Abstract
Background
Premature birth is the leading cause of neonatal death and second leading in children under 5. Information on outcomes of preterm babies surviving the early neonatal period is sparse although it is considered a major determinant of immediate and long-term morbidity.
Methods
Systematic review of studies reporting outcomes for preterm babies in low and middle income settings was conducted using electronic databases, citation tracking, expert recommendations and “grey literature”. Reviewers screened titles, abstracts and articles. Data was extracted using inclusion and exclusion criteria, study site and facilities, assessment methods and outcomes of mortality, morbidity, growth and development. The Child Health Epidemiology Reference Group criteria (CHERG) were used to assess quality.
Findings
Of 197 eligible publications, few (10.7%) were high quality (CHERG). The majority (83.3%) report on the outcome of a sample of preterm babies at time of birth or admission. Only 16.0% studies report population-based data using standardised mortality definitions. In 50.5% of studies, gestational age assessment method was unclear. Only 15.8% followed-up infants for 2 years or more. Growth was reported using standardised definitions but recommended morbidity definitions were rarely used. The criteria for assessment of neurodevelopmental outcomes was variable with few standardised tools - Bayley II was used in approximately 33% of studies, few studies undertook sensory assessments.
Conclusions
To determine the relative contribution of preterm birth to the burden of disease in children and to inform the planning of healthcare interventions to address this burden, a renewed understanding of the assessment and documentation of outcomes for babies born preterm is needed. More studies assessing outcomes for preterm babies who survive the immediate newborn period are needed. More consistent use of data is vital with clear and aligned definitions of health outcomes in newborn (preterm or term) and intervention packages aimed to save lives and improve health.
Introduction
The proportion of all deaths in children under-five years that occur in the first four weeks of life (neonatal death) has increased from 36% in 1990 to 43% in 2011 and 75% of neonatal deaths occur in the first week of life [1]. Of the estimated 7.6 million deaths in children under 5 years of age, an estimated 17%, are attributed to prematurity [1]. and approximately 35% are attributed to preterm birth (before 37 completed weeks or 259 days of pregnancy) [2], making prematurity the leading cause of neonatal death and the second leading cause of death in children under five years old [1].
Globally, around 10–11% of all births, or an estimated 15 million births per year, are estimated to be born preterm (before 37 weeks gestation) [3,4]. The incidence of preterm birth is around 10.6% in North America and 6.2% in Europe [3]. There are fewer reliable estimates from low and middle income settings because of uncertainty around assessment of gestational age and reliance on low birth weight as a proxy measure. However the incidence of preterm birth in these settings is considerably higher with estimates of between 15 and 24% in ultrasound dated population studies in African settings [5–8]. Although the reported rates of preterm birth are highest in sub Saharan Africa, the highest absolute number of preterm births occurs in Asia [4].
Preterm birth accounts for more than one million neonatal deaths per year. Information on the outcomes of babies born preterm but who survive the early neonatal period is very sparse [9,10]. Preterm birth is considered to be a major determinant of immediate as well as long term morbidity and is associated with growth and developmental delay. To be able to determine the relative contribution of preterm birth to the burden of disease in children under five years and to inform the planning of healthcare interventions to address this burden, a renewed understanding of the assessment and documentation of outcomes for babies born preterm is needed [11].
We undertook a systematic review of studies which report on outcomes for babies reported to be born preterm in low and middle income settings. For each study, we included the method of assessment of prematurity and outcomes including mortality, morbidity, growth and development.
Methods
Search strategy
A systematic search of all published literature using the following databases without language restrictions was conducted: Pubmed, Cochrane, Scopus, Ovid SP, Embase, WHO Regional Databases, CINAHL, American Psychological Association and Google. Search terms used included the MeSH terms and are shown below:
"Premature Birth" AND "Infant, Low Birth Weight" [MeSH] AND "Developing Countries" [Majr] AND “Outcome studies” OR “outcome assessment” OR “outcome measures” OR “treatment outcome” OR “outcome*” OR “endpoint”
We included the MeSH term of “low birth weight” as some papers used low birth weight as a proxy measure for prematurity. The search was repeated using a definitive list of 150 developing countries from the International Monetary Fund’s World Economic Outlook Report [12]. Snowball searching was done to identify additional key papers missed.
Study selection
Inclusion criteria for final review were primary research articles published after 1980 up to July 2014 which included 1) information on the follow up and any outcomes (survival, morbidity, growth and development) of prematurely born infant in any low and middle income country (according to World Bank criteria above) and 2) longitudinal cohort studies, randomised controlled trials and cross sectional studies and 3) full text articles for evaluation of all study components. Papers were excluded if they 1) had no information on outcomes of preterm birth (survival, morbidity, growth and development) 2) were inadvertently from a non-developing country setting 3) were systematic reviews with no direct data to inform our research question and 4) were case studies.
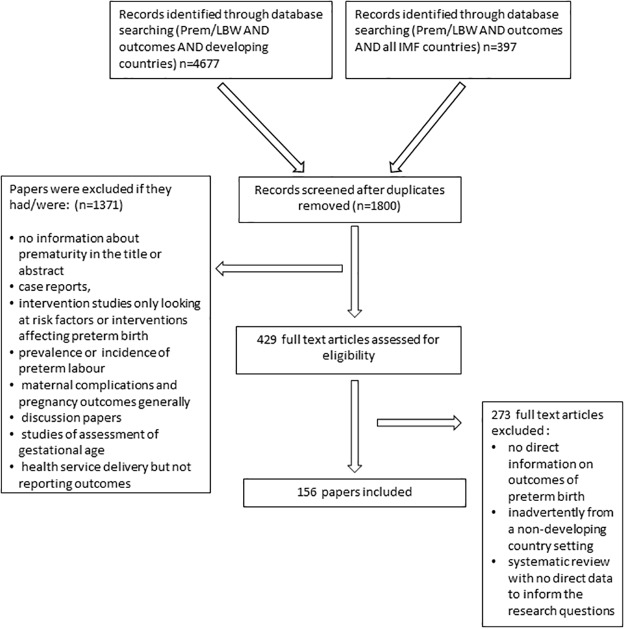
We identified a total of 5321 titles through our searches. Two investigators screened these by title. Of 2023 eligible papers identified after duplicates had been removed, 902 full abstracts were then screened with 456 full text articles reviewed by the two investigators. Data items sought were information regarding recruitment, gestational age, methods of assessing and outcomes relating to mortality, morbidity, growth and development of infants (see S1 Dataset). Data was then extracted into a table which was piloted and reconfigured particularly relating the categorisation of place and time of recruitment, level of neonatal care which could be accessed and categorisation of mortality of infants (PNMR, NNMR, IMR). Where there were discrepancies in assessment, a third reviewer was consulted. In total, 197 publications were considered eligible for inclusion in this review (Fig. 1). We used the Child Health Epidemiology Reference Group (CHERG) criteria to assess the quality of articles [13].
Fig 1. PRISMA Diagram.
Results
A summary table of all included studies is provided (S1 Dataset).
Quality of articles
Papers were assessed for quality using the CHERG criteria: the majority were of low quality (101/197, 51.3%) with 21 papers judged to be of high quality. Many were case series or small cohort studies particularly those from neonatal facilities in low income settings. For observational studies, if the research took into account all plausible confounders, then they would be upgraded.
Study Setting
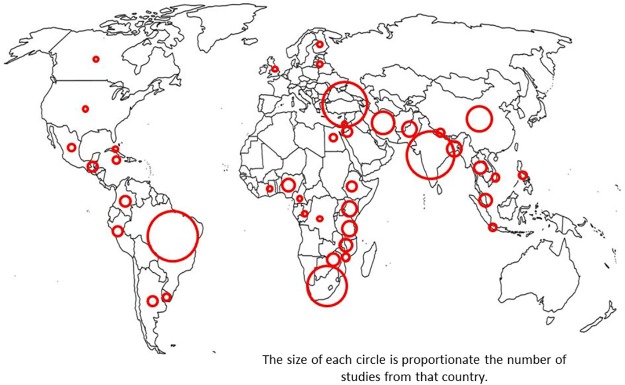
Equal numbers were from Asia (62/197, 31.5%) and Sub-Saharan Africa (56/197, 28.4%); 32 (16.2%) papers from Latin America and the West Indies, 43 (21.8%) from the Middle East and Eastern Europe and two reported on outcomes from multiple settings (Fig. 2). Out of studies from a single setting, 41/197 (20%) were from a low income setting, 48/197 (24%) from a low-middle income setting and just over half (103/197 (52%)) were from a higher middle income setting.
Fig 2. Geographical Distribution of Included Studies.
