Graphical abstract
Abbreviations: S. mansoni, Schistosoma mansoni; norUDCA, 24-nor-ursodeoxycholic acid; UDCA, ursodeoxycholic acid; Abcb4/Mdr2, canalicular phospholipid export pump/multidrug resistance protein 2; B. glabrata, Biomphalaria glabrata; ALT, alanine aminotransferase; AP, alkaline phosphatase; BMDC, bone marrow derived dendritic cells; BMDM, bone marrow derived macrophages; HP, hydroxyproline; M-CSF, macrophage colony-stimulating factor
Keywords: Liver fibrosis, Bile acids, Schistosoma mansoni infection, Hepatic granulomas, Anti-inflammatory/anti-fibrotic therapy
Abstract
Background & Aims
Intrahepatic granuloma formation and fibrosis characterize the pathological features of Schistosoma mansoni infection. Based on previously observed substantial anti-fibrotic effects of 24-nor-ursodeoxycholic acid (norUDCA) in Abcb4/Mdr2−/− mice with cholestatic liver injury and biliary fibrosis, we hypothesized that norUDCA improves inflammation-driven liver fibrosis in S. mansoni infection.
Methods
Adult NMRI mice were infected with 50 S. mansoni cercariae and after 12 weeks received either norUDCA- or ursodeoxycholic acid (UDCA)-enriched diet (0.5% wt/wt) for 4 weeks. Bile acid effects on liver histology, serum biochemistry, key regulatory cytokines, hepatic hydroxyproline content as well as granuloma formation were compared to naive mice and infected controls. In addition, effects of norUDCA on primary T-cell activation/proliferation and maturation of the antigen-presenting-cells (dendritic cells, macrophages) were determined in vitro.
Results
UDCA as well as norUDCA attenuated the inflammatory response in livers of S. mansoni infected mice, but exclusively norUDCA changed cellular composition and reduced size of hepatic granulomas as well as TH2-mediated hepatic fibrosis in vivo. Moreover, norUDCA affected surface expression level of major histocompatibility complex (MHC) class II of macrophages and dendritic cells as well as activation/proliferation of T-lymphocytes in vitro, whereas UDCA had no effect.
Conclusions
This study demonstrates pronounced anti-inflammatory and anti-fibrotic effects of norUDCA compared to UDCA in S. mansoni induced liver injury, and indicates that norUDCA directly represses antigen presentation of antigen presenting cells and subsequent T-cell activation in vitro. Therefore, norUDCA represents a promising drug for the treatment of this important cause of liver fibrosis.
Introduction
Liver fibrosis represents an overwhelming wound-healing process, characterized by excessive deposition of extracellular matrix, to chronic injury which is frequently driven by inflammation [1], [2]. Soluble factors produced by invading inflammatory cells such as proinflammatory and profibrogenetic cytokines and chemokines play a pivotal role in the activation and transformation process of hepatic stellate cells as major cellular source for extracellular matrix production within the injured liver [3]. Schistosoma mansoni (S. mansoni) infection demonstrates a leading cause for liver fibrosis, portal hypertension and its sequels include variceal bleeding and ascites [4]. Portal hypertension in S. mansoni infected individuals results from increased hepatic resistance to blood flow primarily related to a sustained inflammatory-driven fibrotic process as a consequence of egg-induced granuloma formation [5]. Although antihelminthic therapy is effective to treat S. mansoni infection in many patients, portal hypertension and its complications may persist, reflecting the urgent need for novel treatment strategies for S. mansoni induced liver fibrosis.
