165 | 0.298 | 0.205 | 0.018 | 0.829 | 0.440* | 0.039 |
| Corolc | 0.01 | 0.908 | −0.090 | 0.508 | 0.150 | 0.211 | 0.297** | 0.009 |
| Dusp3 | 0.52** | 0.001 | 0.008 | 0.853 | 0.674* | 0.024 | 0.035 | 0.722 |
| Jls1 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Niam | 0.45** | 0.001 | 0.321 | 0.107 | 0.440 | 0.062 | −0.119 | 0.531 |
| Und1 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Und2 | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Zfp639 | 0.34* | 0.048 | 0.035 | 0.686 | 0.730** | 0.008 | 0.55 | 0.091 |
| Bax | 0.14 | 0.096 | 0.053 | 0.620 | 0.004 | 0.918 | 0.019 | 0.796 |
| Bcl2 | 0.01 | 0.718 | 0.102 | 0.485 | 0.311 | 0.329 | 0.673* | 0.046 |
| Catsper1 | 0.35** | 0.004 | 0.471 | 0.053 | −0.183 | 0.799 | −0.146 | 0.580 |
| Cox2 | 0.22* | 0.037 | 0.042 | 0.658 | 0.806** | 0.006 | 0.102 | 0.067 |
| 3βHsd | 0.18 | 0.067 | 0.008 | 0.846 | 0.018 | 0.775 | 0.159 | 0.434 |
| Hspa2 | 0.19* | 0.030 | −0.200 | 0.987 | 0.048 | 0.306 | 0.486 | 0.075 |
| Pgk2 | 0.44** | 0.001 | −0.107 | 0.545 | 0.476 | 0.052 | 0.529 | 0.062 |
| Srepb2 | 0.55** | 0.001 | 0.251 | 0.252 | 0.746** | 0.010 | 0.580 | 0.078 |
| Tnp2 | 0.32** | 0.006 | −0.012 | 0.380 | 0.397 | 0.077 | −0.234 | 0.829 |
Testicular mRNA levels were assessed by RT-PCR for hamsters (n = 5-7) exposed to long days (LD, 14L: 10D for 16 weeks), short days during photosensitivity (SD-S, 10L:14D for 16 weeks), or SD during photorefractoriness (SD-R, 10L:14D for 22 weeks). Linear regression analyses of mRNA levels versus testicular mass were performed over all three treatment groups and individual groups. The correlation coefficient (r2) and p values are shown. N/D, not determined.
p < 0.050
p < 0.010
3.3. RT-PCR Analysis of Reference cDNA Expression
In addition to candidate cDNA clones isolated by ACP-DD-PCR screening, mRNA levels were assessed for reference cDNA clones that influence various cell functions (Table 4) that were deemed to be important for fertility. Table 5B contains results from one-way ANOVA analyses of means and SEM for reference mRNA levels. Results of linear regression analyses for testis mass and reference mRNA levels from separate treatment groups are described on Table 6.
We used multiple linear regression modeling of testis weight and mRNA levels for Aqp11, Zfp639, At7l1, Dusp3, and Niam with forward stepwise selection. We set significance levels for admission into, retention in, and exclusion from the model at 0.05, 0.35, and 0.15, respectively. Values for Bax, Bcl2, 3βHsd, Cox2 and Srebp2 were forced into the model because their expression levels are known markers of testicular function. In other words, they were included in the model whether or not they predicted testis weight. The model admitted only Aqp11 and Zfp639 (F7,4=48.65, p=0.001, adjusted r2 =0.97). Only Aqp11 had a statistically significant relationship with testis mass in the model (F6,11= 9.57, p = 0.013, adjusted r2 = 0.8237).
