Abstract
Purpose of review
To review the recent literature on risk factors for chronic rhinosinusitis (CRS) with an emphasis on genetic, comorbid diseases and environmental factors associated with CRS. Through identifying potential risk factors for CRS, we glean insights into the underlying pathogenic mechanisms and essential for developing effective therapeutic strategies.
Recent findings
Recent findings demonstrate that genetics, comorbid medical conditions including airway diseases, gastroesophageal reflux disease, inflammatory and autoimmune diseases and various demographic and environmental factors are associated with having a CRS diagnosis. Limitations of current studies include, variable application of disease definitions, lack of prospective longitudinal studies and a disproportionate focus on tertiary care populations.
Summary
CRS has a broad spectrum of associations ranging from genetics to comorbid diseases and environmental factors. These predisposing factors provide valuable information for possible designing therapeutic and preventive interventions. However, to better understand whether these associations cause CRS, further studies are needed to independently replicate findings, establish temporal relationships between exposure and disease onset, evaluate the influence of exposure dose on disease severity, and to understand the biological effects of these risk factors in the context of CRS.
Keywords: Sinusitis, Comorbidity, Risk factors, Epidemiology, Genetics, Environment
Introduction
Chronic rhinosinusitis (CRS) is defined as symptomatic inflammation of the sinonasal mucosa that persists for at least 12 weeks is one of the most common chronic diseases of adults in the United States.[1–3] CRS is typically classified clinically into two distinguishable phenotypes: chronic rhinosinusitis without nasal polyposis (CRSsNP) and chronic rhinosinusitis with nasal polyposis (CRSwNP). CRS is estimated to affect about 13% of the population in the United States[4] and 10.9% of the population in Europe with an incidence of 1.13 per 100 person-years.[5, 6**] Patients with CRS suffer from significantly impaired quality of life including decreased health utility, emotional distress, and decreased physical and social activity with disease-specific expenditures totaling approximately $6 billion annually.[7–11]
Although the pathogenesis of CRS has been under active investigations, the etiology of CRS still remains controversial.[12] CRSsNP has traditionally been considered a consequence of bacterial infection similar to acute rhinosinusitis, whereas CRSwNP is most frequently characterized by Type 2 inflammation in Western populations. Other potential risk factors for both forms of CRS have included anatomical obstruction of the osteomeatal complex, impaired mucociliary clearance, osteitis, microbes, biofilms, superantigen effects, immune dysfunctions, impaired epithelial defense, genetic factors, and environmental exposures such as inhaled allergens and irritants (Fig. 1).[1, 13–15]
Figure 1.
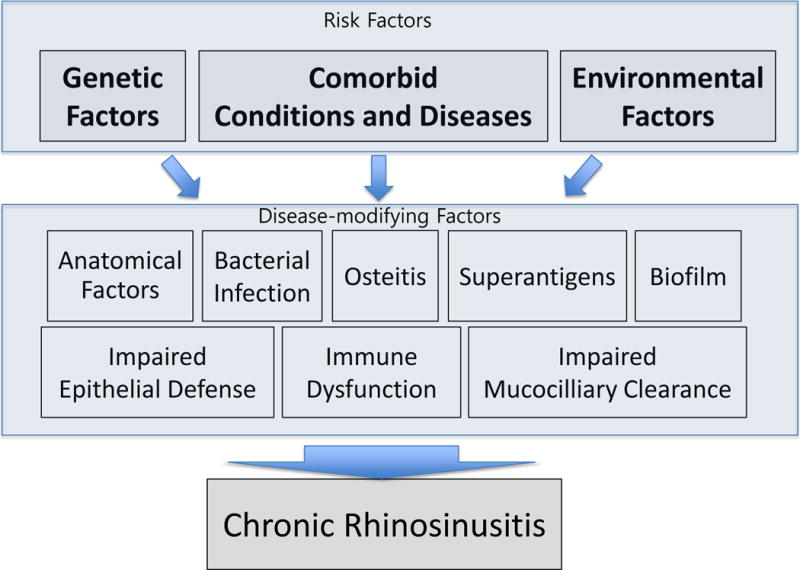
Risk factors for the development of CRS
Given the significant health and economic impact, and lack of widely accepted representative animal models to study CRS mechanistically, understanding risk factors for CRS might provide insights into the underlying pathogenic mechanisms and may facilitate preventive interventions that mitigate risk factors to reduce progression to CRS. This review will briefly summarize the recent literature with an emphasis on genetic factors, comorbid conditions and environmental factors associated with adult CRS.
Genetics associated with CRS
While specific monogenic disorders including cystic fibrosis, primary immunodeficiencies and primary ciliary dyskinesia (Kartagener’s syndrome) are associated with a high prevalence of CRS, their contribution to overall prevalence is low.[16, 17] Recently, Hsu et al. performed a comprehensive and current review of the literature examining the genetics of CRS- in this section we will briefly summarize her major findings and highlight pertinent emerging literature published in the interim.[18**] As pointed out in her excellent review, interpretation of genetic studies in CRS has been limited by the resolution to which CRS patients were phenotyped, over-representation of CRS patients undergoing sinus surgery, sparse replication studies, differences in study design, and inadequate accounting for linkage disequilibrium and multiple testing. Nonetheless, genes found to significantly associate with CRS can broadly be categorized into genes involved with ion channels (eg, CFTR); genes encoding human leukocyte antigens (eg, HLA-A, -B, -C, -DR and -DQ); genes involved in innate immunity (eg, CD14, IRAK4, LFT, MET, NOS1, NOS1AP, NOS2A, SERPINA1 and TLR2); genes involved in Type 2 inflammation (eg, IL1RL1, IL4, IL13 and IL33); genes involved in inflammation (eg, IDO1, IL1A, IL1B, IL1R2, IL1RN, IL6, IL22RA1, LTA, TNF and TNFA1P3); genes involved in tissue remodeling (eg, MMP9, POSTN and TGFB1); genes involved in arachidonic acid metabolism (eg, LTC4S, PTGDR and PTGS2), and other genes significantly associated with CRS (eg, ADRB2, AOAH, CACNA1I, DCBLD2, EMID2, GSTT1, KIAA1456, LAMA2, LAMB1, MSRA, MUSK, NAV3, PARS2, PTGS2, RYBP, TP73 and TRIP12). In Table 1 [19–38], we highlight genes, which showed association with specific phenotype of CRS or high odds ratio in paper from Hsu et al. In most of these studies, CRSwNP patients served as cases and candidate gene approaches were used although several studies demonstrate that genes including CFTR, IL1RL1 and AOAH associate with CRSsNP.[18**–38] Until recently, outside of CFTR, there were no replication studies validating these prior findings and no mechanistic studies demonstrating their biological relevance.
Table 1.