The majority of studies (164/197, 83.7%) report on outcomes of a sample of babies born preterm and recruited into the study at the time of birth or at the time of admission to a special care baby unit at a health facility. For the purpose of this review we have referred to these studies as ‘facility based studies’. Only 16.0% (31/197) of studies were from a community setting i.e. babies and/or mothers not recruited at time of visit to a health care facility but from among the general population usually at antenatal visits within a representative area serving a population. Two thirds of these studies (N = 22) came from low or low middle income settings. A total of 17.8% (35/197) studies reported population based data (sample size studied was all babies in the general population studied or a representative sample of babies from the general population) such as studies from Ghana [14,15], Nepal [16], Tanzania [17], Malawi [18] and Guatemala [19]. This included facility based studies from Brazil, China and Chile where it was reported that almost all births in the area would have taken place in a hospital [20–25]. In general, facility based studies tended to be small case control or cohort studies whereas community based studies were often larger population based observational cohort studies.
Just over half of the facility based studies reported on babies who had been admitted to a special/intensive care baby unit (114/197, 58.2%). This included 19 (9.7%) studies for babies from a special care unit with no ventilation (almost all from low or low middle income settings) and 63 (32.1%) from a neonatal intensive care unit with ventilator facilities. Papers reporting on outcomes from settings where neonatal care (including ventilation) was available were predominantly from the high middle income countries particularly the Middle East but included papers from Turkey, China, South Africa, Zimbabwe, India and Pakistan. In 32/114 (28.0%) of the studies reporting on babies admitted to a baby care unit, it was not clear what type of neonatal care was available. Often this was labelled as “neonatal intensive care” or ‘neonatal care facilities’ without specific description of content.
Population
The numbers of infants included in the studies varied from 10–12 in one study of outcomes of preterm infants receiving continuous positive airway pressure (CPAP) in a neonatal intensive care unit [26] to 2.9 million infants born in a national study of neonatal survival conducted over 10 years in Chile [27]. The majority of papers (169/197, 86.2%) included in the review were cohort studies. Only three studies followed up on only the preterm infants born from a representative sample of the population [3,20,28]. More commonly, preterm infants were followed up as part of a subset of a larger study looking at neonatal outcomes of all infants (or those born low birth weight) in a community.
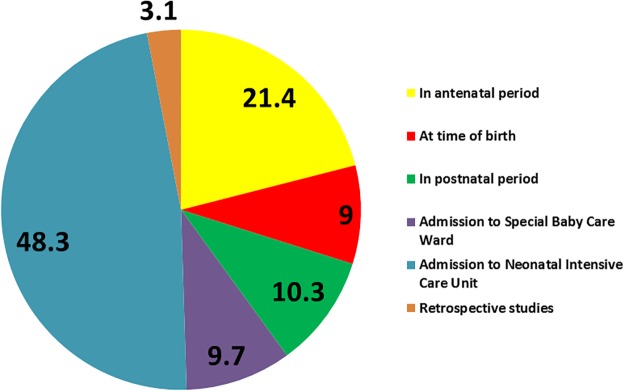
In 20% of studies, the parent cohort was identified during the antenatal period. For other studies, preterm birth was determined at the time of birth, in the post natal period or at time of recruitment to a newborn care facility (Fig. 3).
Fig 3. Setting and timing of recruitment for studies reporting on outcome of preterm birth in low and middle income settings (% studies).
In the majority of studies (114/197, 58%) outcomes are reported for babies admitted to a neonatal intensive care or special care unit (or unit named as a neonatal unit but with facilities not specified). Frequently, studies reported on a subgroup that had complications such as respiratory distress syndrome, intra-ventricular haemorrhage or retinopathy of prematurity.
The majority of studies were prospective but did not often include comparison groups and were unclear about the inclusion or exclusion of twin infants, a likely confounder in studies of outcomes of prematurity [29]. Just over a third (58/156, 37.2%) reported including twins.
Length of follow up
Length of follow up varied from only 24–72 hours [30,31] to 15 years in the recent cohort studies from Brazil, India, Philippines, South Africa, Guatemala [32,33]. In half of all studies, the period of follow up was not clear. This includes a quarter (50/197, 25.5%) of studies with the period of follow up defined as “until discharge” from a health care facility and 23.9% (47/197) with no information at all provided about length of follow up. 15.8% (31/197) of studies followed up infants for 2 years or more. One recent study reports on the long term growth, blood glucose level and blood pressure outcomes in cohorts of children for over 15 years in Brazil, India, Philippines, South Africa and Guatemala, demonstrating some differences in long term height attainment of those born premature [32,33]. Two groups reported on long term cognitive and educational outcomes of infants born prematurely—the group in India [34] and that from the recent Stein paper [35].
Gestational age at birth
51.0% (100/197) of all studies provided information on the gestational age at birth with an equal spread across income settings. The majority of studies included all babies born at <37 weeks gestation but eight studies specifically targeted only late-preterm infants (34+0–36+7 weeks gestation) and studied outcomes including general morbidity [35–37], transcutaneous bilirubin [38], growth [30] neuro-behavioural outcomes [39] respiratory outcomes with antenatal steroids [40] or surfactant therapy [41]. A total of 47 studies reported only on babies born below 33 or 34 weeks with only a few of these concentrating on the extremely preterm [25,26,42].
A variety of criteria and methods of assessment were used to define gestational age at birth. In approximately half the studies (99/197, 50.5%), the method of assessment was unclear. 22% (44/197) of studies used a combination of methods with 7.2% (14/197) using a combination of Ballard/Dubowitz and last menstrual period (LMP), 15.3% (30/197) of studies used antenatal USS in combination with other methods—mainly LMP. USS dating has been used in some community settings in low income settings such as Malawi, Mozambique, Tanzania and Guatemala [6,8,28,42,43], studies originating from bigger teaching hospitals such as those from Brazil, Ghana, Zambia, India and South Africa [21,44,45] and studies where babies were recruited at health care facilities with neonatal units in both low and middle income settings such as Turkey, Malaysia, Oman and Congo [46–49]. Almost a third of studies used a single method of assessment to determine gestational age at birth. This consisted of: fundal height (two Malawi studies) [18,50], the Capurro score in two South American studies [19,51], Ballard score in six studies (6/197) [52–57] and Dubowitz score in six studies (6/197) [22,58–62]. LMP was the only information used to estimate gestational age at birth in 22 studies (22/197). Low birth weight was used as a proxy for prematurity in two studies [56,63].
Only eight studies consistently used USS dating of the pregnancy as the basis for calculation of gestational age at birth [54,63], one study specifically looking at Doppler flow in an Indian perinatal centre [64].
Mortality
59% (116/197) of studies measured survival as an outcome with 49 of these (25%) using uniform definitions for describing mortality rates (perinatal, early neonatal, neonatal and infant) [65]. More than half of all studies (53.7%) 106/197 reported in-hospital mortality or survival during the period of follow up ‘post discharge’. 29 studies reported mortality but never specified a time period for which survival was assessed. In many studies, no denominator was reported and therefore it was unclear whether measurements were a rate (per 1000 live births) or a percentage of the babies included in the study. This makes comparison across studies difficult.
Out of 20 identified population based studies which reported on mortality rates, 19 reported mortality using the internationally agreed definitions for Perinatal Mortality Rate (PNMR), Neonatal Mortality Rate (NMR), Early and Late NMR. Data is summarised in Table 1. Almost all of these studies came from low and low middle income settings with a few population based studies from high middle income settings using systems for birth and death registration (studies from Chile and China). The others relied solely on follow up through the research studies, of mothers who had attended antenatal clinic. Most studies compared preterm births with term births. A few studies reported outcome separately by gestational age at birth [14,15,20,27,31,66,67] and some just look at outcomes of late preterm birth or compare this to term births [23,24,39,40].