3.4. Aqp11 mRNA Knockdown
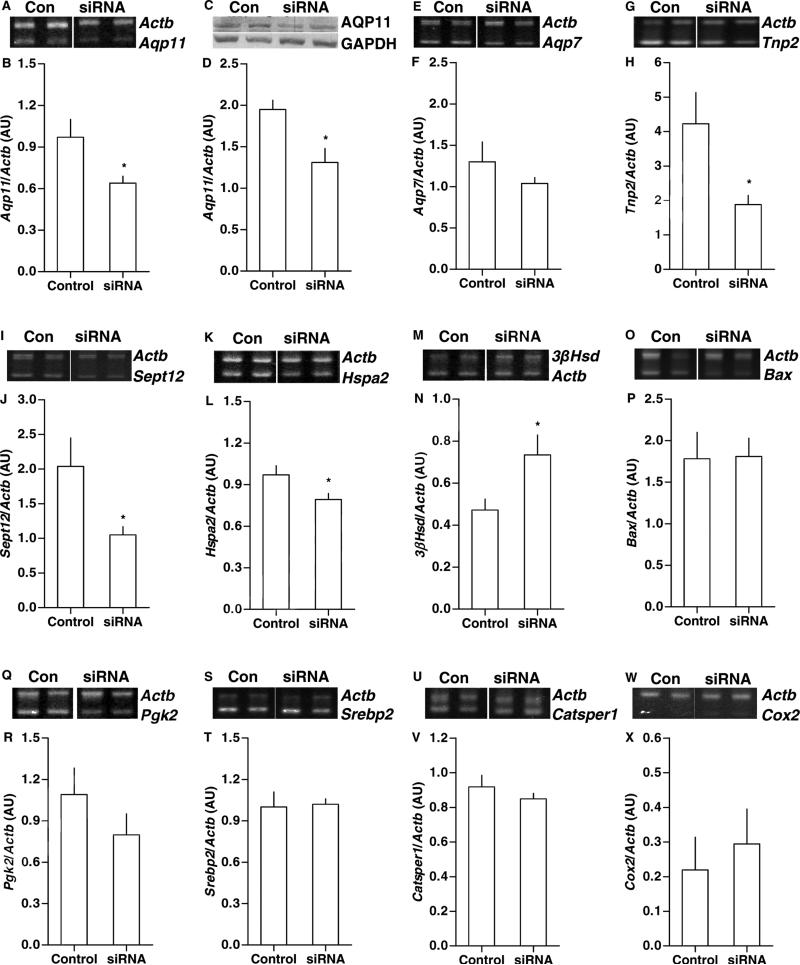
Hamsters received unilateral intra-testicular injections of Aqp11 small interfering RNA (siRNA), and contralateral injections of vehicle control. The results are described, relative to vehicle control. Aqp11 siRNA reduced Aqp11/Actb mRNA levels 34% (Fig. 2A-2B), and it reduced AQP11 protein levels 33% (Fig. 2C-2D). Candidate and reference mRNA levels were also assessed. Aqp7 mRNA levels, an index of off-target effects of Aqp11 siRNA, did not change (Fig. 2E-2F). Aqp7 has the highest homology to Aqp11 of aquaporin family members expressed in testis, and it is thought to govern spermatogenesis (Calamita et al., 2001a).
Figure 2. Aqp11 Knockdown effects on testicular expression.
Hamsters were injected with vehicle control (n = 8) or Aqp11 siRNA (n = 16), and assessed for experimental and reference mRNA or protein levels by RT-PCR (with beta-actin (Actb) co-amplification) or Western blot (normalized to GAPDH protein), respectively. Representative bands are shown for agarose or polyacrylamide gel analyses. (A) Aqp11 and Actb. (B) Aqp11/Actb mRNA levels decreased. (C) Western blot analysis of AQP11 and GAPDH. (D) AQP11/GAPDH protein levels decreased. (E) Aqp7 and Actb. (F) Aqp7/Actb mRNA levels did not change. (G) Tnp2 and Actb. (H) Tnp2/Actb mRNA levels decreased. (I) Sept12 and Actb. (J) Sept12/Actb mRNA levels decreased. (K) Hspa2 and Actb. (L) Hspa2/Actb mRNA levels decreased. (M) 3βHsd and Actb. (N) 3βHsd/Actb mRNA levels increased. (O) Bax and Actb. (P) Bax/Actb mRNA levels did not change. (Q) Pgk2 and Actb. (R) Pgk2/Actb mRNA levels did not change. (S) Srebp2 and Actb. (T) Srebp2/Actb mRNA levels did not change. (U) Catsper1 and Actb. (V) Catsper1/Actb mRNA levels did not change. (W) Cox2 and Actb. (X) Cox2/Actb mRNA levels did not change. Means ± SEM are shown in graphs. For siRNA vs control, *p < 0.05.