Genes significantly associated with CRS in prior studies
| Study [Ref] | Gene | Chromosome Location | Variation Surveyed | CRS Phenotype | Relevant Results OR (P value) | Involved Function |
|---|---|---|---|---|---|---|
| Pinto et al. 2008 [19] | CFTR | 7q31 | Multiple SNPs | CRSsNP | NA (.0023) | Chloride ion transport |
| Raman et al. 2002 [20] | CFTR | 7q31 | Multiple SNPs | CRS | 3.5 (<.05) | Chloride ion transport |
| Keles et al. 2008 [21] | HLA-C | 6p21 | Multiple SNPs | CRSwNP | NA (<.05) | Encoding human leukocyte antigens |
| Takeuchi et al. 1999 [22] | HLA-B | 6p21 | Multiple SNPs (HLA-B54) | CRS | 3.23 (<.037) | Encoding human leukocyte antigens |
| Luxenberger et al. 2000 [23] | HLA-A | 6p21 | Multiple SNPs (HLA-A74) | CRSwNP | 71.3 (<0.03) | Encoding human leukocyte antigens |
| Zhai et al. 2007 [24] | HLA-DR | 6p21 | HLA-DR*16 | CRSwNP | 8.9 (.03) | Encoding human leukocyte antigens |
| Schubert et al. 2004 [25] | HLA-DQ | 6p21 | HLA-DQB1*03 | CRSwNP | 4.25 (.001) | Encoding human leukocyte antigens |
| Monar-Gabor et al. 2000 [26] | HLA-DR | 6p21 | HLA-DR*7 | CRSwNP | 2.55 (<.05) | Encoding human leukocyte antigens |
| Fajardo-Dolci et al. 2006 [27] | HLA-DQ | 6p21 | HLA-DQA1*0201 | CRSwNP | 6.79 (.0027) | Encoding human leukocyte antigens |
| Pascual et al. 2008 [28] | NOS2A | 17q11-q12 | Promoter VNTR | CRSwNP | 14.39 (.001) | Innate immunity |
| Yazdani et al. 2012 [29] | CD14 | 5q31 | rs2569190 | CRSwNP | 1.88 (.04) | Innate immunity |
| Erbek et al. 2007 [30] | IL1A | 2q14 | rs17561 | CRSwNP | 2.743 (<.001) | Inflammation |
| Batikhan et al. 2010 [31] | TNF | 6q21 | rs1800629 | CRSwNP | 3.68 (.016) | Inflammation |
| Zhang et al. 2011 [32] | NOS1 | 12q24 | Multiple SNPs | CRS | Varied (.0023-.0129) | Innate immunity |
| Zhang et al. 2011 [32] | NOS1AP | 1q23 | rs4657164 | CRS | 1.67(.0178) | Innate immunity |
| Zielinska-Blizniewska et al. 2012 [33] | POSTN | 13q13 | −33C>G | CRSwNP | 4.56 (<.001) | Tissue remodeling |
| Kim et al. 2007 [34] | TGFB1 | 19q13 | rs1800469 | CRSsNP | NA (.012) | Tissue remodeling |
| Benito et al. 2012 [35] | PTGDR | 14q22 | Diplotype | CRSwNP | 2.44 (.043) | Arachidonic acid metabolism |
| Zhang et al. 2012 [36] | AOAH | 7q14-p12 | rs4504543 | CRSsNP | 0.03 (<.0001) | Others |
| Ozcan et al. 2010 [37] | GSTT1 | 22q11 | Homozygous deletion | CRSwNP | 2.03 (.05) | Others |
| Bosse et al. 2009 [38] | PARS2 | 1p32 | Rs2873551 | CRS | 0.49(.000026) | Others |
CRS, chronic rhinosinusitis; OR, odds ratio; CRSsNP, chronic rhinosinusitis without nasal polyp; NA, not applicable; CRSwNP, chronic rhinosinusitis with nasal polyp
Recently, Henmyr et al. conducted a study of single nucleotide polymorphisms (SNP) associations of 613 patients with CRS compared against a publically available, though racially unmatched, control population comprised of 1588 participants.[39*] They found significant (P < .05) associations between genetic variations in only 7 genes (eg, PARS2, TGFB1, NOS1, NOS1AP, IL22RA1, DCBLD2 and ALOX5AP) of the 53 SNPs previously associated with CRS status.[39*] Of these, only the association between the rs2873551 SNP in PARS2 gene was significant after a Bonferroni correction. PARS2 is a mitochondrial prolyl t-RNA synthase for which limited research exists- further validation of these genetic association studies and research on the function of PARS2 gene may shed light on reasons for its association with CRS. In analysis comparing CRSwNP against the CRSsNP patient populations, the authors also found the rs4504543 SNP in the acyloxyacyl hydrolase (AOAH) gene with the CRSwNP phenotype (P = .022). This SNP had previously been associated with CRS in a pooling-based genomewide association study (pGWAS) and replicated in a Chinese population with CRS.[36, 40]
Another emerging line of research suggests that polymorphisms in the bitter taste receptor T2R38 gene (TAS2R38 gene) may also be associated with CRS status. In human sinonasal epithelial cells, microbial secretions activate the T2R38 receptor that regulates calcium-flux dependent NO production, stimulates mucociliary clearance and increases direct antibacterial effects. The activity of these innate responses is dependent on three common polymorphisms and the polymorphisms segregate together to form two common haplotypes of the TAS2R38 gene.[41] The functional PAV haplotype combines with the non-functional AVI haplotype to generate three genotypes- PAV/PAV, PAV/AVI and AVI/AVI with the AVI/AVI genotype showing significantly impaired pseudomonas aeruginosa killing in vitro cultured epithelial cells. More recently, Adappa et al. examined the relative frequency of the AVI/AVI genotype and PAV/PAV genotypes in medically recalcitrant CRS patients compared to a geographically matched control population and found a significantly higher rate of AVI/AVI genotype patients among the medically recalcitrant CRS group (P = .0383) suggesting that the PAV/PAV genotype may decrease CRS risk.[42] Separately, Mfuna Endam et al. used a pGWAS and demonstrated SNPs associated with the TAS2R38 gene were found at a higher frequency in the CRS population compared to controls although statistics and odds ratios were not reported.[43] In other lines of research, Purkey et al. examined the association between selected potassium channels and CRS status in a study comparing 828 children with CRS against 5083 controls and found that two epithelial potassium channels KCNMA1 and KCNQ5 were associated with CRS status in Caucasian and African American children respectively.[44] This line of research, together with the TAS2R38 findings, and known associations with CFTR suggest that genes regulating ion flux in epithelial cells may confer significant genetic predisposition to CRS.
Comorbid diseases and conditions associated with CRS
In addition to genes specifically associated with CRS, it has been recognized that CRS is associated with specific comorbid medical conditions that directly affect the airway. Other studies also suggest gastroesophageal reflux disease (GERD) and autoimmune/inflammatory diseases, and various demographic factors may similarly be associated with having CRS. These factors have been recently reviewed by Lam et al. in a recently published review article but are further summarized in our discussion and in Table 2.[5, 6**, 45**, 46, 47**, 48]
Table 2.