Table 1. Mortality rates for babies born preterm and term with method of assessment of gestational age at birth.
| Author | Country | Sample size | Assessment gestational age | Gestationalage range (wks) | PNMRPerinatal mortality rate | NMR (Neonatal mortality rate) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENMR- Early neonatal mortality rate | Late neonatal mortality rate | Reported NMR (authors do not distinguish between early and late NNM) | ||||||||||
| Rate/1000 | RR or OR | Rate/1000 | RR or OR | Rate/1000 | RR or OR | Rate/1000 | RR or OR | |||||
| Slyker et al 2014(83) | Kenya | 468 singleton pregnancies of HIV + women from ANC | LMP, Dubowitz and fundal height | All gest | Infant death incidence rate (IR) for preterm 7-fold higher than infants born at term (IR = 5.8 vs 0.81 per 1000 person-days, incidence rate ratio (IRR) = 7.1, 95%CI = 1.5–30, p = 0.008). | |||||||
| Ades et al 2013(84) | Uganda | 351 live born infants | LMP and sonic biometry | All gest | <37 wks OR—12.7 (3.8–42.7). Increased GA assd with decreased OR of death—0.45 (0.2–0.9) | |||||||
| Welaga et al 2013(3) | Ghana | 17751 births | LMP | All gest | Overall ENMR—16 |
<32 wks—62
32–36–23 >36 wks—19 Overall NMR—24 |
<32 weeks-3.4 (2.66–4.32)
<36 weeks—1.2 (0.95–1.54) |
|||||
| Engmann et al 2012(2) | Ghana | 18,852 births (10.8% < 32 22.3%- 32–36 weeks) | LMP | All gest |
<32 wks—186
32–36 wks—33.7 >36wks—16.4 |
<32 wks—13.8 (11.5–16.38)
32–36 wks—2.1 (1.69–2.60) |
<32 wks—37.3
<36 wks—16.8 >36 wks—12.1 |
<32 wks OR—3.16 (2.35–4.22)
<36 wks OR—1.39 (1.05–1.84) |
||||
| Barros et al 2012(23) | Brazil | 1577 births followed up | LMP | All gest |
<34 wks—168
34–36 wks—19 <37 wks—11 38 wks—8 39–41 wks—4 Overall NMR (all gest)– 12 |
<34 wks—34.4(21.6–54.8)
34–36 wks—3.4 (1.8–6.6) <37 wks—2.7 (1.3–5.6) 38 wks—2.0 (1.1–3.5) 39–41–1.0 *Adjusted RR only provided |
||||||
| Schmiegelowl 2012 (85) | Tanzania | 872 women from ANC | USS | <37 | 52 (all gestations) |
Crude OR: <37 wks: 23.07 (10.8–49.12)
Adjusted OR: <37 wks 14.47 (3.2–64.8) |
||||||
| Graner et al 2010 (86) | Vietnam | 5521 births | Unclear | < 37 | 25(all gestations) | Adjusted OR: <37 wks 9.15 (CI 4.7–17.8) | 11.6 (all gestations) |
Adjusted OR <37 wks
7.83 (4.1–14.9) |
||||
| Pileggi et al 2010 (87)* | Brazil | 15,377 births 19 hospitals | Unclear | <37 |
<30 wks-430
<37 wks- 71 |
|||||||
| Engmann 2009 (88) | DR Congo | 7959 births—women from ANC | LMP | 24 - <37 |
<37wks- 738
>37 wks—38 |
OR (multivariate analysis) 71.1 (47.6–106.3) |
<37 wks—573
>37 wks—19.1 |
OR (multivariate analysis) 68.8 (39.9–118.6) | ||||
| Gonzalez et al 2006 (35) | Chile | 29.4 million births (national database) | Unclear | All |
*Rates given for 1990–2000
27 wks: 405–276 30 wks: 231–142.6 34 wks: 59–29.4 37 wks: 59–29.4 38 wks: 4–2.4 41 wks: 2.7–2 Overall NMR (all gest): 8.3–5.7 |
|||||||
| Barros et al 2005 *(89) | Brazil | 6011 (1984)5304 (1993)2427 (2004)Births | LMP | <37 |
1982
<34 wks—490 >34–36+ wks-33 1993 <34 wks 153 34–36+ wks-13 |
|||||||
| Van den Broek et al 2005 (90) | Malawi | 449 babies—ANC in Malawian districts | USS | 24–37 |
21.7% prems died vs 3.4% term
>32–37 wks 6.9% vs 3.4% died |
Risk ratio 6.32 (3.2–12.45) term vs preterm | ||||||
| Osman et al 2001 (91) | Mozambique | 908 women—ANC | USS | 21 - <37 |
<37 wks Adj OR 8.48 (3.4–20.9)
OR—23.47 (11.5–47.9) |
|||||||
| Kulmala et al 2000 (6) | Malawi | 813 births (all women in ANC) | Fundal height | <38 | 65.3 (all gestations) |
If <38 wks
Adj OR 9.6 (4.4–21) p<0.001 |
37- (all gestations) | If <38 wks adj OR 11 (3.6–32.4) p<0.001 | ||||
| Xu et al 1998 (92)* | China | 9207 births from birth records | >28 |
Preterm < 2.5 kg—306.9
Preterm >2.5 kg-26.3 |
||||||||
| Kapoor et al 1996 (93) | India | 966 births (live and still) | Unclear | <37 | <37 wks—273 | RR 1.95 (1.36–2.8) | ||||||
| Schreiber et al 1994 (94) | Guatemala |
120/120 cases & controls
From civil registry |
Unclear | Not clear | OR of 17.1 (5–59.3) Coeff-2.8(SE 0.6) | |||||||
| Gray 1991 (95)* | Brazil | 11,171 live births | Capurro score | Proxy:b-wt <2.5kg |
591 preterm SGA
318 preterm AGA 25 (all gest) |
OR—preterm/LBW—45.1 (32.4–62.9)
Adj OR preterm LBW 52.1(33.8–80.4) |
||||||
| Zhang et al 1991 (22)* | China/ Shanghai | 1134—every birth in Shanghai | Not clear | >28 |
28–36 wks—137.7
37–41 wks—9.2# Overall all gest—15 |
28–36 wks—70.2
37–41 wks—3.9 Overall all gest- 6.9 |
||||||
RR—Relative risk OR—odds ratio
All studies report an increased mortality rate among babies born preterm or report prematurity as the leading cause of death in neonates with lower gestational ages associated with increased mortality.
Morbidity
There is currently no agreed set of criteria to define neonatal or infant morbidity. In the included studies, morbidity was defined in a variety of ways. The Simplified Newborn Illness Severity and Mortality Risk Score II (SNAPP II) was used in two studies from tertiary neonatal units in Brazil [68,69].
Measures of morbidity used particularly in community based studies included; need for hospitalisation and prevalence of wheezing and pneumonia [23], number of routine and additional clinic visits in the post natal period [19] or a pictorial diary with specific measurements of diarrhoea and ways of measuring body temperature and respiratory rate [17]. Studies with an emphasis on facility based care used proxy measures for morbidity such as need for transfer to NICU [49], length of time in NICU [70,71,72] time spent in oxygen [73] and time spent on ventilator [41,74–76], number of routine and additional clinic visits in the post natal period [19].
Specific morbidities relating to preterm in neonatal intensive care units (mainly in the higher middle income countries) used well defined criteria in some cases. For example intra-ventricular haemorrhage [77,78] was defined using the Levene staging system [79] or the Papile classification [80–82]. Studies looking at necrotising enterocolitis [53,81,83–88] used the Bells (or modified Bell’s) criteria [89] and some studies [77] using the Gidieon classification for respiratory distress syndrome [90] or a classification for, broncho-pulmonary dysplasia [52,91–96]. Some studies looking at retinopathy of prematurity [75,76,92–95,98–100] used the International Classification of Retinopathy of Prematurity (ICROP) [75,97,99–101]. These well-defined outcomes tools were not however systematically used across all studies in neonatal units which reported these morbidities.
The majority of these facility based studies report an increased risk of morbidity in preterm infants compared to babies born at term. This includes increased risk of respiratory distress syndrome (RDS) [52,59,102,103], broncho-pulmonary dysplasia (BPD) [68,97,104–108], retinopathy of prematurity (ROP) [73,101,106,109] intraventricular haemorrhage (IVH) [72,110], periventricular leukomalacia (PVL) [111] and cerebral palsy [55,58,112]. Studies are however not comparable due to differences in diagnostic approach and level of care available in the different settings. In addition, lack of information on the range of gestational age at birth even in hospital populations studied makes comparison difficult. Some studies specifically looked at morbidity in the late preterm group and report an increased risk of morbidity including hyperbilirubinaemia, sepsis, wheezing and hospital admissions post discharge [23,30,35–37,39,103,113].
Growth
Less than 50% of all included studies report on growth outcomes. Where growth was reported, it was in a mixture of both low and middle income studies, this is usually done using standard internationally agreed methods and includes measurement of head circumference, height, weight (and in combination) using CDC or WHO standard growth curves for comparison.
Overall studies report that babies born preterm do not meet the same growth targets as babies born at term, continue to remain below the standard growth curve and demonstrate reduced ability for catch up growth. This is particularly well documented in the larger prospective community studies—almost all which had a follow up period of two years—from Malawi [28], Tanzania [114], India [115], Pakistan (3 year follow up) [116] China [117] and Brazil [23] as well as the more recent longer term cohort studies from India, Philippines, Brazil, Guatemala and South Africa [33]. This demonstrated lack of complete catch up growth at 15 years in those born premature or born at term but who were small for gestational age. It also demonstrated how those who did have catch up growth in the post natal period did make gains in height and schooling regardless of birth status. Interestingly, the studies documenting growth outcomes for babies who had received care in a well-equipped health care facility Cooper in South Africa [55] and Ho in Hong Kong [70] reported that there was less evidence of differences in growth between babies born preterm and term. In Kenya, where neonatal special care facilities are much more limited, only 20–28% of infants born preterm reached the lower limit of normal growth by term [118].