Recently, several novel bile acid derivatives, including side chain-shortened bile acids such as 24-nor-ursodeoxycholic acid (norUDCA) [6], were identified as promising new treatment options for common and important diseases such as arteriosclerosis, metabolic syndrome, and liver fibrosis [7], [8], [9], which has fostered a rebirth of bile acid research [10], [11]. Data obtained in Abcb4/Mdr2−/− (canalicular phospholipid export pump/multidrug resistance protein 2) mice, representing a well characterized model system for sclerosing cholangitis and biliary type of liver fibrosis, suggest potential direct anti-inflammatory and anti-fibrotic effects of norUDCA [6], [12], [13]. However, since Abcb4−/− mice suffer from defective bile formation (i.e., the lack of biliary phospholipid secretion with consecutive toxic bile formation) and norUDCA also has substantial effects on bile formation and composition [14], [15], it remains unclear whether this promising compound is able to exert beneficial mechanisms in non-cholestatic types of liver fibrosis. S. mansoni related pathology is mainly induced by cellular immune responses and orchestrated by CD4-positive T-lymphocytes [16], [17]. TH1 dominates the early inflammatory infectious reaction, which shifts towards an egg-driven TH2-reaction later on. Based on the findings of these longitudinal studies, it is suggested that an imbalanced TH1/TH2-response may represent a pivotal trigger for S. mansoni induced liver fibrosis [18], [19]. However, the therapeutic potency of novel bile acid analogues such as norUDCA in counteracting the inflammatory and/or fibrotic response in a murine model of hepatic schistosomiasis has not been explored so far.
Therefore, the aim of this study was to explore the therapeutic efficacy of norUDCA in vivo, in a mouse model of S. mansoni induced liver fibrosis, compared to the effectiveness of its mother compound UDCA.
Materials and methods
S. mansoni mouse model and infection
Six- to eight-week-old NMRI mice (Charles River Laboratories, Germany) were housed in an animal facility with a 12:12 h light/dark cycle, with ad libitum water and free access to standard chow (SSNIFF, Soest, Netherlands). The experimental protocols were performed according to the German animal protection law and approved by the local animal care and use committee. Schistosomal cercariae were generated in a Brazilian S. mansoni strain held in a cycle with NMRI mice and Biomphalaria glabrata (B. glabrata) water snails (Puerto Rico) [20]. S. mansoni cercariae were obtained by mass shedding of 5–10 infected B. glabrata after light exposure. Mice were exposed to parasites for 90 min by sitting in a water bath enriched with approximately 50 S. mansoni cercariae. Infection grade was estimated by determination of hepatic granuloma count in hematoxylin/eosin-stained liver slices using a conventional microscope (magnification 100×).
Bile acid treatment
Twelve weeks following S. mansoni infection, a time point when liver fibrosis is already fully established, mice were fed either standard chow (control, n = 7), 0.5% (wt/wt) norUDCA-supplemented diet (norUDCA, n = 14) or 0.5% (wt/wt) UDCA-supplemented diet (UDCA, n = 12) for four weeks. In addition, naive mice (naive, n = 7) on standard chow were studied. Thereafter, mice were euthanized and liver samples and sera were processed as described previously in detail [21].
Serum analysis
Enzymatic assays for detection of serum alanine aminotransferase (ALT), alkaline phosphatase (AP) and total serum bile acid levels were performed and analyzed using a cobas® 6000 analyzer (Roche Diagnostics, Mannheim, Germany).
Liver histology and hepatic hydroxyproline measurement
One part of the right lobe of each liver (lobe 3) was fixed in 4% neutral buffered formaldehyde solution and embedded in paraffin. Four-μm thin sections were stained with hematoxylin/eosin (H&E) and Sirius Red (SR) for detection of collagen fibers. Morphometric analysis of granuloma size was performed using ImageJ software (v1.47v; National Institute of Health). For biochemical quantification of liver fibrosis, hepatic hydroxyproline (HP) content was determined as described previously [6].
Statistical analysis
Statistical analysis was performed using SPSS (Release 14.0, 2005, SPSS Inc., Chicago, IL). For single time point comparison of two groups the Student’s t-test or, where appropriate, the non-parametric Mann–Whitney U-test was used. The number of animals per group was as follows: naive, n = 7; control, n = 7; UDCA, n = 12; norUDCA, n = 14. A p value of less than 0.05 was considered significant.
Additional Materials and methods are provided in the Supplementary material section.