Summary of selected studies on comorbid diseases associations with CRS status
| Study [Ref] | Design | Criteria for CRS Diagnosis | Criteria for Comorbidity Diagnosis | Relevant Results aOR (95%CI) | Comments |
|---|---|---|---|---|---|
| Airway disease: Allergic Rhinitis | |||||
| Jarvis et al. 2012 [5] | Cross-sectional Prospective | Patient-reported symptoms | Patient-reported symptoms | CRS vs non-CRS: 5.7 (5.3–6.2) | European general population based on survey responses (n=53,185) |
| Tan et al. 2013 [6**] | Case-control Retrospective | ICD-9 | ICD-9 | CRSsNP vs control: 2.4 (1.6–1.9)* CRSwNP vs control: 2.8(2.3–3.5)* |
Based on a US primary care population (n=446,480) |
| Kim et al. 2011 [46] | Cross-sectional Prospective | Symptoms and physical examination performed by clinicians | Patient-reported symptoms | CRS vs non-CRS: AR: 1.4 (0.9–2.2) Intermittent/moderate-severe AR: 1.6 (0.9–3.0) Persistent/mild AR: 2.6 (1.3–5.0)* Persistent/moderate-severe AR: 8.2 (4.7–14.4)* |
Korean general population (n=4,098) |
| Airway disease: Asthma | |||||
| Jarvis et al. 2012 [5] | Cross-sectional Prospective | Patient-reported symptoms | Patient-reported symptoms | CRS vs non-CRS Asthma: 2.7 (2.3–3.2) Asthma & AR: 11.9 (10.6–13.2) |
European general population based on survey responses (n=53,185) |
| Tan et al. 2013 [6**] | Case-control Retrospective | ICD-9 | ICD-9 | CRSsNP vs control: 1.7 (1.6–1.9)* CRSwNP vs control: 2.8 (2.3–3.5)* |
Based on a US primary care population (n=446,480) |
| Kim et al. 2011 [46] | Cross-sectional Prospective | Symptoms and physical examination performed by clinicans | Patient-reported symptoms clinicans | CRS vs non-CRS: 1.9(0.8–4.6) | Korean general population (n=4,098) |
| Chung et al. 2014 [47*] | Cross-sectional Prospective | ICD-9 Diagnosed by certified otolaryngologist | Elixhauser Comorbidity Index | CRS vs non-CRS: 3.1(2.8–3.4)* |
Taiwan’s Longitudinal Health Insurance Database 2000 (LHID2000) (n=5,734) |
| GERD and other Gastrointestinal diseases | |||||
| Tan et al. 2013 [6**] | Case-control Retrospective | ICD-9 | ICD-9 | GERD CRSsNP vs control: 1.7 (1.6–1.8) * CRSwNP vs control: 1.5 (1.2–1.8) * |
Based on a US primary care population (n=446,480) |
| Chung et al. 2014 [47*] | Cross-sectional Prospective | ICD-9 Diagnosed by certified otolaryngologist | Elixhauser Comorbidity Index | CRS vs non-CRS: Peptic ulcer: 1.9 (1.7–2.0)* Liver disease: 1.4 (1.3–1.6)* |
Taiwan’s Longitudinal Health Insurance Database 2000 (LHID2000) (n=5,734) |
| Wong et al. 2004 [48] | Cross-sectional Prospective | Symptoms and evaluation by rhinologist | 24-hour pH measurement via probe in nasopharynx and hypopharynx | Of included CRS patients, 32.4% were diagnosed with GERD. Only 0.2% had reflux episodes recorded in the nasopharynx. |
Australian tertiary care population consisting of patients with CRS (n=40) |
| Inflammatory and autoimmune disease | |||||
| Tan et al. 2013 [6**] | Case-control Retrospective | ICD-9 | ICD-9 | Rheumatoid arthritis CRSsNP vs control: 1.5 (1.1–2.0) CRSwNP vs control: 1.0 (0.5–2.2) Systemic lupus erythematosus CRSsNP vs control: 2.2 (1.1–4.3) CRSwNP vs control: 3.6 (1.0–12.8) |
Based on a US primary care population (n=446,480) |
| Chung et al. 2014 [47*] | Cross-sectional prospective | ICD-9 Diagnosed by certified otolaryngologist | Elixhauser Comorbidity Index | CRS vs non-CRS: Rheumatoid arthritis 1.9 (1.6–2.1)* Systemic lupus erythematosus 1.5 (0.9–2.7) Ankylosing spondylitis 1.4 (1.1–1.8)* |
Taiwan’s Longitudinal Health Insurance Database 2000 (LHID2000) (n=5,734) |
| Psychiatric and neurologic disease | |||||
| Tan et al. 2013 [6**] | Case-control Retrospective | ICD-9 | ICD-9 | Anxiety CRSsNP vs control: 1.7 (1.5–2.0)* CRSwNP vs control: 1.7 (1.2–2.4) Depression CRSsNP vs control: 1.3 (1.1–1.5) CRSwNP vs control: 1.1 (0.8–1.6) Headache CRSsNP vs control: 1.8 (1.7–2.0)* CRSwNP vs control: 2.1 (1.7–2.6) | Based on a US primary care population (n=446,480) |
| Chung et al. 2014 [47*] | Cross-sectional Prospective | ICD-9 Diagnosed by certified otolaryngologist | Elixhauser Comorbidity Index | CRS vs non-CRS: Depression 2.0 (1.7–2.2)* Psychosis 1.4 (1.2–1.7)* Headaches 1.7 (1.6–1.8)* Migraines 2.3 (2.0–2.6)* Parkinson’s disease 1.7 (1.3–2.3)* | Taiwan’s Longitudinal Health Insurance Database 2000 (LHID2000) (n=5,734) |
CRS, chronic rhinosinusitis; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; CRSsNP, chronic rhinosinusitis without nasal polyp; CRSwNP, chronic rhinosinusitis with nasal polyp; AR, allergic rhinitis
statistically significant
Airway diseases
Tan et al. recently published the findings of a nonspecialty care-based, population representative, case–control study using the electronic health records of the Geisinger Health System (referred to subsequently as the GHS study).[6**] They compared the premorbid conditions of patients during a period 0–24 months prior to a new diagnosis of CRS with those of a control group matched on age, sex and incident encounter.[6**] This study was able to reveal patterns of care and diagnosis prior to receiving a CRS diagnosis and contrasts from prior studies that have used specialty care populations with more treatment refractory severe disease, or have used only assessments without information regarding the temporal relationships of diagnosis. In this study, allergic rhinitis (AR) was specifically found to have a significant association with CRS [[adjusted odds ratio (aOR)= 2.4 and 2.6 for CRSsNP and CRSwNP respectively]. Although high (30–80%) rates of atopic sensitization among CRS patients have long been reported, few studies have compared these results against those of the general population even though rates of atopic sensitization in the general population have been reported as high as 40–55%.[49, 50] Furthermore, there are significant differences in the threshold for atopic testing among the various published studies of atopy amongst CRS patients. In our own studies, we found higher rates of multiple sensitization among tested patients with CRSwNP compared with a control population of patients with rhinitis symptoms.[51] Other larger population-based studies have found strong associations between AR and comorbid CRS although the timing of the AR diagnosis relative to CRS onset was not elucidated (Table 2).[5, 46] In the study by Kim et al., the aOR of having CRS among patients with moderate-severe persistent AR was 8.2 when compared to mild intermittent AR. One limitation of epidemiologic studies of AR and CRS is that the symptoms of these two conditions overlap substantially and may be difficult to differentiate using a questionnaire. Despite these associations, other studies do not find that AR status affects management of CRS, need for surgical intervention or correlations with clinical symptom or radiographic severity.[52–54] Thus, while a history of atopic sensitization is more common among patients with CRS, its effect on CRS severity and treatment response rates remains unclear.