Development
In total, only 38/197 (19.4%) of the studies found reported on development and/or neurological outcomes of babies born preterm. Studies came equally from low, low middle and high middle income settings. 5 of these studies were from neonatal intensive care units in countries such as China, South Africa and Turkey with some of these studies examining specific cohorts of children such as those with periventricular echogenicities [46], cord pH at birth [119], the use of ferritin [120] or absent end diastolic flow [83]. Only five studies were population based: Malawi [28], Pakistan [116], Ethiopia [121], Guatemala [43] and a recent large study in Nepal [16]. The majority of other studies were from babies who had been admitted to a neonatal unit including India [34,58,122–125], Bangladesh [126], Kenya [112] and Taiwan were developmental care was provided [97]. Outcome measures include a wide range of developmental cognitive educational or neurological assessments (Table 2). Some studies conducted comprehensive neurological and developmental assessments as well as vision and hearing testing [55,93,121,126,127]. Only a few of these studies clarified criteria for neurological impairment or cerebral palsy [93,112]. 82% (31/38) of those studies assessing development or cognition used the Bayley Scales of Infant Development II or III with two studies using an adapted Indian version. Other developmental tools used were the Griffiths Mental Development Scales [54,70], the Denver II [22] or the Gesell Developmental Scales [117] (some adapted or validated for a specific setting). Some used tools created for a specific region such as the Malawi Developmental Assessment Tool [128] or the Ankara Developmental Screening Inventory [129]. Some used specific tools for one area of development or ability such as the Peabody Developmental Motor Scale, the Movement ABC or the Reynell Developmental Language Scales. Cognitive measures which were used varied and included the WISC III, WISC-R Bender Gestalt and Human figure drawing tests, WAIS, Kaufman ABC and the Stanford Binet. Behavioural measures were used in a minority of studies and included the Achenbach questionnaires and Raval’s scales of social maturity. Neurological assessments were conducted in a few studies with some using specific classification systems such as those by Costello [130], Robertson [131], Saigal and Rosenbaum [132] or Amiel-Tison and Gosselin [133].
Table 2. Assessment of neuro-development for babies born preterm in low and middle income countries.
| Developmental outcome tools used | Cognitive outcome measures used | Neurological and sensory assessments | Specific outcome measures eg. Speech and language or OT | Classification and identification of specific disability |
|---|---|---|---|---|
| Denver Development Screening TestDevelopment Screening Inventory Denver II [22] Gesell developmental scales for 0–3 yrs revised by Chinese Pediatric Association and Beijing Mental Development Cooperative Group [117] Bayley Scales of Infant Development (Indian Norms) Bayley Scales of Infant Development II (BSID II) Peabody Developmental Motor Scale Alberta Infant Motor Scale Development delay determined by Dorothy Egan's Model Malawi Developmental Assessment Tool [128] Griffith’s Developmental Assessment Scales (also for Columbia) [54,70] Ankara Developmental Screening Inventory [129] 17 milestone gross motor development scale (Jahari) Munich functional developmental diagnostics: gross motor and fine motor skills, perception, active speech, comprehension of speech, age of social interaction and independence [96] |
Stanford Binet Intelligence scales Bender Gestalt Test and the Human figure drawing test “School progress reports/performance” Weschler's inteligence scale (WISC-Revised) Wide-range Achievement Test (WRAT) Universal Non Verbal Intelligence Test (UNIT) Stroop and Backward Digit span (Weschler) |
“Assessment in high risk clinic, hearing and vision assessment” “Hearing and ophthalmic assessment” Tympanomtetry and free field audiometry with a pure tone audiometer “Examined for neurodevelopmental impairments and disabilities. If problems, EEG or USS used to investigate” Spontaneous movements (Prechtls qualitative assessment of general movements) “hearing screening, vision (squint), ROP, Assessed for CP and IVH (USS)” Hammersmith neonatal neurological assessment Neonatal Neurobehavioural Exam—Chinese (NNE-C) BSEAs—Brain stem evoked auditory potentials Neurobehavioural Asseement of the Preterm Infant (NAPI) [99] |
Combined Amiel Tison [133] Methods Raval's Scale of Social Maturity OT assessment “Social and environmental assessment” Draw-a-person Screening Procedure for Emotional Disturbance (DAP-SPED) Movement Assessment Battery for Children (ABC) Finger tapping test Early language milestone (ELM) scale Amiel Tison & Gosselin [133] method for describing specific upper motor neurone abnormalities such as muscle tone and reflexes Achenbach's Child Behaviour Check List |
Neurodevelopmental status assessed by physio providing neurodevelopmental score (NDS) to child. Gross Motor Function (GMF) and GMFCS Functional disability (physical and cognitive development) determined by Saigal and Rosenbaum's method [132] Cerebral palsy defined as 'presence of abnormal muscle tone or power in one or more limbs or the trunk. CP defined as a “nonoprogressive CNS disorder with abnormal muscle tone with classification system for mild moderate or severe CP. Bilateral severe hearing loss—permanent hearing loss that required amplification in both ears. Bilateral blindness—absence of functional vision in either eye. Neuro-developmental impairment—mod to severe CP, an MDI or PDI of < 70, bilateral deafness or bilateral blindness. “Profound impairment”—MDI < 50 or GMFCS level 4 or 5. “Minimal impairment” defined as an MDI or PDI score 70–84 & not having moderate to severe cerebral palsy, bilateral severe hearing loss or blindness. |
Although studies varied in terms of outcomes and are difficult to compare, generally there were poorer developmental outcomes in babies born preterm compared to term.
Discussion
Prematurity is the leading cause of neonatal mortality worldwide and one of the limiting factors for achieving a two thirds reduction in under five mortality rate between 1990 and 2015 (Millennium Development Goal 4) [134].
Alongside this, reports of a very high incidence of prematurity from reliable studies in low and middle income settings (with good estimates of gestational age at delivery and representative population samples) are now emerging.
This review identified almost 200 studies which report on outcome for babies born preterm. Studies represent a good geographical spread. Very few studies are from a community setting or provide population based data; most studies report outcomes for babies born in a health care facility and/or accessing health care because of identified health problems associated with preterm birth. With an estimated 47% of babies born with skilled birth attendance in low income countries and 60% in lower middle income countries [1] this means that there is currently no information about the majority of babies born preterm who have no access to health care and for whom outcomes might be worse than those reported in this review. Even for those where health care was available, the outcome for babies born preterm will depend on the availability and uptake of newborn care. In almost 30% of all studies reporting on babies who had received care at a ‘baby care unit’ there was no information about the level of clinical care and in many more cases, the information was inferred by reading the article carefully rather than it being reported clearly within the text.
We noted considerable variation in recruitment of participants, location of study, method of assessing gestational age and whether still births were included in the figures. Another key determinant of survival for babies born preterm is gestational age at time of birth. Only 51% of studies reported on the gestational ages of the infants. Furthermore the actual method of assessment was not clear in fifty percent of the studies. Gestational age, where reported, was most frequently estimated only after birth via assessment of appearance of the baby with/ without taking into account recall of the date of the last menstrual period. Only eight studies consistently used ultrasound scan dating.
For studies reporting ‘mortality rates’ both the denominator and nominator were not clear and did not fit with standard definitions, for example, perinatal, early neonatal, late neonatal or infant mortality. After extensive examination of the data it became clear that comparison of these data and conduct of a meta-analysis is currently not possible. A recent paper published by Katz includes a meta-analysis from original datasets obtained specifically for this purpose. This study does provide pooled overall relative risks for preterm neonatal mortality at 6.82 and 2.50 for post neonatal mortality with higher rates for those born both preterm and small for gestational age (SGA) 15.42 [135]. Similarly in studies we identified in this systematic review mortality rates are consistently higher in preterm births than in term births.
There are no currently agreed standard criteria to capture neonatal morbidity. In this systematic review, key morbidities in facilities in low and middle income settings do not seem to be dissimilar from those seen in high income settings [136]. Tools used for measuring neonatal morbidity in neonatal units such as the SNAPPE II [137] uses physiological indicators which cannot be feasibly obtained in most low income settings. Some standard definitions for morbidities such as intraventricular haemorrhage or retinopathy or prematurity are present but are not useful in community settings where little facilities are present for diagnoses. Some studies used criteria for assessing sepsis. None of these were defined according to internationally recognised criteria. However, it is likely that some are similar to that defined in the Young Infants Clinical Signs Study [138] which could be used more frequently. In contrast, growth was assessed using comparable criteria across the majority of studies. Similar to high income settings, growth falters with inadequate feeding in the preterm period.
The assessment of neuro-developmental outcomes was extremely variable with a variety of tools measuring a range of domains of neurodevelopment including general development, specific cognitive outcomes, physical examination measurements or specific sensory outcomes.
Apart from growth as an outcome, there are no standard definitions for criteria for morbidity and development and even though there are standard definitions for mortality, these are often not used.