Results
NorUDCA treatment improves liver histology and reduces granuloma size in hepatic schistosomiasis without any antihelminthic effect
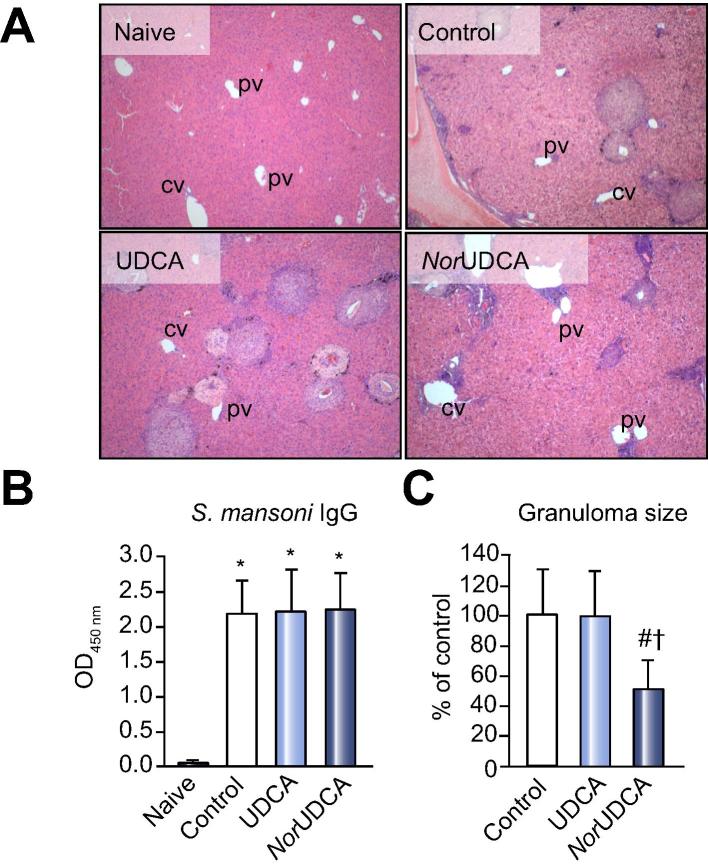
Infection with S. mansoni cercariae was uniformly accomplished in all experimental groups, proven microscopically (by a board certified expert pathologist, C.L.), by determination of granuloma count/per low power field (control: 7.6 ± 2.3; UDCA: 5.5 ± 1; norUDCA: 6 ± 1.5) and via quantification of antibodies against parasite eggs (Fig. 1A and B). Morphometric analysis of liver slices demonstrated a significant reduction (up to 50%) of the granuloma size after norUDCA treatment, compared to UDCA and control (% of control: UDCA, 98 and norUDCA, 51) (Fig. 1C). Serum parameters for hepatocellular injury (ALT) remained unchanged 16 weeks following infection, while bile acid feeding resulted in significantly higher levels of serum AP levels compared to naive and control mice (Table 1). Notably, the local ductular reaction (in response to granuloma formation), reflected by keratin 19 (K19) staining, was enhanced after UDCA treatment but attenuated after norUDCA treatment (Supplementary Fig. 1).
Fig. 1.
NorUDCA ameliorates liver histology of chronically Schistosoma mansoni infected NMRI mice. (A) Representative liver histology images (HE; original magnification 100×), of female NMRI mice, 16 weeks after infection with 50 S. mansoni cercariae, receiving control diet and UDCA (0.5% wt/wt) or norUDCA (0.5% wt/wt) enriched diet for 4 weeks, are shown (original magnification 100×). In addition, a naive (uninfected) group was studied. (B) Infection with 50 S. mansoni cercariae resulted in uniformly infection levels in all infected groups, confirmed by antibody detection directed against parasite eggs. (C) Morphometric analysis of granuloma diameter revealed a significant reduction of granuloma size after norUDCA treatment compared to control and UDCA group. ∗p <0.05 (vs. naive), #p <0.05 (vs. control), †p <0.05 (vs. UDCA).
Table 1.
Serum liver biochemistry.