Given Aqp11 expression and potential importance in spermatids (Yeung, 2010; Yeung and Cooper, 2010), four spermatid-specific reference cDNAs were also included to evaluate Aqp11 siRNA. Human cation channel sperm associated protein (CATSPER1), in sperm flagellum, has been shown to be activated by elevated intracellular pH, and extracellular progesterone and prostaglandins (Lishko et al., 2011). Expression of human CATSPER1 and murine Catsper1 are required for sperm hyperactivation and fertility (Lishko et al., 2011; Carlson et al., 2003). Low expression levels of heat shock protein A2 (HSPA2) in human semen have been associated with infertility (Motiei et al., 2012) and previous studies suggest murine Hspa2 is required for meiosis and spermatogenesis (Zhu et al., 1997). Phosphoglycerate kinase 2 (Pgk2), which catalyzes an ATP-generating step in glycolysis and is expressed only during spermatogenesis, has been found to be essential for normal sperm motility and male fertility in mice (Danshina et al., 2010). Transition protein (Tnp2) is required for chromatin condensation and histone removal, and it helps maintain fertility in mice (Zhao et al., 2001; Shirley et al., 2004). Septin 12 (SEPT12) is testis-specific and crucial for differentiation of male germ cells, and SEPT12 mutations have been found in infertile men (Kuo et al., 2012).
Aqp11 siRNA reduced Tnp2/Actb mRNA levels 54% (Fig. 2G-2H), and there was a linear relationship for Tnp2 and Aqp11 mRNA levels across both treatment groups (r2=0.39, F1,22=15.67, p < 0.001) and in siRNA samples alone (r2=0.34, F1,14=8.77, p=0.010). Aqp11 siRNA reduced Sept12/Actb mRNA levels 49% (Fig. 2I-2J), and there was a linear relationship with Aqp11 mRNA levels across both treatment groups (r2=0.71, F1,22=57.85, p < 0.001), and with siRNA alone (r2=0.34, F1,14=114.57, p < 0.001). Aqp11 siRNA reduced Hspa2/Actb mRNA levels 18% (Fig. 2K-2L), and there was a linear relationship across treatment groups (r2=0.23, F1,22=7.83, p=0.010), but not in siRNA samples alone. Aqp11 siRNA increased 3βHsd/Actb mRNA levels 36% (Fig. 2M-2N), but there was no linear relationship for 3βHsd and Aqp11 mRNA levels across treatment groups or in siRNA samples alone. There were no effects of Aqp11 siRNA on Bax (Fig. 2O-2P), Bcl2 (data not shown), Pgk2 (Fig. 2Q-2R), Srebp2 (Fig. 2S-2T), Catsper1 (Fig. 2U-2V), or Cox2 (Fig. 2W-2X) mRNA levels, normalized to Actb.
Finally, because Aqp11 has been shown to be expressed in mouse and rat spermatids (Yeung and Cooper, 2010), expression levels of the four spermatid-specific reference genes were assessed for photoperiodic regulation (Table 5). The mRNA levels of Catsper1/Actb, Pgk2/Actb, and Tnp2/Actb, but not Hspa2/Actb were influenced by photoperiod. The mRNA levels for each of these four genes were lowest during SD-S exposure, and the levels for Hspa2/Actb did not differ between SD-S and SD-R.
5. DISCUSSION
In the course of identifying novel changes in the hamster testicular transcriptome that correlate with photoperiodic modulation of fertility, two main findings were produced. First, after a small set of cDNA candidates was identified by ACP-DD-PCR, multiple linear regression modeling indicated a high goodness-of-fit for changes in Aqp11 mRNA levels during exposure to three photoperiodic conditions. Semi-quantitative RT-PCR allowed for verification of the correlation between Aqp11 expression and testicular weight. Consequently, for the remainder of the study the focus was on the potential role of Aqp11 in testicular functions. Second, siRNA-mediated knockdown indicated the important role of Aqp11 in the tonic regulation of signals required for male fertility.
Photoperiodic manipulation has been established as a strategy for disrupting and restoring fertility in hamsters. Marked reductions in testis weight during SD-S exposure, and its recovery during SD-R exposure, have been shown previously to correlate with steroidogenesis, spermatogenesis, and sperm motility (Kawazu et al., 2003; Gaston and Menaker, 1967; Morgan et al., 2003b; Mayerhofer et al., 1989). Thus, it was expected that photoperiodic manipulation would induce near-global changes in the testicular transcriptome of hamsters. This prediction was supported by results presented herein. Specifically, even partial interrogation of the testicular transcriptome by ACP-DD-PCR allowed rapid identification of Aqp11 as a candidate to regulate testicular function. Thus, testicular regression and recrudescence in hamsters have continued to provide information to inform the study of reproductive capacity.