This review has highlighted the need for more robust studies assessing the outcomes for babies born preterm but who survive the immediate newborn period. It is vital that more consistent use of data is encouraged with clear and aligned definitions of both health outcomes in the newborn (preterm or term) and the intervention packages aimed to save lives and improve health. Methods of gestational age assessment, care packages available and outcomes to be assessed will need to be clearly defined and standardised to truly measure the burden of disease associated with preterm birth as well as to assess the effect of interventions to prevent or reduce morbidity and developmental delay in babies who survive. Similar to the call for core outcome indicators for trials [139] and the CROWN initiative [140] asking for core outcome measures in Women’s Health, we would recommend the development and adaptation of an agreed framework and indicators for the reporting of outcomes following preterm birth (Fig. 4). With the global burden of disease pertaining not only to mortality but also to morbidity, it is important that indicators assess these outcomes as well. Without this we will continue to lack the evidence needed to decide which interventions are most effective to improve outcomes for the large number of preterm babies born in low and middle income countries.
Fig 4. Recommended reported measures for studies on neonatal outcomes.

Supporting Information
(XLS)
Acknowledgments
The authors would like to thank Caroline Hercod for her editorial support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. WHO (2013) World Health Statistics 2013. Geneva: World Health Organization; Available at: http://www.who.int/gho/publications/world_health_statistics/2013/en/ [Google Scholar]
- 2. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed] [Google Scholar]
- 3. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. 10.2471/BLT.08.062554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 5. van den Broek NR, White SA, Ntonya C, Ngwale M, Cullinan TR, et al. Reproductive health in rural Malawi: a population-based survey. Brit J Obstet Gynaec. 2013;110:902–908. [DOI] [PubMed] [Google Scholar]
- 6. van den Broek N, Ntonya C, Kayira E, White S, et al. Preterm birth in rural Malawi: high incidence in ultrasound dated population. Hum Reprod. 2005;20:3235–3237. [DOI] [PubMed] [Google Scholar]
- 7. van den Broek NR, White SA, Goodall M, Ntonya C, Kayira E, Molyneux ME, et al. The APPLe study: a randomized, community-based, placebo-controlled trial of azithromycin for the prevention of preterm birth, with meta-analysis. PLoS Med. 2009;6:e1000191 10.1371/journal.pmed.1000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osman NB, Challis K, Cotiro M, Nordahl G, Bergstrom S. Perinatal outcome in an obstetric cohort of Mozambican women. J Trop Pediatr. 2001;47:30–38. [DOI] [PubMed] [Google Scholar]
- 9. Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Preg Childb. 2010;10:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 11. WHO. Part 4:Burden of disease, DALYs. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 12. IMF. World Economic Outlook. Washington, DC, USA: World Economic Studies Division Research Department; 2012. [Google Scholar]
- 13. Steinglass R, Cherian T, Vandelaer J, Klemm RDW, Sequeira J. Special Issue: Development and use of the Lives Saved Tool (LiST): a model to estimate the impact of scaling up proven interventions on maternal, neonatal and child mortality. Int J Epidemiol 2011;39:i1–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engmann C, Walega P, Aborigo RA, Adongo P, Moyer CA, Lavasani L, et al. Stillbirths and early neonatal mortality in rural Northern Ghana. Trop Med Int Health. 2012;17:272–282. 10.1111/j.1365-3156.2011.02931.x [DOI] [PubMed] [Google Scholar]
- 15. Welaga P, Moyer CA, Aborigo R, Adongo P, Williams J, Hodgson A, et al. Why Are Babies Dying in the First Month after Birth? A 7-Year Study of Neonatal Mortality in Northern Ghana. PloS One. 2013;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christian P, Murray-Kolb LE, Tielsch JM, Katz J, LeClerq SC, Khatry SK. Associations between preterm birth, small-for-gestational age, and neonatal morbidity and cognitive function among school-age children in Nepal. BMC Pediatrics. 2014;14:58 10.1186/1471-2431-14-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald CM, Kupka R, Manji KP, Okuma J, Bosch RJ, Aboud S, et al. Predictors of stunting, wasting and underweight among Tanzanian children born to HIV-infected women. Eur J Clin Nutr. 2012;66:1265–1276. 10.1038/ejcn.2012.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kulmala T, Vaahtera M, Ndekha M, Koivisto AM, Cullinan T, Salin ML, et al. The importance of preterm births for peri- and neonatal mortality in rural Malawi. Paediatr Perinat Ep. 2000;14:219–226. [DOI] [PubMed] [Google Scholar]
- 19. Bartlett AV, Paz de Bocaletti ME, Bocaletti MA. Neonatal and early postneonatal morbidity and mortality in a rural Guatemalan community: the importance of infectious diseases and their management. Pediatr Infect Dis J. 1991;10:752–757. [DOI] [PubMed] [Google Scholar]
- 20. Barros FC, Rossello JLD, Matijasevich A, Dumith SC, Barros AJD, dos Santos I, et al. Gestational age at birth and morbidity, mortality, and growth in the first 4 years of life: findings from three birth cohorts in Southern Brazil. BMC Pediatr. 2012;12:169 10.1186/1471-2431-12-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barros FC, Victora CG, Barros AJ, Santos IS, Albernaz E, Matijasevich A, et al. The challenge of reducing neonatal mortality in middle-income countries: findings from three Brazilian birth cohorts in 1982, 1993 and 2004. Lancet. 2005;365:847–854. [DOI] [PubMed] [Google Scholar]
- 22. Morris SS, Victora CG, Barros FC, Halpern R, Menezes AM, César JA, et al. Length and ponderal index at birth: associations with mortality, hospitalizations, development and post-natal growth in Brazilian infants. Int J Epidemiol. 1998;27:242–247. [DOI] [PubMed] [Google Scholar]
- 23. Santos IS, Matijasevich A, Domingues MR, Barros AJ, Victora CG, Barros FC. Late preterm birth is a risk factor for growth faltering in early childhood: a cohort study. BMC Pediatr. 2009;9:71 10.1186/1471-2431-9-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu B, Rantakallio P. Low birth weight in China and Finland. Scand J Soc Med. 1998;26:10–7. [PubMed] [Google Scholar]
- 25. Zhang J, Cai WW, Chen H. Perinatal mortality in Shanghai: 1986–1987. Int J Epidemiol. 1991;20:958–963. [DOI] [PubMed] [Google Scholar]
- 26. Pieper CH, Smith J, Maree D, Pohl FC. Is nCPAP of value in extreme preterms with no access to neonatal intensive care? J Trop Pediatr 2003;49:148–152. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez R, Merialdi M, Lincetto O, Lauer J, Becerra C, Castro R, et al. Reduction in neonatal mortality in Chile between 1990 and 2000. Pediatrics. 2006;117:e949–954. [DOI] [PubMed] [Google Scholar]
- 28. Gladstone M, Neilson JP, White S, Kafulafula G, van den Broek N. Post-neonatal mortality, morbidity, and developmental outcome after ultrasound-dated preterm birth in rural Malawi: A community-based cohort study. PLoS Med 2011;8:e1001121 10.1371/journal.pmed.1001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marston L, Peacock JL, Yu K, Brocklehurst P, Calvert SA, Greenough A, et al. Comparing methods of analysing datasets with small clusters: case studies using four paediatric datasets. Paediatr Perinat Ep. 2009;23:380–392. 10.1111/j.1365-3016.2009.01046.x [DOI] [PubMed] [Google Scholar]
- 30. Barros MC, Mitsuhiro S, Chalem E, Laranjeira RR, Guinsburg R. Neurobehavior of late preterm infants of adolescent mothers. Neonatology. 2011;99:133–139. 10.1159/000313590 [DOI] [PubMed] [Google Scholar]
- 31. De Almeida MFB, Guinsburg R, Martinez FE, Procianoy RS, Leone CR, Marba STM, et al. Perinatal factors associated with early deaths of preterm infants born in Brazilian Network on Neonatal Research centers. Jornal de pediatria. 2008;84:300–307. 10.2223/JPED.1787 [DOI] [PubMed] [Google Scholar]