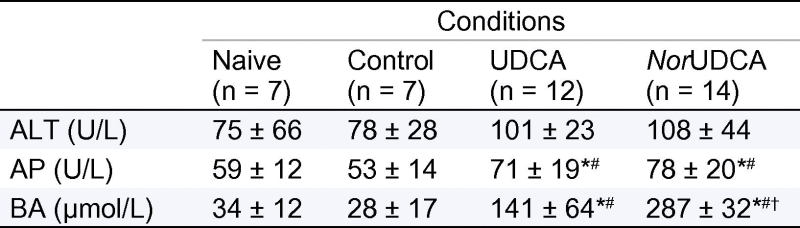 |
Serum biochemistry of naive mice (healthy; n = 7) vs. control (infected, untreated; n = 7), UDCA (infected, UDCA-treated, n = 12) and norUDCA (infected, norUDCA-treated, n = 14). Results are given as mean ± SD.
ALT, alanine aminotransferase; AP, alkaline phosphatase; BA, bile acids.
∗p <0.05 (vs. naive), #p <0.05 (vs. control), †p <0.05 (vs. UDCA).
Furthermore, in vitro studies were designed to explore potential direct antihelminthic effects of bile acids. All isolated adult S. mansoni worms died within 6 h by control incubation with Praziquantel. Monitoring of bile acid treated groups over 5 days showed no antihelminthic effects of norUDCA or UDCA (% of vital worms after 5 days of incubation: Praziquantel, 0; control, 95.8; UDCA, 93.3; norUDCA, 95.5).
UDCA and norUDCA treatment attenuates inflammatory reaction in hepatic schistosomiasis
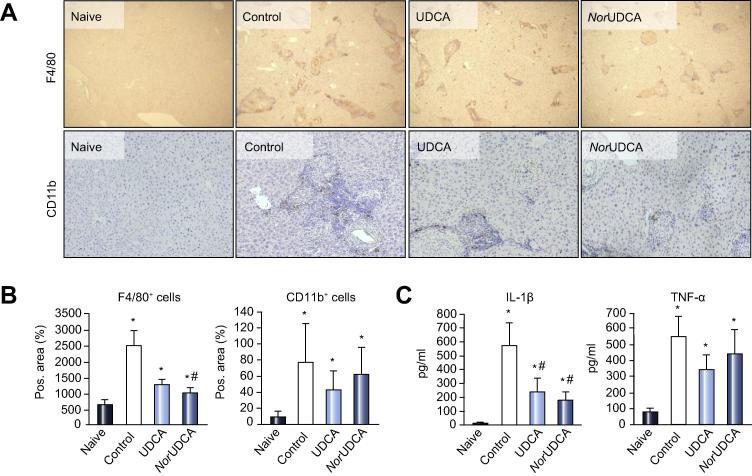
Immunohistochemical analysis of liver slices revealed a substantial accumulation of F4/80+ and CD11b+ monocytes/macrophages and granulocytes around tissue entrapped eggs, in untreated controls compared to naive mice (Fig. 2A). Treatment with both bile acids led to a significant reduction of F4/80+ (but not CD11b+) cells compared to the untreated control (Fig. 2B).
Fig. 2.
NorUDCA and UDCA suppress inflammatory cell infiltration and inflammatory response. (A) Representative immunohistochemistry for F4/80+ (original magnification 40×) and CD11b+ (original magnification 100×) cells within liver specimens of all subjects is shown. NorUDCA treatment leads to a pronounced disaggregation of inflammatory cell infiltrate. (B) Quantification of F4/80+ macrophages (ImageJ analysis) revealed a significant reduction of macrophages within S. mansoni granulomas after norUDCA treatment while CD11b+ cell count did not show bile acid specific differences. (C) Serum levels of TH1 cytokines IL-1 beta and TNF-alpha were measured by ELISA. Both cytokines were significantly elevated after S. mansoni infection. Four-week feeding with UDCA and norUDCA treatment counteracted this elevation for IL-1 beta but not for TNF-alpha. Protein values were calculated by standard curve concentrations of recombinant IL-1 beta and TNF-alpha. Concentrations were expressed as means ± SD of duplicates. ∗p <0.05 (vs. naive), #p <0.05 (vs. control).
