Abstract
For patients with end-stage heart failure, cardiac transplantation persists to be the gold standard. Nevertheless, the availability of organs remains a main constraint to the treatment. Through mounting usage of ex vivo heart perfusion an increase in organ availability was achieved by reconditioning of organs formerly not regarded as appropriate for transplantation. We propose the future standard application of this state-of-the-art technology to improve the pool of donor organs by evaluating hearts outside standard acceptability criteria.
MeSH Keywords: Death, Sudden, Cardiac; Donor Selection; Heart Transplantation
Due to the rate of medically applicable donors staying comparatively fixed worldwide, clinicians have explored new approaches to cope with the ever-increasing demand of heart transplant lists globally. Consequently, the transplant community has turned to various methods to counterbalance this discrepancy. Technological developments targeting the ex vivo “preservation” period may prove supportive in enlarging the donor pool by increasing the usage as well as by advancing the results of marginal donor hearts.
As the first commercially accessible system, the TransMedics Organ Care System (OCS; TransMedics, Inc., Andover, MA, USA) makes it possible for the beating donor heart to be preserved in a warm (34°C) perfused oxygenated condition throughout transfer from donor to recipient, thus enabling a prolonged “out of body” period of minimum 8 hours with extending potential geographic zones for organ procurement and reducing the damaging effects of cold ischemic storage. Additionally, the OCS can be applied to perfusion, ventilation, and monitoring of donor lungs between the donor and recipient locations in a near-physiologic state.
Our group released the first report of heart transplantation applying the OCS in connection with an adverse donor-recipient risk profile [1]. The very promising early results showed the clinical efficacy of this method. Allografts subjected to lengthier downtimes may have shown minimized left ventricular function, augmented troponin levels, or regional wall motion abnormalities. In particular, the OCS is highly suitable for evaluation of these “extended criteria” donor hearts with reduced left ventricular ejection fraction, left ventricular hypertrophy, previous donor cardiac arrest, prolonged predicted ischemic time (> 4 hours), alcohol/substance abuse, and unknown coronary artery disease status due to a lack of coronary angiography.
In general, the OCS facilitates improvement of logistics, meaning: valuation of extended criteria allografts and careful preparation of recipients, especially those who have had LVAD implantation with previous sternotomies. In these cases, we detected a low occurrence of right heart failure, improved allograft function, reduced blood transfusion requirement, as well as shorter intensive care unit and hospital stays. The OCS has proven beneficial to “resuscitate” marginal organs by decreasing ischemia/reperfusion injury. Furthermore, it may contribute to enhancing function of “marginal” heart allografts. It extends the donor pool by utilizing organs formerly not regarded suitable for transplantation and diminishes the risk of primary allograft failure – a promising fact considering the combined risk profile of donor and recipient. As a consequence of highly auspicious postoperative results, usage of the OCS is now standard of care at our institution. Currently, a multicenter Food and Drug Administration (FDA)-approved single-arm non-randomized trial is in progress in the United States that will assess the degree of usage of extended criteria donor hearts by application of the OCS and early results subsequently to transplantation.
In addition, we were able to show that hearts from non-heparinized, donation after cardiac death (DCD) porcine donors can be successfully resuscitated by applying the OCS in a setting that accurately simulates clinical conditions [2]. We have conducted a feasibility study on grounds of ex vivo resuscitation and valuation of the graft succeeding circulatory death which complies with DCD donation practice in the UK.
By using a pilot swine model we could validate that hearts can be procured subsequently to “circulatory death” with agonal times in excess of 20 minutes as well as resuscitated in the OCS, leading to comparable metabolic and hemodynamic parameters to those of hearts that have been transplanted successfully in humans. We showed that three out of five organs sustained hemodynamic stability, good contractility on visual assessment with minimizing lactate levels in the perfusate throughout ex vivo perfusion, myocardial lactate extraction with lower venous than arterial concentration and perfusate lactate concentration below 2 mmol/L by the second hour and consequently would have been regarded appropriate for transplantation according to criteria of acceptability.