Similar to AR, asthma is strongly associated with CRS status, especially CRSwNP (Table 2). In the GHS study, the aOR of having an asthma diagnosis preceding the incident CRSwNP diagnosis was 2.8 and 1.7 for CRSsNP compared to control patients without an incident CRS diagnosis and was further increased to 4.4 and 2.5 respectively in the 6-months prior to the CRS diagnosis. In the population-based Global Allergy and Asthma European Network (GA2LEN) study of European adults, Jarvis et al. revealed an overall aOR of 3.5 (95% CI: 3.2–3.8) when comparing subjects with CRS to those without. This association increased further to 11.9 (95% CI: 10.6–13.2) among those adults with both asthma and AR.[5] Chung et al., using Taiwan’s Longitudinal Health Insurance Database 2000 (LHID2000), recently reported that of the 38 chronic conditions evaluated, asthma (aOR=3.1, 95% CI: 2.8–3.4) had the strongest with CRS when compared to control patients without CRS.[47*] However, unlike AR, studies do demonstrate that asthma severity may correlate with radiographic CRS severity and that the frequency and severity of CRS affects the severity of coexistent asthma.[55, 56] In some patients, CRSwNP, asthma and aspirin sensitivity form a well-established association known as aspirin-exacerbated respiratory disease (AERD) or Samter’s triad. Following exposure to aspirin, patients with AERD presumably overproduce cysteinyl leukotrienes and prostaglandin D2 that are mediators of eosinophilic inflammation and downregulate anti-inflammatory prostaglandin E2.[17, 57]
In addition to asthma and AR, the GHS study also revealed a pattern of episodic and chronic lower airway illnesses preceding the diagnosis of CRS and supported to the hypothesis of the unified airway. Interestingly, premorbid bronchitis and pneumonia were significantly more common in patients who developed CRS. These findings suggest that acute episodic respiratory diseases may modify host susceptibility to CRS, perhaps similarly to the relationship between rhinovirus (RV) and respiratory syncytial virus infections and subsequent asthma development.[58, 59] These findings have also been supported by the more recently published study utilizing the insurance claims-based LHID2000 dataset in which “chronic pulmonary disease” was the second most strongly associated condition, after asthma, with Otolaryngologist diagnosed CRS (aOR = 3.0, 95% CI: 2.8–3.3).[47*]
Gastroesophageal reflux disease and other Gastrointestinal diseases
GERD is known to play some roles in various diseases of the upper and lower airway diseases such as chronic cough,[60] asthma[56] and otitis media with effusion, especially in young children.[61] There is some evidence to suggest an association between GERD and CRS.[48, 61–64] For example, the GHS study showed modest, but significant, relationship between GERD and CRS. The European Community Respiratory Health Study (ECRHS), a multinational longitudinal study, has similarly suggested that GERD, particularly the nocturnal subtype, is strongly associated with the development of lower respiratory tract diseases, such as asthma, but has not specifically examined its effect on rhinosinusitis.[65] Others have suggested that omeprazole-responsiveness of sinusitis symptoms was indicative of a role for GERD in CRS pathogenesis.[66] However, intriguing research from the eosinophilic esophagitis literature suggests that omeprazole may have anti-inflammatory independent of its effects on suppressing acid production in gastric parietal cells.[67] It should be noted that eosinophilic esophagitis shares histologic, and immunopathologic, parallels to CRSwNP.
Inflammatory and autoimmune diseases
Other premorbid diseases associations with CRS include inflammatory and autoimmune diseases.[47*, 68–70] In the GHS study, the association between lupus and CRS had an aOR of 3.6 although this was not statistically significant at the stringent P < .0005 level used in the study. These associations are remarkable given recent findings that local immune responses in CRSwNP seem to generate autoantibodies, particularly against nuclear and epithelial antigens.[71, 72] In a more recently published paper using the LHID2000 database from Taiwan, the investigators found significant comorbid associations between a rheumatoid arthritis diagnosis (aOR=1.85, 95% CI: 1.63–2.1) and CRS although the association between lupus and CRS was not statistically significant due to the relative rarity of lupus in the Taiwanese population.
Other comorbidities and demographic factors
Comorbid psychiatric conditions like anxiety and depression may increase rates of CRS diagnosis and health care utilization associated with CRS.[6**, 47*, 73, 74] Another condition strongly associated with CRS includes headache disorders like migraine potentially due to the overlapping symptoms and symptom vocabulary of chronic migraine and CRS.[47*, 75] In both the LHID2000 study and the GHS study, the strength of co/pre-morbid associations between CRS and headache diagnoses was moderate with aOR between 2.0 and 2.3. However, other lines of research suggest that while migraine affects CRS risk, it does not affect symptomatic improvement following treatment for CRS.[76]
Besides comorbid disease, epidemiologic studies demonstrate underlying demographic factors that are associated with CRS. These studies demonstrated that the peak incidence of CRS among adults was between ages 45 and 54 years [6**] while others subsequently demonstrate that peak prevalence is between 50–59[77]. Sex also had an effect of CRS phenotype with more females developing CRSsNP, while males were more likely to exhibit the CRSwNP. The age of onset and gender predisposition for CRS phenotypes may provide a basis for future research to determine possible hormone or aging-specific effects on the pathogenesis of CRS.
Environmental factors associated with CRS
Environmental factors are known to have significant effects in the pathogenesis of various airway diseases as best illustrated in studies on asthma, tobacco exposure and hypersensitivity pneumonitis. Since airway diseases are unified by the responses of airway epithelial cells and exposure to inhaled pollutants, allergens, irritants and toxins, it is tempting to speculate that many of the environmental exposures linked to lower airway disease will be of relevance to CRS (Table 3).
Table 3.