Serum levels of IL-1 beta and TNF-alpha were significantly increased 16 weeks following S. mansoni infection in control mice compared to the naive group. Both bile acids significantly antagonized this effect for IL-1 beta (Fig. 2C). This goes in line with mRNA expression profile of classical proinflammatory genes (Supplementary Table 2). Both bile acids tended to reduce classical inflammation markers to similar degrees, although this was not reflected by fibrosis with UDCA. In addition, markers for alternatively activated macrophages were assessed. Arg1 expression profile displayed a significant reduction following S. mansoni infection (control) compared to naive mice, whereas UDCA and norUDCA only tended to reduce Arg1. Notably, we observed a significant reduction of Retnla expression after norUDCA treatment comparable to Retnla levels of naive mice. Both bile acids exert similar effects on classical proinflammatory genes whereas only norUDCA affected alternatively activated macrophages in case of Retnla. Moreover, norUDCA (but not UDCA) reduced interleukin (IL)-13 and -4 serum levels (Fig. 3D).
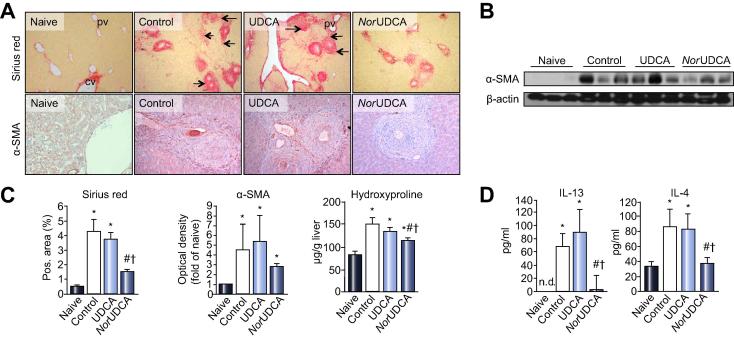
Fig. 3.
NorUDCA significantly reduces hepatic fibrosis in chronically infected Schistosoma mansoni mice. (A) Representative liver histology (Sirius Red staining, SR; original magnification 100×), of female NMRI mice 16 weeks after infection with 50 S. mansoni cercariae, receiving control diet and UDCA (0.5% wt/wt) or norUDCA (0.5% wt/wt) enriched diet for 4 weeks, is shown (original magnification 100×). Hepatic fibrosis with pronounced expansion of connective tissue between hepatic granulomas was most conspicuous in the control and UDCA group, and was reduced after norUDCA feeding. Immunohistochemistry for alpha-SMA (original magnification 200×) revealed a pronounced number of positive cells within fibrotic areas and around egg granulomas in non-treated (control) and UDCA fed mice. Reduced numbers of alpha-SMA+ cells under norUDCA treatment can be observed. (B) Representative Western blot for alpha-SMA and beta-actin (as loading control). (C) Fibrosis and alpha-SMA levels were further analyzed by computerized quantification of SR positive areas of liver sections, Western blot densitometry, and hydroxyproline measurements. (D) Serum levels of profibrotic TH2 cytokines IL-13 and IL-4 were measured by ELISA. Both cytokines were significantly elevated 16 weeks following S. mansoni infection. NorUDCA (but not UDCA) feeding for 4 weeks resulted in a significant reduction of IL-13 and IL-4 serum level. Protein values were calculated by standard curve concentrations of recombinant IL-13 and IL-4. Protein concentrations were expressed as means ± SD of duplicates. ∗p <0.05 (vs. naive), #p <0.05 (vs. control), †p <0.05 (vs. UDCA).
