Abstract
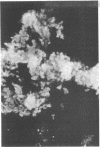
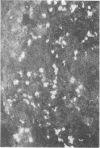
Immunofluorescent techniques were utilized to identify the types of infiltrating lymphocytes adjacent to human malignant tumors arising from a wide range of anatomic sites. 24 of 29 primary tumors and 5 of 8 metastatic lesions showed varying degrees of lymphocytic infiltration. T cells predominated in the infiltrates in primary tumors (mean 80%, range 50-100%) and this pattern was evident regardless of anatomic site or the presence or absence of metastatic spread. By contrast, B cells predominated at the margins of three of five tumor metastases. Mononuclear cells bearing the Fc receptor were not a prominent component of the infiltrates associated with either primary tumors or metastases, but tumor cell binding of fluoresceinated IgG aggregates was observed in 12 of 29 primary tumors. A significant reduction in peripheral blood T cell numbers occurred in a third of the patients studied. This decrease was not clearly related either to the extent of local tumor T cell infiltration or to the presence of disseminated disease. These preliminary findings provide a descriptive analysis of the local and systemic distributions of immunocompetent cells in cancer.
Full text
PDF

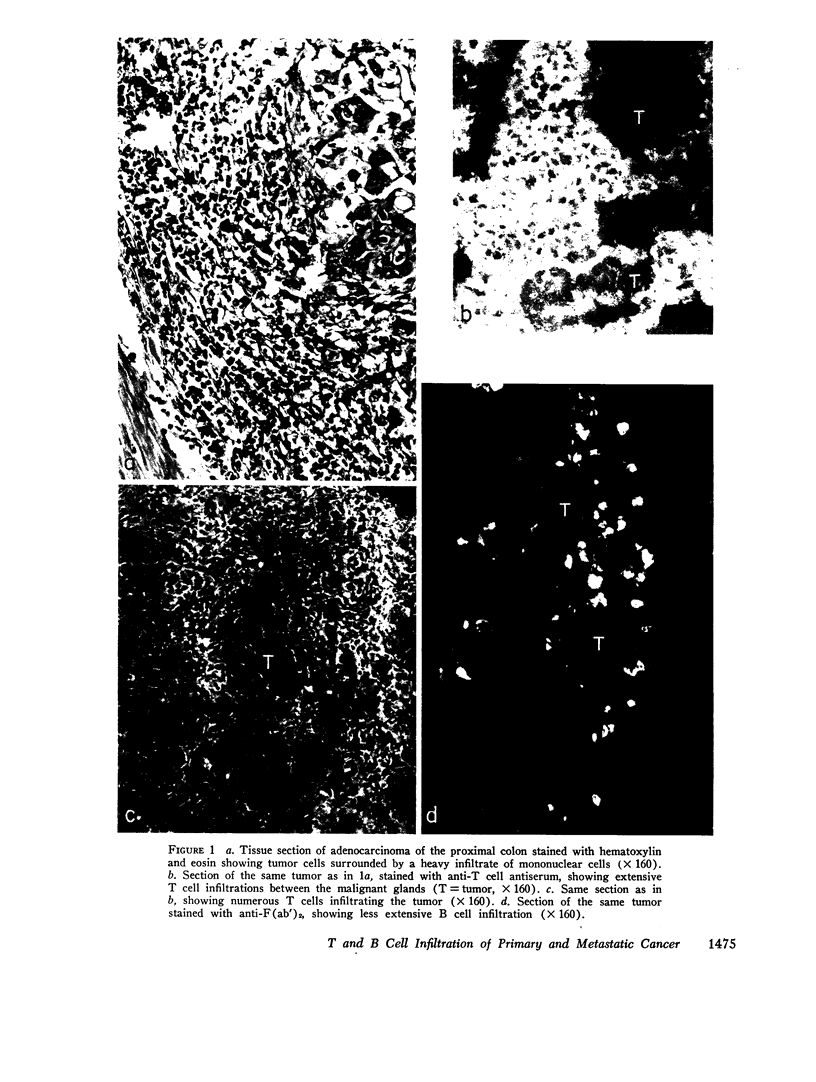
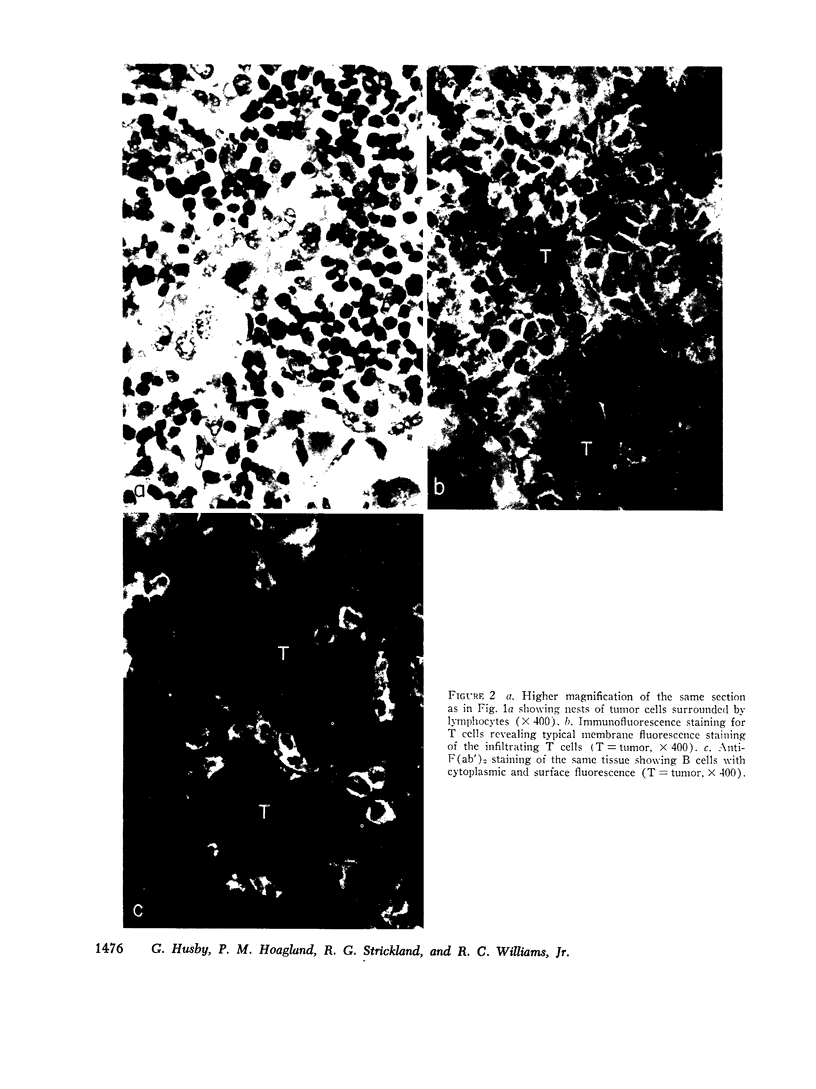

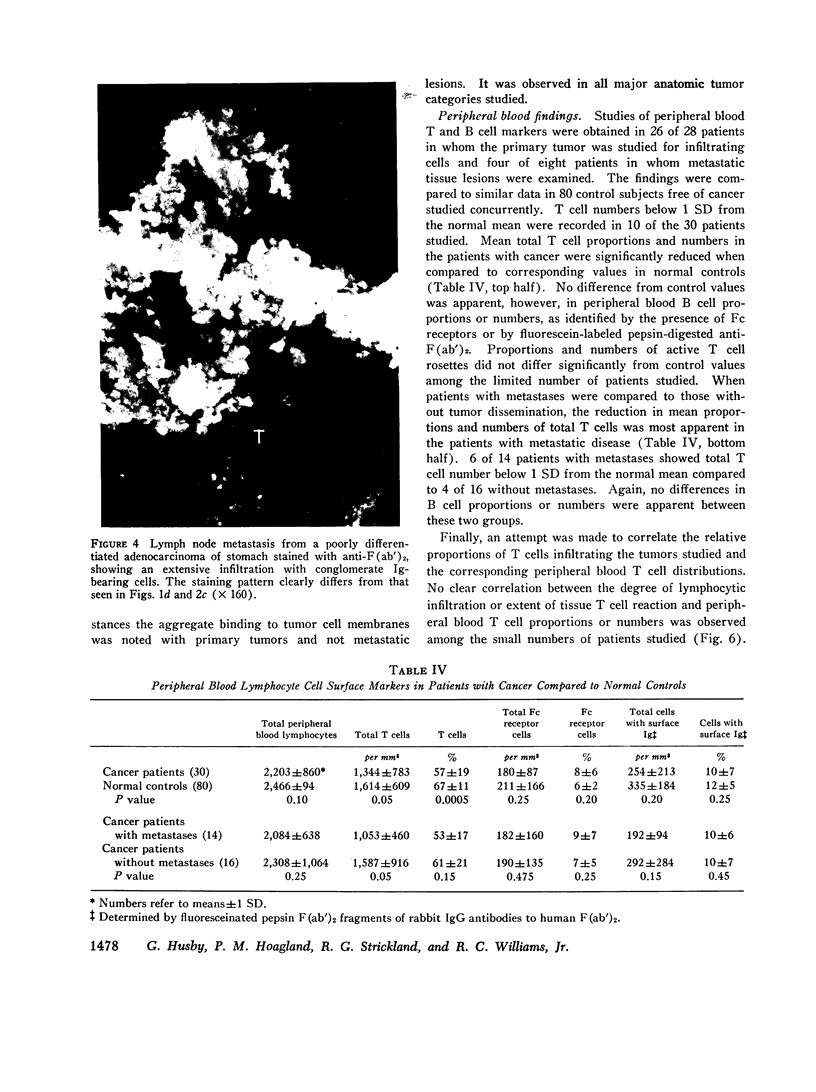
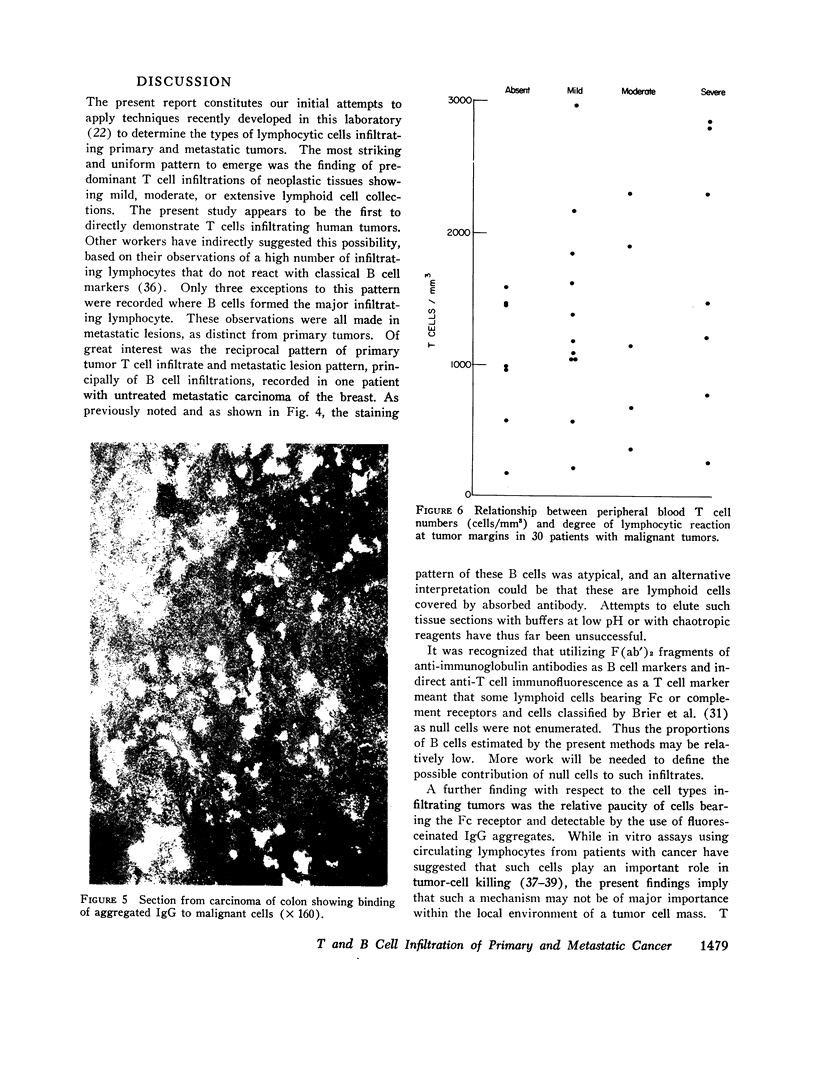



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony H. M., Kirk J. A., Madsen K. E., Mason M. K., Templeman G. H. E and EAC rosetting lymphocytes in patients with carcinoma of bronchus I. Some parameters of the test and of its prognostic significance. Clin Exp Immunol. 1975 Apr;20(1):29–40. [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- BLACK M. M., SPEER F. D. Sinus histiocytosis of lymph nodes in cancer. Surg Gynecol Obstet. 1958 Feb;106(2):163–175. [PubMed] [Google Scholar]