Environmental factors significantly associated with CRS in prior studies
| Study [Ref] | Design | Criteria for CRS Diagnosis | Criteria for Environmental Factors Exposure Confirmation | Relevant Results aOR (95%CI) | Comments |
|---|---|---|---|---|---|
| Tobacco | |||||
| Jarvis et al. 2012 [5] | Cross-sectional prospective | Patient-reported symptoms | Patient response | CRS vs non-CRS Current smoker: 1.0 (0.9–1.2) Former smoker: 1.2 (1.1–1.3) |
European general population based on survey responses (n=53,185) |
| Tan et al. 2013 [6**] | Case-control Retrospective | ICD-9 | ICD-9 | CRSsNP vs control: 1.3 (1.1–1.6) CRSwNP vs control: 1.2 (0.8–1.9) |
Based on a US primary care population (n=446,480) |
| Chen et al. 2003 [77] | Cross-sectional Prospective | Patient-reported symptoms | Patient response | CRS vs non-CRS In male with allergy, without allergy Current smoker: 0.8 (0.5–1.3), 1.7 (1.1–2.4) Former smoker: 0.7 (0.4–1.1), 1.0 (0.6–1.5) In female with allergy, without allergy Current smoker: 1.7 (1.2–2.4), 1.4 (1.0–2.0) Former smoker: 1.2 (0.9–1.6), 1.2 (0.9–1.7) |
Canadian second cycle of National Population Health Survey (NPHS) (n=73,364) |
| Hastan et al. 2011 [78] | Cross-sectional Prospective | Patient-reported symptoms | Patient response | CRS vs non-CRS Current smoker: 1.7 (1.6–1.9)* Former smoker: 1.2 (1.0–1.3) |
European general population based on survey responses (n=57,128) |
| Thilsing et al. 2012 [79**] | Cross-sectional Prospective | Patient-reported symptoms | Patient response | CRS vs non-CRS (aRR, 95% CL) In male Current smoker: 1.9 (1.3–2.8)* Former smoker: 1.1 (0.7–1.8) In female Current smoker: 2.5 (1.7–3.7)* Former smoker: 1.6 (1.0–2.6)* |
Part of a trans-European GA2LEN (Global Asthma and Allergy European Network)-based study, Danish population (n=4,554) |
| Tammemagi et al. 2010 [80] | Case-control retrospective | ICD-9 with confirmation by CT or nasal endoscopy | Patient response | CRS vs non-CRS: SHS 2.2 (1.5–3.2)* | CRS patients from a specialty clinic and controls from primary care (n=612) |
| Lieu et al. 2000 [81] | Cross-sectional prospective | Patient-reported symptoms | Patient response | CRS vs non-CRS (aRR, 95%CI) Current smoker: 1.2 (1.1–1.4) Former smoker: 1.1 (0.9–1.3) | Natuinal Health And Nutritional Examination Survey (NHANES III) (n=20,050) |
| Air Pollution | |||||
| Min et al. 1996 [86] | Cross-sectional prospective | Symptoms and physical examination performed by clinicans | Patient response | CRS vs non-CRS Rural 0.7 (0.4–1.1) | Korean general population based on survey responses (n=9,069) |
| Wolf 2002 [87] | Case-control retrospective | Diagnosed by otolaryngologist | Air quality measurement | In total, no correlation between air pollution levels and CRS rates In the districts with above average air pollution levels, positive correlation between air pollution levels and CRS rates (adjusted R2=0.3) | Tertiary care hospital in Cologne, Germany (n=1,435) |
| Occupational factors | |||||
| Thilsing et al. 2012 [79**] | Cross-sectional prospective | Patient-reported symptoms | Patient response | CRS vs non-CRS (aRR, 95% CL) High molecular weight agents 0.9 (0.6–1.4) Animal dander 1.9 (1.0–3.6) Fish or shellfish 2.07 (1.4–3.0)* Latex 1.1 (0.7–1.7) Pharmaceutical products 0.5 (0.1–2.0) Low molecular weight agents 1.3 (0.9–1.9) Highly reactive chemicals 1.4 (1.0–2.2) Isocyanides 3.1 (0.9–10.7) Reactive cleaning disinfectants 1.3 (0.8–2.2) Metal and metal fumes 0.9 (0.4–1.8) Mixed environments 0.9 (0.4–1.7) Agriculture 0.9 (0.4–2.0) | Part of a trans-European GA2LEN (Global Asthma and Allergy European Network)-based study, Danish population (n=4,554) |
CRS, chronic rhinosinusitis; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; CRSsNP, chronic rhinosinusitis without nasal polyp; CRSwNP, chroni rhinosinusitis without nasal polyp; aRR, adjusted risk ratios; 95%CL, 95% confidence limits; SHS: second hand smoke
statistically significant
Tobacco exposure
Tobacco exposure is one of the best studied risk factors for various airway diseases, including CRS. There is some evidence that active cigarette smoking is more prevalent among patients with CRS.[78, 79**, 80]. The GA2LEN determined that there is a strong association between CRS and active tobacco use (aOR=1.9, 95% CI: 1.8–2.1), with a significant, but smaller, association of CRS with former smokers (aOR=1.3, 95% CI: 1.2–1.4).[78] In the subset of GA2LEN patients from Denmark, Thilsing et al. revealed that smoking status (non-, former-, or current smoker) significantly affected the CRS prevalence (P = .000) while current smoking associated with an increased CRS prevalence in women [relative risk (RR)=2.5], as well as men (RR=1.9), compared to nonsmokers.[79] Separately, analysis of the third National Health and Nutrition Examination Survey (NHANES III) study found more modest associations between self-reported sinusitis and active smokers. Even at the highest exposure to more than 40 cigarettes per day, the adjusted RR for CRS was only ~1.20.[81] Neither of these studies, however, specifically separated associations for CRSsNP and CRSwNP. The few studies examining the relationship between second hand smoke exposure or environmental tobacco smoke (ETS) and adult CRS have produced conflicting results, while studies of CRS in children have demonstrated a consistent association between ETS and CRS among children who have had sinus surgery.[80–83] Studies examining the pathophysiologic effects of tobacco smoke on sinonasal mucosa suggest the reported associations are biologically plausible given findings that smoke decreases the mucociliary clearance of sinonasal mucosa by altering ionic transport mechanisms, impairs ciliogenesis, and suppresses innate immunity through alterations in toll-like receptors (TLRs), effector proteins (eg, β-defensins) and complement components.[84, 85]
Outdoor and indoor air quality
While patients and the lay media frequently ascribe sinusitis to air quality, there are surprisingly few previous studies that examine the relationship between air pollution and sinusitis and fewer yet that use the commonly accepted diagnostic criteria for CRS. Min et al., in the first epidemiologic survey to determine CRS prevalence in South Korea, found no difference between rural and urban areas with regard to the prevalence of CRS.[86] Wolf analyzed the addresses of 1435 patients who sought treatment for CRS in a tertiary care hospital in Cologne, Germany and correlated this against outdoor air quality measures that were collected through a program monitoring air quality in the Rhine and Ruhr area with 1km2 resolution.[87] It was found that when air pollution levels were correlated against CRS patient rates, no correlation was found, but if analysis was done only in the districts with above average air pollution levels, a weak (adjusted R2=0.3), but significant, positive correlation was found between air pollution levels and CRS case rates.[87] The author acknowledged that only SO2, NO2 and total suspended particulate matter were analyzed, only a convenience sample of patients was used, no information on individual outdoor exposure was collected, and there was no attempt to correlate air pollution levels with disease severity. Another study by Bhattacharyya correlated national rates of self-reported sinusitis in the United States with average national ambient SO2, NO2, CO and particulate matter and found significant, but weak, correlations in the prevalence of sinusitis with all air pollution parameters despite the lack of geographic resolution.[88] Asian sand dust (ASD), originating in the arid deserts of Mongolia and China brings large amounts of windborne soil particles to countries in the Asia-Pacific area has been associated with inducing respiratory illness in recent years.[89] ASD and spreads over large areas of several countries, especially Korea, Japan and China. To our knowledge, while no direct studies have examined the effects of ASD exposure and CRS status but Yeo et al. showed that ASD directly increased RV replication and aggravated RV-induced inflammation in human nasal epithelial cells by enhancing IFN-γ, IL-1β, IL-6, and IL-8 secretion providing a possible mechanism by which dust exposure, nasal microbiology and the immunologic barrier may be intertwined.[90]
In addition to outdoor air pollution, the effects of indoor air pollutions on respiratory disease can be substantial- in fact, studies suggest that indoor pollutant levels are substantially higher than outdoor pollutant levels and further, do not intercorrelate. Studies in asthma suggest that indoor endotoxin; allergen exposure to mouse, cockroach, pets, dust mite and mold; as well as indoor pollutants such as particulate matter and NO2 levels have been significantly effects on asthma morbidity.[91] Similarly, some volatile organic compounds (eg, formaldehyde, benzene, toluene and chlorobenzene) which are irritants and indoor sources (eg, solvents, floor adhesive, paint, cleaning products, furnishings, polishes and room fresheners) effects on development of asthma.[92, 93] To our knowledge, there has been only one study focused on indoor environments and nasal polyps. Kim and Hanley reported an association between the use of woodstoves as a principal source of heat and nasal polyposis.[94] No study has examined the association between indoor pollutants and CRS without polyps, outside of occupational exposure studies.