Donation following cardiac death has resulted in an increase of available organs; the retrieval of hearts from said donors, however, has been controversial [3,4]. Four categories of DCD were defined as follows by the Maastricht group [5]: type I are dead on arrival and have not been resuscitated; type II are unsuccessfully resuscitated, type III are typical controlled DCD, with planned cardiac arrest; and type IV are planned donations following brain death (DBD) that suddenly arrest during or after the brain death determination. Of these, type I, II and IV are regarded uncontrolled DCD. For these donors, cardiopulmonary resuscitation is typically conducted until organ recovery procedures are employed.
Recently, the Sydney group published their preclinical results describing a porcine orthotopic heart transplant DCD asphyxia model. The hearts were exposed to 30 minutes of warm ischemia and preserved with either OCS or cold storage Celsior solution. The primary endpoint of the study was the ability to wean off cardiopulmonary bypass (CPB) after orthotopic heart transplant and maintain hemodynamic stability for at least 3 hours post weaning from CPB. Following preservation, the OCS group demonstrated favorable lactate profiles and all hearts out of this group were successfully transplanted. By contrast, no pig could be weaned off CPB in the Celsior preservation group [6].
These excellent results were followed by the world’s first series of successful adult heart transplant procedures from DCD donors performed by the St. Vincent’s Hospital heart transplant team in Australia using the OCS Heart technology. This crucial step represents a landmark in heart transplantation and promises a new era in donor pool expansion [7].
Moreover, retrieving hearts from this donor group can have a substantial effect on transplant activity, especially in countries such as the UK where the availability of donor organs has been stagnant in the recent past. A variety of scenarios have been suggested in which DCD hearts could be utilized; most of them include re-establishing blood flow within the donor through several methods, which led to the emergence of noteworthy ethical concerns due to the physiological status of the donor. Other methods for cardiac procurement involve resuscitation of the heart within the donor by re-establishing the circulation by either external support or by reanimating the heart itself. Both within and outside the transplant community there have been intense discussions arising about the ethical and legal matters and the potential opposing response of the general public to a methodology of that kind.
If these donors turn out to offer a feasible source for transplantable organs, protocols allowing for in situ perfusion prior to consent may be taken into consideration. It is a promising fact that centers that have devised protocols of this sort have reported high percentage of family consent and recovery rates. Controlled DCD protocols that comprise cannulation or donor pretreatment ahead of death declaration have resulted in ethical concerns within society, which have been adequately evaluated and accepted by both the transplant community as well as the public.
Apart from ethical concerns, devising complex uncontrolled DCD programs calls for considerable resource dedication, as well as broad cooperation between transplant centers, field providers and emergency departments. Whereas the usage of in situ perfusion may lead to deferred recovery from uncontrolled DCD in situations where instant operating room availability is unfeasible, this alternative may only be available to transplant centers and donor hospitals with the respective technology and swift access to admission teams. The possible size of this donor pool remains unidentified and is likely to differ with regards to geography and demographics. Nonetheless, comparable difficulties have been successfully tackled in the development of controlled DCD transplantation [8].