- 32. Adair LS. Filipino children exhibit catch up growth from age 2 to 12 yrs. J Nutr. 1999;129:1140–1148. [DOI] [PubMed] [Google Scholar]
- 33. Stein AD, Barros FC, Bhargava SK, Hao W, Horta BL, Lee N, et al. Birth status, child growth, and adult outcomes in low- and middle-income countries. J Pediatr. 2013;163:1740–1746 10.1016/j.jpeds.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaudhari S, Otiv M, Chitale A, Pandit A, Hoge M. Pune low birth weight study—cognitive abilities and educational performance at twelve years. Indian Pediatr. 2004;41:121–128. [PubMed] [Google Scholar]
- 35. Celik I, Demirel G, Campolat F, Dilmen U. A common problem for neonatal intensive care units: late preterm infants, a prospective study with term controls in a large perinatal centre. J Matern-Fetal Neonatal Med. 2013;26:459–462. 10.3109/14767058.2012.735994 [DOI] [PubMed] [Google Scholar]
- 36. Abu-Salah O. Unfavourable outcomes associated with late preterm birth: observations from Jordan. J Pakistan Med Assoc. 2011;61:769–772. [PubMed] [Google Scholar]
- 37. Ma X, Huang C, Lou S, Lv Q, Su W, Tan J, et al. The clinical outcomes of late preterm infants: a multi-center survey of Zhejiang, China. J Perinatal Med. 2009;37:695–9. 10.1515/JPM.2009.130 [DOI] [PubMed] [Google Scholar]
- 38. Lavanya KR, Jaiswal A, Reddy P, Murki S. Predictors of significant jaundice in late preterm infants. Indian Pediatr. 2012;49:717–720. [DOI] [PubMed] [Google Scholar]
- 39. Porto AM, Coutinho IC, Correia JB, Amorim MM. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. Brit Med J. 2011;342:d1696 10.1136/bmj.d1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sürmeli-Onay O, Korkmaz A, Yiğit S, Yurdakök M. Surfactant therapy in late preterm infants: respiratory distress syndrome and beyond. Turkish J Pediatr. 2012;54:239–246. [PubMed] [Google Scholar]
- 41. Sallakh-Niknezhad A, Bashar-Hashemi F, Satarzadeh N, Ghojazadeh M, Sahnazarli G. Early versus late trophic feeding in very low birth weight preterm infants. Iran J Pediatr. 2012;22:171–176. [PMC free article] [PubMed] [Google Scholar]
- 42. Schmiegelow C, Minja D, Oesterholt M, Pehrson C, Suhrs HE, Boström S, et al. Factors associated with and causes of perinatal mortality in northeastern Tanzania. Acta Obstet Gyn Scand. 2012;91:1061–1068. 10.1111/j.1600-0412.2012.01478.x [DOI] [PubMed] [Google Scholar]
- 43. Kuklina EV, Ramakrishnan U, Stein AD, Barnhart HH, Martorell R. Growth and diet quality are associated with the attainment of walking in rural Guatemalan infants. J Nutr. 2004;134:3296–3300. [DOI] [PubMed] [Google Scholar]
- 44. Nkyekyer K, Enweronu-Laryea C, Boafor T. Singleton preterm births in Korle Bu Teaching Hospital, Accra, Ghana—origins and outcomes. Ghana Med J. 2006;40:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ballot DE, Potterton J, Chirwa T, Hilburn N, Cooper PA. Developmental outcome of very low birth weight infants in a developing country. BMC Pediatr. 2012;12:11 10.1186/1471-2431-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duman N, Kumral A, Gulcan H, Ozkan H. Outcome of very-low-birth-weight infants in a developing country: a prospective study from the western region of Turkey. J Matern-Fetal Neonatal Med. 2003;13:54–58. [DOI] [PubMed] [Google Scholar]
- 47. Al-Riyami N, Al-Shezawi F, Al-Ruheili I, Al-Dughaishi T, Al-Khabori M. Perinatal Outcome in Pregnancies with Extreme Preterm Premature Rupture of Membranes (Mid-Trimester PROM). Sultan Qaboos Univ Med J. 2013;13:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sonkusare S, Rai L, Naik P. Preterm birth: mode of delivery and neonatal outcome. Med J Malaysia. 2009;64:303–306. [PubMed] [Google Scholar]
- 49. Pambou O, Ntsika-Kaya P, Ekoundzola JR, Mayanda F. Naissances avant terme au CHU de Brazzaville [Preterm births at Brazzaville University Hospital]. Sante. 2006;16:185–189. [PubMed] [Google Scholar]
- 50. Vaahtera M, Kulmala T, Ndekha M, Koivisto AM, Cullinan T, Salin ML, et al. Antenatal and perinatal predictors of infant mortality in rural Malawi. Arch Dis Child-Fetal. 2000;82:F200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gray RH, Ferraz EM, Amorim MS, de Melo LF. Levels and determinants of early neonatal mortality in Natal, northeastern Brazil: results of a surveillance and case-control study. Int J Epidemiol. 1991;20:467–473. [DOI] [PubMed] [Google Scholar]
- 52. Boo NY. Outcome of very low birthweight neonates in a developing country: experience from a large Malaysian maternity hospital. Singap Medical J. 1992;33:33–37. [PubMed] [Google Scholar]
- 53. Worku B, Tilahun T, Schneider J. Occurrence of intra-ventricular hemorrhage among preterm infants admitted in Tikur Anbessa Hospital. Ethiop Med J. 2004;42:109–114. [PubMed] [Google Scholar]
- 54. Charpak N, Ruiz-Pelaez JG, Figueroa de CZ, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics. 2001;108:1072–1079. [DOI] [PubMed] [Google Scholar]
- 55. Cooper PA, Sandler DL. Outcome of very low birth weight infants at 12 to 18 months of age in Soweto, South Africa. Pediatrics. 1997;99:537–44. [DOI] [PubMed] [Google Scholar]
- 56. Mokhachane M, Saloojee H, Cooper PA. Earlier discharge of very low birthweight infants from an under-resourced African hospital: a randomised trial. Ann Trop Paed. 2006;26:43–51. [DOI] [PubMed] [Google Scholar]
- 57. Rylance S, Ward J. Early mortality of very low-birthweight infants at Queen Elizabeth Central Hospital, Malawi. Paediatr Int Child Healt. 2013;33:91–6. 10.1179/2046905513Y.0000000053 [DOI] [PubMed] [Google Scholar]
- 58. Chaudhari S, Kinare AS, Kumar R, Pandit AN, Deshpande M. Ultrasonography of the brain in preterm infants and its correlation with neurodevelopmental outcome. Indian Pediatr. 1995;32:735–742. [PubMed] [Google Scholar]
- 59. Mlay GS, Manji KP. Respiratory distress syndrome among neonates admitted at Muhimbili Medical Centre, Dar es Salaam, Tanzania. J Trop Pediatr. 2000;46:303–307. [DOI] [PubMed] [Google Scholar]
- 60. Manji KP, Massawe AW, Mgone JM. Birthweight and neonatal outcome at the Muhimbili Medical Centre, Dar es Salaam, Tanzania. E Afr Medical J. 1998;75:382–387. [PubMed] [Google Scholar]
- 61. Sloan NL, Ahmed S, Mitra SN, Choudhury N, Chowdhury M, Rob U, et al. Community-based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics. 2008;121:e1047–1059. 10.1542/peds.2007-0076 [DOI] [PubMed] [Google Scholar]
- 62. Wolf MJ, Wolf B, Beunen G, Casaer P. Neurodevelopmental outcome at 1 year in Zimbabwean neonates with extreme hyperbilirubinaemia. Eur J Pediatr. 1999;158:111–114. [DOI] [PubMed] [Google Scholar]
- 63. Burger M, Frieg A, Louw QA. General movements as a predictive tool of the neurological outcome in very low and extremely low birth weight infants—a South African perspective. Early Hum Dev. 2011;87:303–308. 10.1016/j.earlhumdev.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 64. Lakshmi CVS, Pramod G, Geeta K, Subramaniam S, Rao MB, Kallapur SG, et al. Outcome of very low birth weight infants with abnormal antenatal doppler flow patterns: a prospective cohort study. Indian Pediatr. 2013;50:847–852. [DOI] [PubMed] [Google Scholar]
- 65. WHO. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 66. Poudel P, Budhathoki S. Perinatal characteristics and outcome of VLBW infants at NICU of a developing country: an experience at eastern Nepal. J Matern-Fetal Neonatal Med. 2010;23:441–447. 10.1080/14767050903186285 [DOI] [PubMed] [Google Scholar]
- 67. Verma M, Chhatwal J, Jaison S, Thomas S, Daniel R. Refractive errors in preterm babies. Indian Pediatr. 1994;31:1183–1186. [PubMed] [Google Scholar]
- 68. Carvalho PR, Moreira ME, Sa RA, Lopes LM. SNAPPE-II application in newborns with very low birth weight: evaluation of adverse outcomes in severe placental dysfunction. J Perinat Med. 2011;39:343–347. 10.1515/JPM.2010.141 [DOI] [PubMed] [Google Scholar]