Airborne particles and vapors in the occupational environment may play an important role in CRS risk, although studies of these exposures have used definitions that make it difficult to differentiate between CRS and conditions with similar symptoms, such as occupational rhinitis.[95] Thisling et al., using the symptom-based definition of CRS, found that working in a blue collar job significantly increased the CRS prevalence in women but conversely, adjusted risk was significantly lower among male blue collar compared to male white collar workers.[79] Additionally, workplace exposure to “gases, fumes, dust, or smoke” increased the crude risk of CRS, although adjusted RR did not reach statistical significance.[79] Further analysis of 14 specific exposures found that only working with “fish or shellfish” was significantly associated with CRS status.[79] Other lines of work examining occupational rhinitis have also found significant associations between low-molecular weight agent exposure, persulfate salts, latex and animal exposure and rhinosinusitis symptoms in specific occupations. However, none of these studies utilized the symptomatic and duration dependent definition of CRS, without requiring objective evidence of inflammation, and thus, extrapolating these findings to CRS is difficult. [96–100]
Conclusions
Risk factors associated with CRS include genetic and comorbid medical as well as environmental factors. From these insights, we glean insights into the pathophysiology of CRS and can conceptualize studies of novel therapeutic and preventive strategies to reduce the burden of CRS. However, most current study designs establish associations but causality and dose-response cannot yet be established. Improvements in study design, particularly interventional studies or longitudinal cohort studies, and biological validation studies may provide estimates of magnitude these risk factors have on disease severity and prevalence.
Key points.
CRS is associated with a range of genetic factors, comorbid medical conditions and environmental factors.
Identification of risk factors for CRS may provide valuable information regarding the underlying pathogenic mechanisms and developing effective therapeutic strategies and preventive interventions for CRS patients.
As most of current studies of risk factors for CRS include small samples sizes drawn from tertiary care studies and lack of prospective longitudinal studies, general population-based epidemiologic studies are needed for better representation of risk factors for CRS.
Acknowledgments
Disclosure of funding received: This work was supported by the Triological Society, American College of Surgeons, and NIH grants K23DC012067 (BKT) and U19AI106683 (BKT).
Footnotes
Potential conflict of interest: None
References And Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004;131:S1–62. doi: 10.1016/j.otohns.2004.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat 10. 2009:1–157. [PubMed] [Google Scholar]
- 5.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 6**.Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. doi: 10.1016/j.jaci.2013.02.002. A recent study which is the first in the literature to evaluate associations of premorbid medical illness with incident chronic rhinosinusitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler ZM, Wittenberg E, Schlosser RJ, et al. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121:2672–2678. doi: 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2010;144:440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011;120:423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps group. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2008;43:317–320. [PubMed] [Google Scholar]
- 11.Hamilos DL. Chronic rhinosinusitis: Epidemiology and medical management. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–26. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131:933–957. doi: 10.1016/j.jaci.2013.02.023. quiz 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 15.Schleimer RP, Kato A, Peters A, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6:288–294. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan MW. Diseases associated with chronic rhinosinusitis: what is the significance? Curr Opin Otolaryngol Head Neck Surg. 2008;16:231–236. doi: 10.1097/MOO.0b013e3282fdc3c5. [DOI] [PubMed] [Google Scholar]
- 17.Bousquet J, Burney PG, Zuberbier T, et al. GA2LEN (Global Allergy and Asthma European Network) addresses the allergy and asthma ‘epidemic’. Allergy. 2009;64:969–977. doi: 10.1111/j.1398-9995.2009.02059.x. [DOI] [PubMed] [Google Scholar]
- 18**.Hsu J, Avila PC, Kern RC, et al. Genetics of chronic rhinosinusitis: state of the field and directions forward. J Allergy Clin Immunol. 2013;131:977–993. 993 e971–975. doi: 10.1016/j.jaci.2013.01.028. This recent review comprehensively evaluates the current literature regarding the genetics of chronic rhinosinusitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto JM, Hayes MG, Schneider D, et al. A genomewide screen for chronic rhinosinusitis genes identifies a locus on chromosome 7q. Laryngoscope. 2008;118:2067–2072. doi: 10.1097/MLG.0b013e3181805147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raman V, Clary R, Siegrist KL, et al. Increased prevalence of mutations in the cystic fibrosis transmembrane conductance regulator in children with chronic rhinosinusitis. Pediatrics. 2002;109:E13. doi: 10.1542/peds.109.1.e13. [DOI] [PubMed] [Google Scholar]
- 21.Keles B, Cora T, Acar H, et al. Evaluation of HLA-A, -B, -Cw, and -DRB1 alleles frequency in Turkish patients with nasal polyposis. Otolaryngol Head Neck Surg. 2008;139:580–585. doi: 10.1016/j.otohns.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi K, Majima Y, Shimizu T, et al. Analysis of HLA antigens in Japanese patients with chronic sinusitis. Laryngoscope. 1999;109:275–278. doi: 10.1097/00005537-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Luxenberger W, Posch U, Berghold A, et al. HLA patterns in patients with nasal polyposis. Eur Arch Otorhinolaryngol. 2000;257:137–139. doi: 10.1007/s004050050210. [DOI] [PubMed] [Google Scholar]
- 24.Zhai L, Sun Y, Tang L, Liu H. Polymorphism between loci for human leukocyte antigens DR and DQ in patients with nasal polyps. Ann Otol Rhinol Laryngol. 2007;116:66–68. doi: 10.1177/000348940711600111. [DOI] [PubMed] [Google Scholar]
- 25.Schubert MS, Hutcheson PS, Graff RJ, et al. HLA-DQB1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J Allergy Clin Immunol. 2004;114:1376–1383. doi: 10.1016/j.jaci.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Molnar-Gabor E, Endreffy E, Rozsasi A. HLA-DRB1, -DQA1, and -DQB1 genotypes in patients with nasal polyposis. Laryngoscope. 2000;110:422–425. doi: 10.1097/00005537-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Fajardo-Dolci G, Solorio-Abreu J, Romero-Alvarez JC, et al. DQA1 and DQB1 association and nasal polyposis. Otolaryngol Head Neck Surg. 2006;135:243–247. doi: 10.1016/j.otohns.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Pascual M, Sanz C, Isidoro-Garcia M, et al. (CCTTT)n polymorphism of NOS2A in nasal polyposis and asthma: a case-control study. J Investig Allergol Clin Immunol. 2008;18:239–244. [PubMed] [Google Scholar]
- 29.Yazdani N, Amoli MM, Naraghi M, et al. Association between the functional polymorphism C-159T in the CD14 promoter gene and nasal polyposis: potential role in asthma. J Investig Allergol Clin Immunol. 2012;22:406–411. [PubMed] [Google Scholar]