Multiple linear regression modeling showed that Aqp11 fit predetermined criteria for potentially regulating testis function. Although acute changes in Aqp11 expression are not proposed here to regulate testis weight, proximal factors, such as Aqp11, that mediate photoperiodic effects on genes that regulate steroidogenesis and sperm function, are likely to affect structural changes that attend to those effects. The cDNA candidates that were excluded by multiple linear regression modeling were At7l1, Coro1c, Dusp3, Niam, and Zfp639.
Aqp11 is expressed abundantly in testis and kidney (Yeung and Cooper, 2010; Gorelick et al., 2006). Although aquaporins transport water and solutes across cell membranes, Aqp11 has not been show to do so (Gorelick et al., 2006). Heterogeneous expression of aquaporins along the male reproductive tract suggests that they modulate local fluid balance (Pastor-Soler et al., 2001; Arrighi et al., 2010; Calamita et al., 2001a; Calamita et al., 2001b). In the testis, Aqp11 is expressed in elongated spermatids at the time of the shedding of residual bodies before spermiation (Yeung and Cooper, 2010). A reproductive function for Aqp11 has not been determined in Aqp11 knockout mice, as 85% have died of renal failure by postnatal day 60, with cysts forming in their renal cortices and large vacuoles of endoplasmic reticulum origin forming in proximal tubule cells (Morishita et al., 2005). Interestingly, Aqp11 transfected into CHO cells localized to endoplasmic reticulum (Morishita et al., 2004).
As might be expected, six of the reference cDNAs, Catsper1, 3βHsd, Hspa2, Pgk2, Srebp2, and Tnp2, were expressed at their lowest levels during SD-S. Given the extent to which reduction of spermatogenesis, steroidogenesis, and sperm motility are expected to associate with a major reduction of testis weight during SD-S (Butler et al., 2008; Morgan et al., 2003b; Morgan and Shannonhouse, unpublished data; Jin et al., 2002), the observed changes in the expression levels for these genes reflects a global loss of testicular function. Apoptosis occurs extensively during spermatogenesis in hamsters (Pastor et al., 2011). Reduction of both apoptosis-associated genes, Bax and Bcl2, during SD-R likely maintains constitutive and induced apoptosis within optimal ranges.
Aqp11 knockdown with intratesticular siRNA selectively altered gene expression. A heterogeneous siRNA mixture of Aqp11 cDNA fragments was used, as this general strategy has avoided shortcomings of testing a series of single siRNA sequences (Morlighem et al., 2007). In addition to that of Aqp11, expression levels of Hspa2, Sept12, and Tnp2 were reduced by Aqp11 siRNA. Importantly, roles for Hspa2, Sept12, and Tnp2 in promoting sperm motility and spermatogenesis is conserved in humans and rodents (Zhao et al., 2001; Adham et al., 2001; Motiei et al., 2012; Kuo et al., 2012; Lin et al., 2009; Dix et al., 1997). Interestingly, this same treatment increased expression of steroidogenic 3βHsd. Recent studies indicate androgens can reduce spermatogenesis, forming a basis for male contraception (Chen et al., 2005). At 72 h post-injection, treatment with Aqp11 siRNA did not alter testicular weight. A prolonged knockdown of Aqp11 expression might have an effect, as SD exposure requires a few weeks to reduce testicular weight (Morgan, 2012; Morgan et al., 2003a). Under the experimental conditions used in the present study, however, longer siRNA treatment increase the likelihood of inducing a confounding immune response with inflammation and cytotoxicity (Beyerle et al., 2011).
By contrast, expression levels of other genes that influence sperm motility (Catsper1 and Pgk2) and steroidogenesis (Srebp2) were unaltered by Aqp11 siRNA. Furthermore, testicular Aqp11 siRNA did not alter Aqp7 mRNA levels, providing evidence against off-target effects of Aqp11 siRNA. Moreover, absence of effects of Aqp11 siRNA on Cox2, Bax, and Bcl2 mRNA levels provides evidence against global pathological effects, as Cox2 mediates immunogenic responses. Absence of changes in expression of pro-apoptotic Bax and anti-apoptotic Bcl2 genes suggests no effect on basal apoptosis. Although the exploration of mechanisms that underlie Aqp11 regulation of the expression of multiple genes fell outside the scope of this study, it is likely that such coordinate regulation is brought about by changes in a core testicular function.