- 69. Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. [DOI] [PubMed] [Google Scholar]
- 70. Ho JJ, Chang AS. Changes in the process of care and outcome over a 10-year period in a neonatal nursery in a developing country. J Trop Pediatr. 2007;53:232–237. [DOI] [PubMed] [Google Scholar]
- 71. Tanir HM, Sener T, Tekin N, Aksit A, Ardic N. Preterm premature rupture of membranes and neonatal outcome prior to 34 weeks of gestation. Int J Gynaecol Obstet. 2003;82:167–172. [DOI] [PubMed] [Google Scholar]
- 72. Mancini MC, Barbosa NE, Banwart D, Silveira S, Guerpelli JL, Leone CR. Intraventricular hemorrhage in very low birth weight infants: associated risk factors and outcome in the neonatal period. Revista do Hospital das Clinicas. 1999;54:151–154. [DOI] [PubMed] [Google Scholar]
- 73. Feghhi M, Altayeb SMH, Haghi F, Kasiri A, Farahi F, Dehdashtyan M, et al. Incidence of Retinopathy of Prematurity and Risk Factors in the South-Western Region of Iran. Middle E Afr J Ophthal. 2012;19:101–106. 10.4103/0974-9233.92124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Badiee Z. Intraventricular hemorrhage in very low birth weight infants. Associated risk factors in Isfahan, Iran. Saudi J Med. 2007;28:1362–1366. [PubMed] [Google Scholar]
- 75. Guven S, Bozdag S, Saner H, Cetinkaya M, Yazar AS, Erguven M. Early neonatal outcomes of volume guaranteed ventilation in preterm infants with respiratory distress syndrome. J Matern-Fetal Neonatal Med. 2013;26:396–401. 10.3109/14767058.2012.733778 [DOI] [PubMed] [Google Scholar]
- 76. Korkmaz A, Teksam Ö, Yurdakök M, Yigit S, Tekinalp G. Fetal malnutrition and its impacts on neonatal outcome in preterm infants. Turkish J Pediatr. 2011;53:261–268. [PubMed] [Google Scholar]
- 77. Satar M, Ozlu F, Cekinmez EK, Yapicioglu-Yildiztas H, Narli N, Erdem E, et al. Is Retinopathy of Prematurity Decreasing?—Comparison of Two Different Periods in the Same NICU. Turkish J Pediatr. 2014;56:166–170. [PubMed] [Google Scholar]
- 78. Leadford AE, Warren JB, Manasyan A, Chomba E, Salas AA, Schelonka R, et al. Plastic bags for prevention of hypothermia in preterm and low birth weight infants. Pediatrics. 2013;132:e128–134. 10.1542/peds.2012-2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Levene MI, de Crespigny LC. Classification of intraventricular hemorrhage. Lancet. 1983;8325:643. [DOI] [PubMed] [Google Scholar]
- 80. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. [DOI] [PubMed] [Google Scholar]
- 81. Serce O, Benzer D, Gursoy T, Karatekin G, Ovali F. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev. 2013;89:1033–1036. 10.1016/j.earlhumdev.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 82. Adegoke AA, Lawoyin TO, Ogundeji MO, Thomson AM. A Community-based Investigation of the Avoidable Factors of Maternal Mortality in Nigeria: The Pilot Experiences. African Healt Sci. 2007;7:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ertan A, Tanriverdi H, Stamm A, Jost W, Endrikat J, Schmidt W. Postnatal neuro-development of fetuses with absent end-diastolic flow in the umbilical artery and/or fetal descending aorta. Arch Gynecol Obstet. 2012;285:1547–1552. 10.1007/s00404-011-2191-4 [DOI] [PubMed] [Google Scholar]
- 84. Gezer A, Parafit-Yalciner E, Guralp O, Yedigoz V, Altinok T, Madazl R. Neonatal morbidity mortality outcomes in pre-term premature rupture of membranes. J Obstet Gynaec. 2013;33:38–42. [DOI] [PubMed] [Google Scholar]
- 85. Iranpour R, Sadeghnia A, Hesaraki M. High-Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure in the Management of Respiratory Distress Syndrome. J Isfahan Med Sch. 2011;29:1–12. [Google Scholar]
- 86. Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatrica. 2013;102:e560–565. 10.1111/apa.12416 [DOI] [PubMed] [Google Scholar]
- 87. Karagol BS, Zenciroglu A, Okumus N, Polin RA. Randomized controlled trial of slow vs rapid enteral feeding advancements on the clinical outcomes of preterm infants with birth weight 750–1250 g. J Parenter Enteral Nutr. 2013;37:223–228. 10.1177/0148607112449482 [DOI] [PubMed] [Google Scholar]
- 88. Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child. 2013;99:F110–115. 10.1136/archdischild-2013-304745 [DOI] [PubMed] [Google Scholar]
- 89. Bell MJ. Neonatal necrotizing enterocolitis. New Engl J Med. 1978;298:281–282. [PubMed] [Google Scholar]
- 90. Kero PO, Makinen EO. Comparison between clinical and radiological classification of infants with the respiratory distress syndrome (RDS). Eur J Pediatr. 1979;130:271–278. [DOI] [PubMed] [Google Scholar]
- 91. Ho JJ, Amar HS, Mohan AJ, Hon TH. Neurodevelopmental outcome of very low birth weight babies admitted to a Malaysian nursery. J Paediatr Child H. 1999;35:175–180. [DOI] [PubMed] [Google Scholar]
- 92. Cetinkaya M, Ozkan H, Koksal N. Maternal preeclampsia is associated with increased risk of necrotizing enterocolitis in preterm infants. Early Hum Dev. 2012;88:893–898. 10.1016/j.earlhumdev.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 93. Dilli D, Eras Z, Andiran N, Dilmen U, Sakrucu E. Neurodevelopmental evaluation of very low birth weight infants with transient hypothyroxinemia at corrected age of 18–24 months. Indian Pediatr. 2012;49:711–715. [DOI] [PubMed] [Google Scholar]
- 94. Can E, Bülbül A, Uslu S, Cömert S, Bolat F. Effects of aggressive parenteral nutrition on growth and clinical outcome in preterm infants. Pediatr Int. 2012;54:869–874. 10.1111/j.1442-200X.2012.03713.x [DOI] [PubMed] [Google Scholar]
- 95. Wang H, Gao X, Liu C, Yan C, Lin X, Yang C, et al. Morbidity and mortality of neonatal respiratory failure in China: surfactant treatment in very immature infants. Pediatrics. 2012;129:e731–740. 10.1542/peds.2011-0725 [DOI] [PubMed] [Google Scholar]
- 96. Bancalari E, Claure N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Semin Perinatol. 2006;30:164–170. [DOI] [PubMed] [Google Scholar]
- 97. Chen LC, Wu YC, Hsieh WS, Hsu CH, Leng CH, Chen WJ, et al. The effect of in-hospital developmental care on neonatal morbidity, growth and development of preterm Taiwanese infants: A randomized controlled trial. Early Hum Dev. 2013; 89:301–306. 10.1016/j.earlhumdev.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 98. Fortes Filho JB, Fortes BGB, Tartarella MB, Procianoy RS. Incidence and main risk factors for severe retinopathy of prematurity in infants weighing less than 1000 grams in Brazil. J Trop Pediatr. 2013;59:502–506. 10.1093/tropej/fmt036 [DOI] [PubMed] [Google Scholar]
- 99. Fortes Filho JB, Eckert GU, Valiatti FB, Dos Santos PG, da Costa MC, Procianoy RS. The influence of gestational age on the dynamic behavior of other risk factors associated with retinopathy of prematurity (ROP). Graefe Arch Clin Exp. 2010;248:893–900. 10.1007/s00417-009-1248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–999. [DOI] [PubMed] [Google Scholar]
- 101. Ahmed AS, Muslima H, Anwar KS, Khan NZ, Chowdhury MA, Saha SK, et al. Retinopathy of prematurity in Bangladeshi neonates. J Trop Pediatr. 2008;54:333–339. 10.1093/tropej/fmn035 [DOI] [PubMed] [Google Scholar]
- 102. Ayaya SO, Esamai FO, Rotich J, Sidle J. Perinatal morbidity at the Moi Teaching and Referral Hospital, Eldoret. E Afr Med J. 2001;78:544–549. [DOI] [PubMed] [Google Scholar]
- 103. Ma XL, Xu XF, Chen C, Yan CY, Liu YM, Liu L, et al. Epidemiology of respiratory distress and the illness severity in late preterm or term infants: a prospective multi-center study. Chinese Med J. 2010;123:2776–2780. [PubMed] [Google Scholar]
- 104. Al-Abdi S, Abou Mehrem A, Dabelah K, Al-Aghbari M, Al-Aamri M. The effect of low to moderate patient volume on very low-birth-weight outcomes in a level III-B neonatal intensive care unit. Am J Perinatol. 2011;28:219–226. 10.1055/s-0030-1268236 [DOI] [PubMed] [Google Scholar]
- 105. Grupo Collaborative Neocosur. Very-low-birth-weight infant outcomes in 11 South American NICUs. J Perinatol. 2002;22:2–7. [DOI] [PubMed] [Google Scholar]