- 30.Erbek SS, Yurtcu E, Erbek S, et al. Proinflammatory cytokine single nucleotide polymorphisms in nasal polyposis. Arch Otolaryngol Head Neck Surg. 2007;133:705–709. doi: 10.1001/archotol.133.7.705. [DOI] [PubMed] [Google Scholar]
- 31.Batikhan H, Gokcan MK, Beder E, et al. Association of the tumor necrosis factor-alpha -308 G/A polymorphism with nasal polyposis. Eur Arch Otorhinolaryngol. 2010;267:903–908. doi: 10.1007/s00405-009-1167-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Endam LM, Filali-Mouhim A, et al. Polymorphisms in the nitric oxide synthase 1 gene are associated with severe chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:e49–54. doi: 10.2500/ajra.2011.25.3588. [DOI] [PubMed] [Google Scholar]
- 33.Zielinska-Blizniewska H, Sitarek P, Milonski J, et al. Association of the −33C/G OSF-2 and the 140A/G LF gene polymorphisms with the risk of chronic rhinosinusitis with nasal polyps in a Polish population. Molecular biology reports. 2012;39:5449–5457. doi: 10.1007/s11033-011-1345-6. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Park HS, Holloway JW, et al. Association between a TGFbeta1 promoter polymorphism and rhinosinusitis in aspirin-intolerant asthmatic patients. Respiratory medicine. 2007;101:490–495. doi: 10.1016/j.rmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Benito Pescador D, Isidoro-Garcia M, Garcia-Solaesa V, et al. Genetic association study in nasal polyposis. J Investig Allergol Clin Immunol. 2012;22:331–340. [PubMed] [Google Scholar]
- 36.Zhang Y, Endam LM, Filali-Mouhim A, et al. Polymorphisms in RYBP and AOAH genes are associated with chronic rhinosinusitis in a Chinese population: a replication study. PloS one. 2012;7:e39247. doi: 10.1371/journal.pone.0039247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozcan C, Tamer L, Ates NA, Gorur K. The glutathione-S-transferase gene polymorphisms (Gstt1, Gstm1, and Gstp1) in patients with non-allergic nasal polyposis. Eur Arch Otorhinolaryngol. 2010;267:227–232. doi: 10.1007/s00405-009-1066-9. [DOI] [PubMed] [Google Scholar]
- 38.Bosse Y, Bacot F, Montpetit A, et al. Identification of susceptibility genes for complex diseases using pooling-based genome-wide association scans. Human genetics. 2009;125:305–318. doi: 10.1007/s00439-009-0626-9. [DOI] [PubMed] [Google Scholar]
- 39*.Henmyr V, Vandeplas G, Hallden C, et al. Replication study of genetic variants associated with chronic rhinosinusitis and nasal polyposis. J Allergy Clin Immunol. 2014;133:273–275. doi: 10.1016/j.jaci.2013.08.011. The present study investigates the reproducibility of previous SNP associations with chronic rhinosinusitis. [DOI] [PubMed] [Google Scholar]
- 40.Barnes KC, Grant A, Gao P, et al. Polymorphisms in the novel gene acyloxyacyl hydroxylase (AOAH) are associated with asthma and associated phenotypes. J Allergy Clin Immunol. 2006;118:70–77. doi: 10.1016/j.jaci.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. The Journal of clinical investigation. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3–7. doi: 10.1002/alr.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mfuna Endam L, Filali-Mouhim A, Boisvert P, et al. Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol. 2014;4:200–206. doi: 10.1002/alr.21275. [DOI] [PubMed] [Google Scholar]
- 44.Purkey MT, Li J, Mentch F, et al. Genetic variation in genes encoding airway epithelial potassium channels is associated with chronic rhinosinusitis in a pediatric population. PloS one. 2014;9:e89329. doi: 10.1371/journal.pone.0089329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Lam K, Hirsch AG, Tan BK. The association of premorbid diseases with chronic rhinosinusitis with and without polyps. Curr Opin Otolaryngol Head Neck Surg. 2014;22:231–241. doi: 10.1097/MOO.0000000000000052. This recent review summarizes the various associations of premorbid disease with chronic sinusitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YS, Kim NH, Seong SY, et al. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25:117–121. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 47*.Chung SD, Chen PY, Lin HC, Hung SH. Comorbidity profile of chronic rhinosinusitis: a population-based study. Laryngoscope. 2014;124:1536–1541. doi: 10.1002/lary.24581. This study uses a national insurance database to examine comorbid conditions associated with chronic rhinosinusitis diagnosed by otolaryngologists. [DOI] [PubMed] [Google Scholar]
- 48.Wong IW, Omari TI, Myers JC, et al. Nasopharyngeal pH monitoring in chronic sinusitis patients using a novel four channel probe. Laryngoscope. 2004;114:1582–1585. doi: 10.1097/00005537-200409000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Blomme K, Tomassen P, Lapeere H, et al. Prevalence of allergic sensitization versus allergic rhinitis symptoms in an unselected population. Int Arch Allergy Immunol. 2013;160:200–207. doi: 10.1159/000339853. [DOI] [PubMed] [Google Scholar]
- 51.Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:88–94. doi: 10.1002/alr.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20:625–628. doi: 10.2500/ajr.2006.20.2907. [DOI] [PubMed] [Google Scholar]
- 53.Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23:145–148. doi: 10.2500/ajra.2009.23.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2014 doi: 10.1002/alr.21258. [DOI] [PubMed] [Google Scholar]
- 55.Bresciani M, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 56.Lin DC, Chandra RK, Tan BK, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:205–208. doi: 10.2500/ajra.2011.25.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung RM, Kern RC, Conley DB, et al. Osteomeatal complex obstruction is not associated with adjacent sinus disease in chronic rhinosinusitis with polyps. Am J Rhinol Allergy. 2011;25:401–403. doi: 10.2500/ajra.2011.25.3672. [DOI] [PubMed] [Google Scholar]
- 58.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. 1061 e1051. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.James KM, Peebles RS, Jr, Hartert TV. Response to infections in patients with asthma and atopic disease: an epiphenomenon or reflection of host susceptibility? J Allergy Clin Immunol. 2012;130:343–351. doi: 10.1016/j.jaci.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carr TF, Koterba AP, Chandra R, et al. Characterization of specific antibody deficiency in adults with medically refractory chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:241–244. doi: 10.2500/ajra.2011.25.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conley D, Pearlman A, Zhou K, et al. The role of point-of-care CT in the management of chronic rhinosinusitis: a case-control study. Ear, nose, & throat journal. 2011;90:376–381. doi: 10.1177/014556131109000812. [DOI] [PubMed] [Google Scholar]
- 62.Fokkens W, Lund V, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology Supplement. 2007:1–136. [PubMed] [Google Scholar]
- 63.el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology. 1997;113:755–760. doi: 10.1016/s0016-5085(97)70168-9. [DOI] [PubMed] [Google Scholar]
- 64.DelGaudio JM. Direct nasopharyngeal reflux of gastric acid is a contributing factor in refractory chronic rhinosinusitis. Laryngoscope. 2005;115:946–957. doi: 10.1097/01.MLG.0000163751.00885.63. [DOI] [PubMed] [Google Scholar]