The collective results elucidate testicular processes that influence infertility. ACP-DDPCR screening identified candidate genes for control of testicular regression and recrudescence. Although there are previous reports of Aqp11 expression in testis, to our knowledge the present study is first to provide molecular evidence of a role for Aq11 in specific testicular functions. The close relationship between Aqp11 expression and testicular weight during photoperiodic modulations suggests that the approach used herein provides a means by which future studies could assess incremental changes in testicular function over finely tuned time courses, in order to elucidate further the proximal control of fertility.
HIGHLIGHTS.
○ Differential display PCR revealed candidate genes for control of fertility in testis during photoperiodic modulation
○ Multiple linear regression modeling helped identify aquaporin-11 as the best fit among the candidate genes
○ RNA interference of Aqp11, using small interfering RNA demonstrated Aqp11 tonically induces markers of fertility
○ The collective results provide the first molecular evidence that Aqp11 regulates fertility
ACKNOWLEDGEMENTS
This work was supported by a Texas AgriLife Research award (CM), a Heep Neuroscience Fellowship (JLS), and National Institutes of Health awards HD-024312 and AG-036670 (HFU).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adham IM, Nayernia K, Burkhardt-Gottges E, Topaloglu O, Dixkens C, Holstein AF, Engel W. Teratozoospermia in mice lacking the transition protein 2 (Tnp2) Mol Hum Reprod. 2001;7:513–520. doi: 10.1093/molehr/7.6.513. [DOI] [PubMed] [Google Scholar]
- Arrighi S, Ventriglia G, Aralla M, Zizza S, Di Summa A, Desantis S. Absorptive activities of the efferent ducts evaluated by the immunolocalization of aquaporin water channels and lectin histochemistry in adult cats. Histol Histopathol. 2010;25:433–444. doi: 10.14670/HH-25.433. [DOI] [PubMed] [Google Scholar]
- Berndtson WE, Desjardins C. Circulating LH and FSH levels and testicular function in hamsters during light deprivation and subsequent photoperiodic stimulation. Endocrinology. 1974;95:195–205. doi: 10.1210/endo-95-1-195. [DOI] [PubMed] [Google Scholar]
- Beyerle A, Braun A, Merkel O, Koch F, Kissel T, Stoeger T. Comparative in vivo study of poly(ethylene imine)/siRNA complexes for pulmonary delivery in mice. J Control Release. 2011;151:51–56. doi: 10.1016/j.jconrel.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Butler MP, Turner KW, Zucker I. A melatonin-independent seasonal timer induces neuroendocrine refractoriness to short day lengths. J Biol Rhythms. 2008;23:242–251. doi: 10.1177/0748730408317135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamita G, Mazzone A, Bizzoca A, Svelto M. Possible involvement of aquaporin-7 and -8 in rat testis development and spermatogenesis. Biochem Biophys Res Commun. 2001a;288:619–625. doi: 10.1006/bbrc.2001.5810. [DOI] [PubMed] [Google Scholar]
- Calamita G, Mazzone A, Cho YS, Valenti G, Svelto M. Expression and localization of the aquaporin-8 water channel in rat testis. Biol Reprod. 2001b;64:1660–1666. doi: 10.1095/biolreprod64.6.1660. [DOI] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT. A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther. 2005;312:546–553. doi: 10.1124/jpet.104.075424. [DOI] [PubMed] [Google Scholar]
- Crosnoe LE, Kim ED. Impact of age on male fertility. Curr Opin Obstet Gynecol. 2013;25:181–185. doi: 10.1097/GCO.0b013e32836024cb. [DOI] [PubMed] [Google Scholar]
- Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, McCarrey JR, Eddy EM, O'Brien DA. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod. 2010;82:136–145. doi: 10.1095/biolreprod.109.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DW, Ring RH, Khawaja XZ, Novak TJ, Aston C. High-throughput confirmation of differential display PCR results using reverse Northern blotting. J Neurosci Methods. 2003;123:47–54. doi: 10.1016/s0165-0270(02)00343-6. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Allen JW, Collins BW, Poorman-Allen P, Mori C, Blizard DR, Brown PR, Goulding EH, Strong BD, Eddy EM. HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development. 1997;124:4595–4603. doi: 10.1242/dev.124.22.4595. [DOI] [PubMed] [Google Scholar]