- 106. Chen Y, Li XX, Yin H, Gilbert C, Liang JH, Jiang YR, et al. Risk factors for retinopathy of prematurity in six neonatal intensive care units in Beijing, China. Br J Ophthalmol. 2008;92:326–330. 10.1136/bjo.2007.131813 [DOI] [PubMed] [Google Scholar]
- 107. Meneguel JF, Guinsburg R, Miyoshi MH, de Araujo Peres C, Russo RH, Kopelman BI, et al. Antenatal treatment with corticosteroids for preterm neonates: impact on the incidence of respiratory distress syndrome and intra-hospital mortality. Sao Paulo Med J. 2003;121:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ruiz-Peláez JG, Charpak N. Bronchopulmonary dysplasia epidemic: incidence and associated factors in a cohort of premature infants in Bogotá, Colombia. / Epidemia de displasia broncopulmonar: incidencia y factores asociados en una cohorte de niños prematuros en Bogotá, Colombia. Biomédica. 2014;34:29–39. [DOI] [PubMed] [Google Scholar]
- 109. Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115:e518–525. [DOI] [PubMed] [Google Scholar]
- 110. Vural M, Yilmaz I, Ilikkan B, Erginoz E, Perk Y. Intraventricular hemorrhage in preterm newborns: risk factors and results from a University Hospital in Istanbul, 8 years after. Pediatr Int. 2007;49:341–344. [DOI] [PubMed] [Google Scholar]
- 111. Liu J, Li J, Qin GL, Chen YH, Wang Q. Periventricular leukomalacia in premature infants in mainland China. Am J Perinatol. 2008;25:535–540. 10.1055/s-0028-1083841 [DOI] [PubMed] [Google Scholar]
- 112. Were FN, Bwibo NO. Two year neurological outcomes of Very Low Birth Weight infants. E Afr Med J. 2006;83:243–249. [DOI] [PubMed] [Google Scholar]
- 113. Olusanya BO, Solanke OA. Maternal and neonatal profile of late-preterm survivors in a poorly resourced country. J Matern-Fetal Neonatal Med. 2012;25:346–352. 10.3109/14767058.2011.577471 [DOI] [PubMed] [Google Scholar]
- 114. Webb AL, Manji K, Fawzi WW, Villamor E. Time-independent maternal and infant factors and time-dependent infant morbidities including HIV infection, contribute to infant growth faltering during the first 2 years of life. J Trop Pediatr. 2009;55:83–90. 10.1093/tropej/fmn068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ke E, Krishnan V, Zachariah P. Auxologic, biochemical and clinical (ABC) profile of low birth weight babies- a 2-year prospective study. J Trop Pediatr. 2007;53:374–382. [DOI] [PubMed] [Google Scholar]
- 116. Cheung YB, Yip PS, Karlberg JP. Fetal growth, early postnatal growth and motor development in Pakistani infants. Int J Epidemiol. 2001;30:66–72. [DOI] [PubMed] [Google Scholar]
- 117. Zhang X, Chen K, Wei XP, Qu P, Liu YX, Chen J, et al. Perinatal vitamin A status in relation to neurodevelopmental outcome at two years of age. International journal for vitamin and nutrition research. Int Z Vitaminforschung. 2009;79:238–249 10.1024/0300-9831.79.4.238 [DOI] [PubMed] [Google Scholar]
- 118. Were FN, Bwibo NO. Early growth of very low birth weight infants. E Afr Med J. 2006;83:84–89. [DOI] [PubMed] [Google Scholar]
- 119. Seth B, Datta V, Bhakhri BK. Umbilical artery pH at birth and neurobehavioral outcome in early preterm infants: A cohort study. J Pedi Neurosciences. 2014;9:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Joy R, Krishnamurthy S, Bethou A, Rajappa M, Ananthanarayanan PH, Bhat BV et al. Early versus late enteral prophylactic iron supplementation in preterm very low birth weight infants: a randomised controlled trial. Arch Dis Child. 2014;99:F105–109. 10.1136/archdischild-2013-304650 [DOI] [PubMed] [Google Scholar]
- 121. Servili C, Medhin G, Hanlon C, Tomlinson M, Worku B, Baheretibeb Y, et al. Maternal common mental disorders and infant development in Ethiopia: the P-MaMiE Birth Cohort. BMC Pub Health. 2010;10:693 10.1186/1471-2458-10-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chaudhari S. Learning problems in children who were "high risk" at birth. Indian Pediatr. 1994;31:1461–1464. [PubMed] [Google Scholar]
- 123. Chaudhari S, Bhalerao MR, Chitale A, Pandit AN, Nene U. Pune low birth weight study-a six year follow up. Indian Pediatr. 1999;36:669–676. [PubMed] [Google Scholar]
- 124. Chaudhari S, Kulkarni S, Barve S, Pandit AN, Sonak U, Sarpotdar N. Neurologic sequelae in high risk infants—a three year follow up. Indian Pediatr. 1996;33:645–653. [PubMed] [Google Scholar]
- 125. Chaudhari S, Kulkarni S, Pajnigar F, Pandit AN, Deshmukh S. A longitudinal follow up of development of preterm infants. Indian Pediatr. 1999;28:873–880. [PubMed] [Google Scholar]
- 126. Khan N, Muslima H, Parveen M, Bhattacharya M, Begum N, Chowdhury S, et al. Neurodevelopmental outcomes of preterm infants in Bangladesh. Pediatrics. 2006;118:280–289. [DOI] [PubMed] [Google Scholar]
- 127. Khan N, Muslima H, Bhattacharya M, Parvin R, Begum N, Jahan M, et al. Stress in mothers of preterm infants in Bangladesh: associations with family, child and maternal factors and children's neuro-development. Child Care Hlth Dev. 2008;34:657–664. 10.1111/j.1365-2214.2008.00873.x [DOI] [PubMed] [Google Scholar]
- 128. Gladstone M, Lancaster GA, Umar E, Nyirenda M, Kayira E, van den Broek NR, et al. The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010;7:e1000273 10.1371/journal.pmed.1000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ozbek A, Miral S, Eminagaoglu N, Ozkan H. Development and behavior of non-handicapped preterm children from a developing country. Pediatr Int. 2005;47:532–540. [DOI] [PubMed] [Google Scholar]
- 130. Costello AM, Hamilton PA, Baudin J, Townsend J, Bradford BC, Stewart AL, et al. Prediction of neurodevelopmental impairment at four years from brain ultrasound appearance of very preterm infants. Dev Medicine Child Neurol. 1988;30:711–722. [DOI] [PubMed] [Google Scholar]
- 131. Robertson CM, Etches PC, Goldson E, Kyle JM. Eight-year school performance, neurodevelopmental, and growth outcome of neonates with bronchopulmonary dysplasia: a comparative study. Pediatrics. 1992;89:365–372. [PubMed] [Google Scholar]
- 132. Saigal S, Rosenbaum P, Stoskopf B, Milner R. Follow-up of infants 501 to 1,500 gm birth weight delivered to residents of a geographically defined region with perinatal intensive care facilities. J Pediatrics. 1982;100:606–613. [DOI] [PubMed] [Google Scholar]
- 133. Amiel-Tison C, Gosselin J. Neurological development from birth to six years. Baltimore, MD: Johns Hopkins University Press; 2001. [Google Scholar]
- 134.United Nations. The Millenium Development Goals Report 2011. New York: United Nations; 2011. Available at: http://www.un.org/millenniumgoals/11_MDG%20Report_EN.pdf
- 135. Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382: 417–425. 10.1016/S0140-6736(13)60993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Harrell FE, Margolis PA, Gove S, Mason KE, Mulholland EK, Lehmann D, et al. Development of a clinical prediction model for an ordinal outcome: the World Health Organization Multicentre Study of Clinical Signs and Etiological Agents of Pneumonia, Sepsis and Meningitis in Young Infants. Stat Med. 1998;17:909–944. [DOI] [PubMed] [Google Scholar]
- 137. Berry MA, Shah PS, Brouillette RT, Hellmann J. Predictors of mortality and length of stay for neonates admitted to children s hospital neonatal intensive care units. J Perinatol. 2008;28:297–302. [DOI] [PubMed] [Google Scholar]
- 138. Mazzi E, Bartos AE, Carlin J, Weber MW, Darmstadt GL. Clinical signs predicting severe illness in young infants (<60 days) in Bolivia. J Trop Pediatr. 2010;56:307–316. 10.1093/tropej/fmq005 [DOI] [PubMed] [Google Scholar]
- 139. Prinsen CA, Vohra S, Rose MR, King-Jones S, Ishaque S, Bhaloo Z, et al. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a 'core outcome set'. Trials. 2014;15:247 10.1186/1745-6215-15-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Khan K (2014) The CROWN Initiative: Journal Editors Invite Researchers to Develop Core Outcomes in Women's Health. International Journal of Gynecology and Obstetrics 126(3):201–202. 10.1016/j.ijgo.2014.06.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.