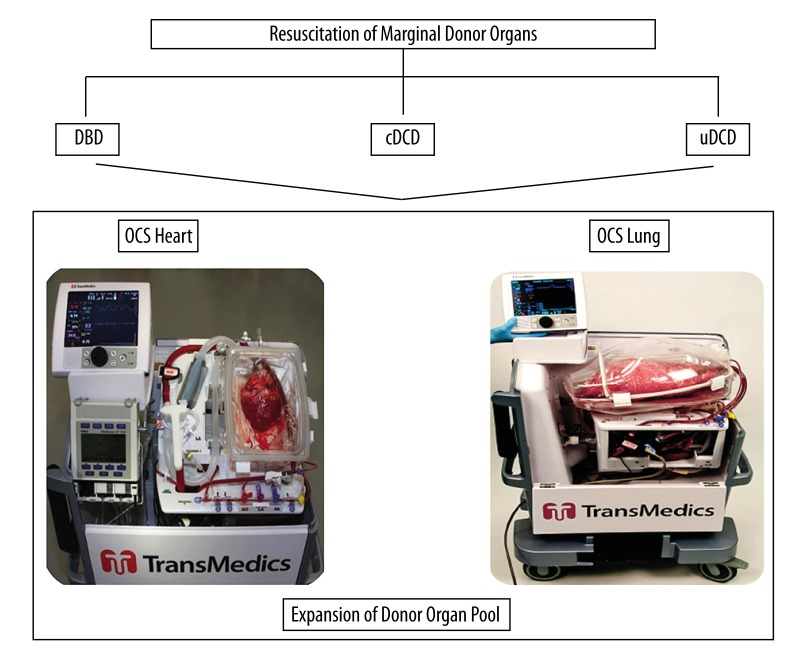
We suggest to obtain the heart in DCD donors together with other organs before ex vivo reconditioning and assessment by means of the OCS (Figure 1). The system permits immediate connection of the graft to the system at the donor site with sustained warm perfusion of a resuscitated organ up until implantation in the recipient hospital, thus reducing the harmful results of cold storage ischemia and supplying continuous assessment of graft viability subsequently to donor death, which is essential due to the organ unavoidably having been exposed to ischemic injury before procurement, the reversibility of which is otherwise indeterminate.
Figure 1.
Schematic illustration of OCS paradigm in donor organ pool expansion. The system permits direct cannulation of the graft to the system at the donor site with uninterrupted warm perfusion of a resuscitated organ until implantation, thus reducing the harmful effects of cold storage, ischemia/reperfusion injury and affording continued assessment of graft viability after donor death with enhancement of “marginal” donor allografts. DBD, donation after brain death; cDCD, controlled donation after cardiac death; uDCD, uncontrolled donation after cardiac death; OCS, Organ Care System
On the grounds of our findings, we anticipate to evaluate the viability of the method in the clinical DCD environment with the eventual goal of enhancing the amount of donor organs for transplantation.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Source of support: Self financing
References
- 1.García Sáez D, Zych B, Sabashnikov A, et al. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann Thorac Surg. 2014;98:2099–105. doi: 10.1016/j.athoracsur.2014.06.098. discussion 2105–6. [DOI] [PubMed] [Google Scholar]
- 2.García Sáez D, Elbetanony A, Lezberg P, et al. Ex vivo heart perfusion after cardiocirculatory death; a porcine model. J Surg Res. 2014 doi: 10.1016/j.jss.2014.12.039. pii: S0022-4804(14)01157-3 [Epubahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Boucek M, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;359:709–14. doi: 10.1056/NEJMoa0800660. [DOI] [PubMed] [Google Scholar]
- 4.Veatch RM. Donating hearts after cardiac death – reversing the irreversible. N Engl J Med. 2008;359:672–73. doi: 10.1056/NEJMp0805451. [DOI] [PubMed] [Google Scholar]
- 5.Daemen JW, Kootstra G, Wijnen RM, et al. Nonheart-beating donors: The Maastricht experience. Clin Transplant. 1994:303–16. [PubMed] [Google Scholar]
- 6.Iyer A, Gao L, Doyle A, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant. 2015;15:371–80. doi: 10.1111/ajt.12994. [DOI] [PubMed] [Google Scholar]
- 7.St Vincent’s Health Australia newsroom. https://svha.org.au/home/newsroom/announcements/first-dcd-heart-transplant.
- 8.Popov AF, Sabashnikov A, Patil NP, et al. Ex vivo lung perfusion – state of the art in lung donor pool expansion. Med Sci Monit Basic Res. 2015;21:9–14. doi: 10.12659/MSMBR.893674. [DOI] [PMC free article] [PubMed] [Google Scholar]