- Francisco NR, Raymond CM, Heideman PD. Short photoperiod inhibition of growth in body mass and reproduction in ACI, BUF, and PVG inbred rats. Reproduction. 2004;128:857–862. doi: 10.1530/rep.1.00390. [DOI] [PubMed] [Google Scholar]
- Gaston S, Menaker M. Photoperiodic control of hamster testis. Science. 1967;158:925–928. doi: 10.1126/science.158.3803.925. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Praetorius J, Tsunenari T, Nielsen S, Agre P. Aquaporin-11: a channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006;7:14. doi: 10.1186/1471-2091-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana K, Samanta PK, Ghosh D. Dose-dependent response to an intratesticular injection of calcium chloride for induction of chemosterilization in adult albino rats. Vet Res Commun. 2002;26:651–673. doi: 10.1023/a:1020976905746. [DOI] [PubMed] [Google Scholar]
- Jin W, Herath CB, Yoshida M, Arai KY, Saita E, Zhanquan S, Ren L, Watanabe G, Groome NP, Taya K. Inhibin B regulating follicle-stimulating hormone secretion during testicular recrudescence in the male golden hamster. J Androl. 2002;23:845–853. [PubMed] [Google Scholar]
- Kawazu S, Kishi H, Saita E, Jin W, Suzuki AK, Watanabe G, Taya K. Inhibin secretion in the golden hamster (Mesocricetus auratus) testis during active and inactive states of spermatogenesis induced by the restriction of photoperiod. J Reprod Dev. 2003;49:87–97. doi: 10.1262/jrd.49.87. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Kwak CI, Gu YY, Hwang IT, Chun JY. Annealing control primer system for identification of differentially expressed genes on agarose gels. Biotechniques. 2004;36:424–426. 428, 430. doi: 10.2144/04363ST02. passim. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Lin YH, Chen HI, Wang YY, Chiou YW, Lin HH, Pan HA, Wu CM, Su SM, Hsu CC, Kuo PL. SEPT12 mutations cause male infertility with defective sperm annulus. Hum Mutat. 2012;33:710–719. doi: 10.1002/humu.22028. [DOI] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lin YH, Lin YM, Wang YY, Yu IS, Lin YW, Wang YH, Wu CM, Pan HA, Chao SC, Yen PH, Lin SW, Kuo PL. The expression level of septin12 is critical for spermiogenesis. Am J Pathol. 2009;174:1857–1868. doi: 10.2353/ajpath.2009.080955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Bartke A, Steger RW. Catecholamine effects on testicular testosterone production in the gonadally active and the gonadally regressed adult golden hamster. Biol Reprod. 1989;40:752–761. doi: 10.1095/biolreprod40.4.752. [DOI] [PubMed] [Google Scholar]
- Morales E, Ferrer C, Zuasti A, Garcia-Borron JC, Canteras M, Pastor LM. Apoptosis and molecular pathways in the seminiferous epithelium of aged and photoinhibited Syrian hamsters (Mesocricetus auratus. J Androl. 2007;28:123–135. doi: 10.2164/jandrol.106.000778. [DOI] [PubMed] [Google Scholar]
- Morgan C. Plasticity in photoperiodic regulation of adrenal, but not testicular, function in Syrian hamsters. Gen Comp Endocrinol. 2012;178:441–449. doi: 10.1016/j.ygcen.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Morgan C, Shannonhouse JL, Morgan C, Thompson RC, Watson SJ, Akil H. Syrian hamster proopiomelanocortin cDNA cloning and early seasonal changes in testicular expression. Gen Comp Endocrinol. 2003a;133:353–357. doi: 10.1016/s0016-6480(03)00189-8. unpublished data. [DOI] [PubMed] [Google Scholar]
- Morgan C, Urbanski HF, Fan W, Akil H, Cone RD. Pheromone-induced anorexia in male Syrian hamsters. Am J Physiol Endocrinol Metab. 2003b;285:E1028–1038. doi: 10.1152/ajpendo.00010.2003. [DOI] [PubMed] [Google Scholar]
- Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, Ikeda M, Yamamoto T, Verkman A, Kusano E, Ookawara S, Takata K, Sasaki S, Ishibashi K. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y, Sakube Y, Sasaki S, Ishibashi K. Molecular mechanisms and drug development in aquaporin water channel diseases: aquaporin superfamily (superaquaporins): expansion of aquaporins restricted to multicellular organisms. J Pharmacol Sci. 2004;96:276–279. doi: 10.1254/jphs.fmj04004x7. [DOI] [PubMed] [Google Scholar]
- Morlighem JE, Petit C, Tzertzinis G. Determination of silencing potency of synthetic and RNase III-generated siRNA using a secreted luciferase assay. Biotechniques. 2007;42:599–600. 602, 604–596. doi: 10.2144/000112444. [DOI] [PubMed] [Google Scholar]
- Motiei M, Tavalaee M, Rabiei F, Hajihosseini R, Nasr-Esfahani MH. Evaluation of HSPA2 in fertile and infertile individuals. Andrologia. 2012 doi: 10.1111/j.1439-0272.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Zucker I. Spontaneous testicular recrudescence of Syrian hamsters: role of stimulatory photoperiods. Physiol Behav. 1987;39:615–617. doi: 10.1016/0031-9384(87)90161-2. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, Brown D. Aquaporin 9 expression along the male reproductive tract. Biol Reprod. 2001;65:384–393. doi: 10.1095/biolreprod65.2.384. [DOI] [PubMed] [Google Scholar]
- Pastor LM, Zuasti A, Ferrer C, Bernal-Manas CM, Morales E, Beltran-Frutos E, Seco-Rovira V. Proliferation and apoptosis in aged and photoregressed mammalian seminiferous epithelium, with particular attention to rodents and humans. Reprod Domest Anim. 2011;46:155–164. doi: 10.1111/j.1439-0531.2009.01573.x. [DOI] [PubMed] [Google Scholar]
- Richardson BA, Petterborg LJ, Vaughan MK, King TS, Reiter RJ. The effect of twice daily gonadotropin-releasing hormone (GnRH) administration and/or renal pituitary homografts on melatonin-induced gonadal atrophy in male Syrian hamsters. Prog Clin Biol Res. 1982;92:129–142. [PubMed] [Google Scholar]
- Shirley CR, Hayashi S, Mounsey S, Yanagimachi R, Meistrich ML. Abnormalities and reduced reproductive potential of sperm from Tnp1- and Tnp2-null double mutant mice. Biol Reprod. 2004;71:1220–1229. doi: 10.1095/biolreprod.104.029363. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Westrom WK, Hamill AI, Goldman BD. Effect of melatonin on the reproductive systems of male and female Syrian hamsters: a diurnal rhythm in sensitivity to melatonin. Endocrinology. 1976;99:1534–1541. doi: 10.1210/endo-99-6-1534. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. A role for N-methyl-D-aspartate receptors in the control of seasonal breeding. Endocrinology. 1990;127:2223–2228. doi: 10.1210/endo-127-5-2223. [DOI] [PubMed] [Google Scholar]
- Wu C, Kang JE, Peng LJ, Li H, Khan SA, Hillard CJ, Okar DA, Lange AJ. Enhancing hepatic glycolysis reduces obesity: differential effects on lipogenesis depend on site of glycolytic modulation. Cell Metab. 2005;2:131–140. doi: 10.1016/j.cmet.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Tran LT. Photoperiod, reproduction, and immunity in select strains of inbred mice. J Biol Rhythms. 2002;17:65–75. doi: 10.1177/074873002129002348. [DOI] [PubMed] [Google Scholar]
- Yeung CH. Aquaporins in spermatozoa and testicular germ cells: identification and potential role. Asian J Androl. 2010;12:490–499. doi: 10.1038/aja.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG. Aquaporin AQP11 in the testis: molecular identity and association with the processing of residual cytoplasm of elongated spermatids. Reproduction. 2010;139:209–216. doi: 10.1530/REP-09-0298. [DOI] [PubMed] [Google Scholar]
- Zhao M, Shirley CR, Yu YE, Mohapatra B, Zhang Y, Unni E, Deng JM, Arango NA, Terry NH, Weil MM, Russell LD, Behringer RR, Meistrich ML. Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice. Mol Cell Biol. 2001;21:7243–7255. doi: 10.1128/MCB.21.21.7243-7255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Dix DJ, Eddy EM. HSP70-2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development. 1997;124:3007–3014. doi: 10.1242/dev.124.15.3007. [DOI] [PubMed] [Google Scholar]