NorUDCA significantly reduces liver fibrosis in hepatic schistosomiasis
SR-stained liver slices of control and UDCA-treated mice showed expanding fibrosis with pronounced portal-portal bridging that was significantly reduced in response to norUDCA treatment (Fig. 3A). This was supported by a reduction of alpha-SMA+ cells on liver slices (Fig. 3A) and reduced alpha-SMA protein levels (Fig. 3B) as well as hepatic hydroxyproline (HP) levels, as markers for biochemical quantification of liver fibrosis by norUDCA (Fig. 3C). IL-13 and -4 serum protein levels were determined since these cytokines have a regulatory importance in TH2 driven liver fibrosis. Serum levels of both profibrotic cytokines were significantly reduced after norUDCA treatment compared to the control and UDCA groups (Fig. 3D). In addition, as shown in Supplementary Table 2, norUDCA reduced TGF-beta expression. Interestingly, infected mice displayed a characteristic increase of matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of metalloproteinase (TIMP-1) mRNA expression levels, which was reduced by both bile acids treatments (Supplementary Table 2). Notably, the TIMP-1 to MMP-2 ratio was reduced only by norUDCA (but not UDCA), which may reflect a beneficial shift favouring matrix degradation (Supplementary Table 3).
Taken together, these data indicate that norUDCA diminished development and ameliorated parameters of hepatic fibrosis, whereas UDCA had no effect on liver fibrosis, in this model system.
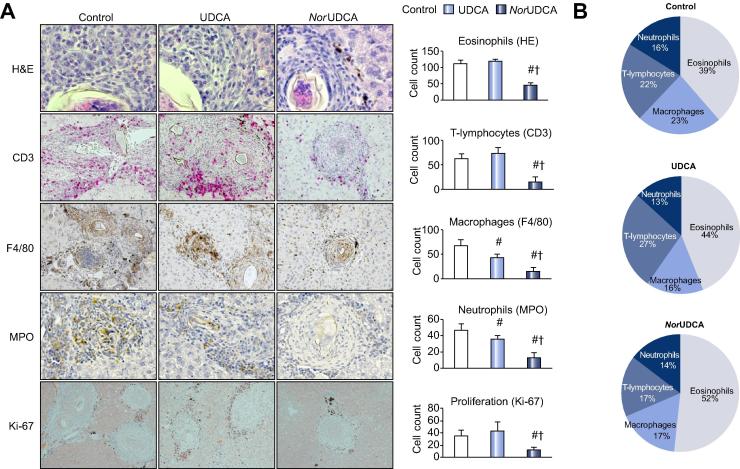
NorUDCA treatment reduces content of inflammatory cell infiltrate and changes cell composition of hepatic S. mansoni granulomas
Since norUDCA treatment resulted in significantly reduced granuloma size, immunohistochemistry was performed to analyze potential changes in cell composition of hepatic S. mansoni granulomas after bile acid treatment. Cell numbers of macrophages (F4/80), T-lymphocytes (CD3), neutrophils (MPO), and eosinophils (HE-staining) were determined microscopically, by counting positive cells in 20 hepatic granulomas per liver slide of each mouse. In norUDCA treated mice, all analyzed cell types were significantly reduced compared to untreated control and UDCA. In addition, the relative cellular composition of inflammatory cells within hepatic granulomas of this group was changed with a significant reduction of CD3+ T-lymphocytes. IHC for Ki-67 revealed reduced cell proliferation within hepatic granulomas of norUDCA treated mice (Fig. 4A and B).
Fig. 4.
NorUDCA treatment reduces content of inflammatory cell infiltrate and changes cell composition of hepatic Schistosoma mansoni granulomas. Immunohistochemistry on liver slices was performed to characterize cell composition of hepatic S. mansoni granulomas with or without bile acid treatment. (A) Representative images for H&E (eosinophils, original magnification 630×), CD3 (T-lymphocytes, original magnification 400×), F4/80 (macrophages, original magnification 200×), and MPO (neutrophils, original magnification 400×) are shown. IHC for Ki-67 (cellular proliferation, original magnification 100×) revealed a significant reduction of dividing cell activity within the norUDCA group compared to UDCA and control. Respective cell numbers were determined microscopically by counting positive cells in 20 hepatic granulomas (hg) per liver slide of each mouse (control: n(hg) = 140; UDCA: n(hg) = 240; norUDCA: n(hg) = 280). Schistosomal hemozoin pigment (brownish pigment) within portal tract and parenchymal interface can be observed. (B) Quantification of cell populations within hepatic granulomas revealed a significant change in cell composition towards a lower content of CD3+ T-lymphocytes. #p <0.05 (vs. control), †p <0.05 (vs. UDCA).