- 65.Emilsson OI, Janson C, Benediktsdottir B, et al. Nocturnal gastroesophageal reflux, lung function and symptoms of obstructive sleep apnea: Results from an epidemiological survey. Respiratory medicine. 2012;106:459–466. doi: 10.1016/j.rmed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 66.DiBaise JK, Olusola BF, Huerter JV, Quigley EM. Role of GERD in chronic resistant sinusitis: a prospective, open label, pilot trial. The American journal of gastroenterology. 2002;97:843–850. doi: 10.1111/j.1572-0241.2002.05598.x. [DOI] [PubMed] [Google Scholar]
- 67.Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–832. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michaud K, Wolfe F. The association of rheumatoid arthritis and its treatment with sinus disease. The Journal of rheumatology. 2006;33:2412–2415. [PubMed] [Google Scholar]
- 69.Chandra RK, Lin D, Tan B, et al. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. American journal of otolaryngology. 2011;32:388–391. doi: 10.1016/j.amjoto.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keller JJ, Wu CS, Lin HC. Increased risk of psoriasis following chronic rhinosinusitis without nasal polyps: a population-based matched-cohort study. The British journal of dermatology. 2013;168:289–294. doi: 10.1111/bjd.12047. [DOI] [PubMed] [Google Scholar]
- 71.Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–1206. e1191. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeffe JS, Seshadri S, Hamill KJ, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123:2104–2111. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macdonald KI, McNally JD, Massoud E. The health and resource utilization of Canadians with chronic rhinosinusitis. Laryngoscope. 2009;119:184–189. doi: 10.1002/lary.20034. [DOI] [PubMed] [Google Scholar]
- 74.Wasan A, Fernandez E, Jamison RN, Bhattacharyya N. Association of anxiety and depression with reported disease severity in patients undergoing evaluation for chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2007;116:491–497. doi: 10.1177/000348940711600703. [DOI] [PubMed] [Google Scholar]
- 75.Hsueh WD, Conley DB, Kim H, et al. Identifying clinical symptoms for improving the symptomatic diagnosis of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:307–314. doi: 10.1002/alr.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeConde AS, Mace JC, Smith TL. The impact of comorbid migraine on quality-of-life outcomes after endoscopic sinus surgery. Laryngoscope. 2014;124:1750–1755. doi: 10.1002/lary.24592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope. 2003;113:1199–1205. doi: 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA(2)LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 79.Thilsing T, Rasmussen J, Lange B, et al. Chronic rhinosinusitis and occupational risk factors among 20- to 75-year-old Danes-A GA(2) LEN-based study. American journal of industrial medicine. 2012;55:1037–1043. doi: 10.1002/ajim.22074. [DOI] [PubMed] [Google Scholar]
- 80.Tammemagi CM, Davis RM, Benninger MS, et al. Secondhand smoke as a potential cause of chronic rhinosinusitis: a case-control study. Arch Otolaryngol Head Neck Surg. 2010;136:327–334. doi: 10.1001/archoto.2010.43. [DOI] [PubMed] [Google Scholar]
- 81.Lieu JE, Feinstein AR. Confirmations and surprises in the association of tobacco use with sinusitis. Arch Otolaryngol Head Neck Surg. 2000;126:940–946. doi: 10.1001/archotol.126.8.940. [DOI] [PubMed] [Google Scholar]
- 82.Briggs RD, Wright ST, Cordes S, Calhoun KH. Smoking in chronic rhinosinusitis: a predictor of poor long-term outcome after endoscopic sinus surgery. Laryngoscope. 2004;114:126–128. doi: 10.1097/00005537-200401000-00022. [DOI] [PubMed] [Google Scholar]
- 83.Ramadan HH, Hinerman RA. Smoke exposure and outcome of endoscopic sinus surgery in children. Otolaryngol Head Neck Surg. 2002;127:546–548. doi: 10.1067/mhn.2002.129816. [DOI] [PubMed] [Google Scholar]
- 84.Bousquet J, Van Cauwenberge P, Bachert C, et al. Requirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma (ARIA) Allergy. 2003;58:192–197. doi: 10.1034/j.1398-9995.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 85.Lee WK, Ramanathan M, Jr, Spannhake EW, Lane AP. The cigarette smoke component acrolein inhibits expression of the innate immune components IL-8 and human beta-defensin 2 by sinonasal epithelial cells. Am J Rhinol. 2007;21:658–663. doi: 10.2500/ajr.2007.21.3094. [DOI] [PubMed] [Google Scholar]
- 86.Min YG, Jung HW, Kim HS, et al. Prevalence and risk factors of chronic sinusitis in Korea: results of a nationwide survey. Eur Arch Otorhinolaryngol. 1996;253:435–439. doi: 10.1007/BF00168498. [DOI] [PubMed] [Google Scholar]
- 87.Wolf C. Urban air pollution and health: an ecological study of chronic rhinosinusitis in Cologne, Germany. Health & place. 2002;8:129–139. doi: 10.1016/s1353-8292(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 88.Bhattacharyya N. Air quality influences the prevalence of hay fever and sinusitis. Laryngoscope. 2009;119:429–433. doi: 10.1002/lary.20097. [DOI] [PubMed] [Google Scholar]
- 89.Yu HL, Chien LC, Yang CH. Asian dust storm elevates children’s respiratory health risks: a spatiotemporal analysis of children’s clinic visits across Taipei (Taiwan) PloS one. 2012;7:e41317. doi: 10.1371/journal.pone.0041317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yeo NK, Hwang YJ, Kim ST, et al. Asian sand dust enhances rhinovirus-induced cytokine secretion and viral replication in human nasal epithelial cells. Inhalation toxicology. 2010;22:1038–1045. doi: 10.3109/08958378.2010.516282. [DOI] [PubMed] [Google Scholar]
- 91.Matsui EC. Environmental exposures and asthma morbidity in children living in urban neighborhoods. Allergy. 2014;69:553–558. doi: 10.1111/all.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rumchev KB, Spickett JT, Bulsara MK, et al. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur Respir J. 2002;20:403–408. doi: 10.1183/09031936.02.00245002. [DOI] [PubMed] [Google Scholar]
- 93.Rumchev K, Spickett J, Bulsara M, et al. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59:746–751. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J, Hanley JA. The role of woodstoves in the etiology of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2002;128:682–686. doi: 10.1001/archotol.128.6.682. [DOI] [PubMed] [Google Scholar]
- 95.Hellgren J. Occupational rhinosinusitis. Current allergy and asthma reports. 2008;8:234–239. doi: 10.1007/s11882-008-0039-1. [DOI] [PubMed] [Google Scholar]
- 96.Riu E, Dressel H, Windstetter D, et al. First months of employment and new onset of rhinitis in adolescents. Eur Respir J. 2007;30:549–555. doi: 10.1183/09031936.00149206. [DOI] [PubMed] [Google Scholar]
- 97.Radon K, Gerhardinger U, Schulze A, et al. Occupation and adult onset of rhinitis in the general population. Occupational and environmental medicine. 2008;65:38–43. doi: 10.1136/oem.2006.031542. [DOI] [PubMed] [Google Scholar]
- 98.Moscato G, Pignatti P, Yacoub MR, et al. Occupational asthma and occupational rhinitis in hairdressers. Chest. 2005;128:3590–3598. doi: 10.1378/chest.128.5.3590. [DOI] [PubMed] [Google Scholar]
- 99.Elliott L, Heederik D, Marshall S, et al. Incidence of allergy and allergy symptoms among workers exposed to laboratory animals. Occupational and environmental medicine. 2005;62:766–771. doi: 10.1136/oem.2004.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buss ZS, Frode TS. Latex allergen sensitization and risk factors due to glove use by health care workers at public health units in Florianopolis, Brazil. J Investig Allergol Clin Immunol. 2007;17:27–33. [PubMed] [Google Scholar]