- Brier A. M., Chess L., Schlossman S. F. Human antibody-dependent cellular cytotoxicity. Isolation and identification of a subpopulation of peripheral blood lymphocytes which kill antibody-coated autologous target cells. J Clin Invest. 1975 Dec;56(6):1580–1586. doi: 10.1172/JCI108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas A., Han T., Takita H., Minowada J. Immunologic assays in lung cancer. Skin tests, lymphocyte blastogenesis, and rosette-forming cell count. N Y State J Med. 1973 Mar 15;73(6):747–750. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Human T lymphocyte "E" rosette function. I. A process modulated by intracellular cyclic AMP. J Exp Med. 1974 Oct 1;140(4):1122–1126. doi: 10.1084/jem.140.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Lymphocyte E rosette inhibitory factor: a regulatory serum lipoprotein. J Exp Med. 1975 Nov 1;142(5):1092–1107. doi: 10.1084/jem.142.5.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Saffer E., Fisher B. Studies concerning the regional lymph node in cancer. VI. Correlation of lymphocyte transformation of regional node cells and some histopathologic discriminants. Cancer. 1973 Jul;32(1):104–111. doi: 10.1002/1097-0142(197307)32:1<104::aid-cncr2820320114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Forman J., Britton S. Heterogeneity of the effector cells in the cytotoxic reaction against allogeneic lymphoma cells. J Exp Med. 1973 Feb 1;137(2):369–386. doi: 10.1084/jem.137.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R. A., Finstad J. Essential relationship between the lymphoid system, immunity, and malignancy. Natl Cancer Inst Monogr. 1969 Jul;31:41–58. [PubMed] [Google Scholar]
- Goodman D. B., Bloom F. E., Battenberg E. R., Rasmussen H., Davis W. L. Immunofluorescent localization of cyclic AMP in toad urinary bladder: possible intercellular transfer. Science. 1975 Jun 6;188(4192):1023–1025. doi: 10.1126/science.167437. [DOI] [PubMed] [Google Scholar]
- Hall M. G., Jr, Chung E. B., Leffall L. D., Jr The probable immunological role of regional lymph nodes in simulated colon carcinoma in the rabbit. J Natl Med Assoc. 1972 Mar;64(2):102–passim. [PMC free article] [PubMed] [Google Scholar]
- Hellström I. E., Hellström K. E., Pierce G. E., Bill A. H. Demonstration of cell-bound and humoral immunity against neuroblastoma cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1231–1238. doi: 10.1073/pnas.60.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Pierce G. E., Fefer A. Studies on immunity to autochthonous mouse tumors. Transplant Proc. 1969 Mar;1(1):90–94. [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Lichtenstein L. M. The role of cyclic AMP in the cytolytic activity of lymphocytes. J Immunol. 1971 Aug;107(2):610–612. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Husby G., Strickland R. G., Caldwell J. L., Williams R. C., Jr Localization of T and B cells and alpha fetoprotein in hepatic biopsies from patients with liver disease. J Clin Invest. 1975 Nov;56(5):1198–1209. doi: 10.1172/JCI108197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALISS N. Immunological enhancement of tumor homografts in mice: a review. Cancer Res. 1958 Oct;18(9):992–1003. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Lueker R. D., Abdin Z. H., Williams R. C., Jr Peripheral blood T and B lymphocytes during acute rheumatic fever. J Clin Invest. 1975 May;55(5):975–985. doi: 10.1172/JCI108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole C., Stejskal V., Perlmann P., Karlsson M. Lymphoid cells mediating tumor-specific cytotoxicity to carcinoma of the urinary bladder. Separation of the effector population using a surface marker. J Exp Med. 1974 Mar 1;139(3):457–466. doi: 10.1084/jem.139.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt D. J., Brynes R. K., Vardiman J. W., Coppleson L. W. Mesocolic lymph node histology is an important prognostic indicator for patients with carcinoma of the sigmoid colon: an immunomorphologic study. Cancer. 1975 May;35(5):1388–1396. doi: 10.1002/1097-0142(197505)35:5<1388::aid-cncr2820350523>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Pilch Y. H., Golub S. H. Lymphocyte-mediated immune responses in neoplasia. Am J Clin Pathol. 1974 Aug;62(2):184–211. doi: 10.1093/ajcp/62.2.184. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Smith A. H., Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A. 1975 May;72(5):1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin C., Tarpley J. L., Chretien B. Thymus-derived lymphocytes in patients with solid malignancies. Clin Immunol Immunopathol. 1975 Mar;3(4):476–481. doi: 10.1016/0090-1229(75)90072-0. [DOI] [PubMed] [Google Scholar]
- Richters A., Kaspersky C. L. Surface immunoglobulin positive lymphocytes in human breast cancer tissue and homolateral axillary lymph nodes. Cancer. 1975 Jan;35(1):129–133. doi: 10.1002/1097-0142(197501)35:1<129::aid-cncr2820350118>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Jaffe E. S., Green I. Receptors for complement and immunoglobulin on human and animal lymphoid cells. Transplant Rev. 1973;16:3–28. doi: 10.1111/j.1600-065x.1973.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Stjernswärd J., Jondal M., Vánky F., Wigzell H., Sealy R. Lymphopenia and change in distribution of human B and T lymphocytes in peripheral blood induced by irradiation for mammary carcinoma. Lancet. 1972 Jun 24;1(7765):1352–1356. doi: 10.1016/s0140-6736(72)91091-4. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N., Sylvester R. A., Daniels T. E., Greenspan J. S., Williams R. C., Jr T and B lymphocytes in peripheral blood and tissue lesions in Sjögren's syndrome. J Clin Invest. 1974 Jan;53(1):180–189. doi: 10.1172/JCI107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonder O., Morse P. A., Jr, Humphrey L. J. Similarities of Fc receptors in human malignant tissue and normal lymphoid tissue. J Immunol. 1974 Oct;113(4):1162–1169. [PubMed] [Google Scholar]
- Tonder O., Thunold S. Receptors for immunoglobulin Fc in human malignant tissues. Scand J Immunol. 1973;2(3):207–215. doi: 10.1111/j.1365-3083.1973.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Tsakraklides V., Anastassiades O. T., Kersey J. H. Prognostic significance of regional lymph node histology in uterine cervical cancer. Cancer. 1973 Apr;31(4):860–868. doi: 10.1002/1097-0142(197304)31:4<860::aid-cncr2820310415>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Stobo J. D., Paul W. E., Green I. Antibody-dependent lymphoid cell-mediated cytotoxicity: no requirement for thymus-derived lymphocytes. Science. 1972 Jan 14;175(4018):194–196. doi: 10.1126/science.175.4018.194. [DOI] [PubMed] [Google Scholar]
- Van D. E., Paxton L. L. Chemotaxis as a preparative technique for human polymorphonuclear leukocytes. J Lab Clin Med. 1975 Aug;86(2):309–314. [PubMed] [Google Scholar]
- Watson J., Epstein R., Cohn M. Cyclic nucleotides as intracellular mediators of the expression of antigen-sensitive cells. Nature. 1973 Dec 14;246(5433):405–409. doi: 10.1038/246405a0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]