NorUDCA impairs surface expression of major histocompatibility complex (MHC) class II of antigen presenting cells and blocks T-cell-receptor dependent and independent activation/proliferation without inducing apoptosis
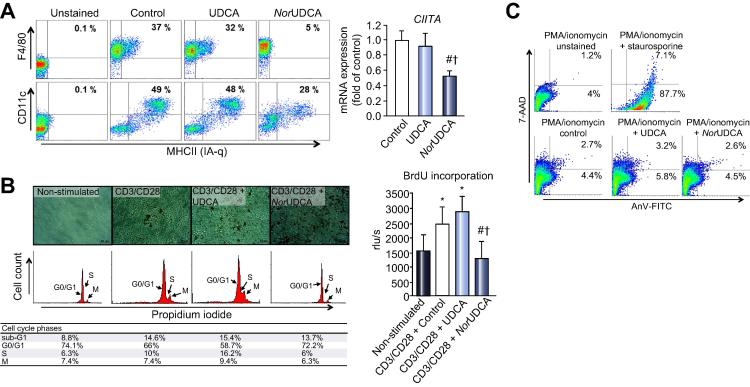
Populations of double positive bone marrow derived macrophages (BMDM: F4/80+/MHC II+) and dendritic cells (BMDC: CD11c+/MHC II+) were analyzed by flow cytometry. Comparable amounts of double positive BMDMs were generated in control and UDCA-treated cells, whereas norUDCA significantly reduced the amount of double positive BMDMs (Fig. 5A). The same pattern was observed in BMDCs. Only half of CD11c+ cells present an MHC II signal on their surface. This was also reflected by significantly reduced CIITA expression levels in BMDMs, after norUDCA incubation (Fig. 5A).
Fig. 5.
NorUDCA significantly reduces expression of major histocompatibility complex class II molecules (MHC II) on bone marrow-derived macrophages (BMDM) and dendritic cells (BMDC) and inhibits proliferation of primary CD4+ T-lymphocytes. (A) Haematopoietic stem cells of uninfected female NMRI mice were incubated with either UDCA (50 μM) or norUDCA (500 μM), according to their achievable intrahepatic concentrations in vivo, and then analyzed for the two directions of APC lineages by flow cytometry. NorUDCA significantly decreased percentage of surface MHC class II expression on APCs, demonstrated by surface double-staining with specific markers for BMDMs (F4/80) and BMDCs (CD11c). mRNA expression level of the key MHC class II regulator CIITA was significantly downregulated after norUDCA incubation, in contrast to UDCA and control. (B) CD4+ T-lymphocytes, isolated from chronically S. mansoni infected NMRI mice, were stimulated with CD3/CD28 dynabeads. NorUDCA significantly inhibited proliferation of T-lymphocytes, confirmed by BrdU incorporation. Stimulated, norUDCA incubated T-lymphocytes did not enter cell cycle interphase (propidium iodide staining); whereas control and UDCA treated cells started to proliferate following stimulation. (C) T-lymphocytes from mesenteric lymph nodes of S. mansoni infected mice were isolated and restimulated with PMA/Ionomycin, and incubated with staurosporine (1 μM, positive control), UDCA (50 μM) or norUDCA (500 μM) for 4 h. AnV-FITC (apoptosis) positive cells were most apparent after incubation with staurosporine (95%), while bile acid sublimation (UDCA and norUDCA) did not induce apoptosis. For validation of the method and reproducibility of the results, the experiments were repeated in two independent series. ∗p <0.05 (vs. unstimulated control), #p <0.05 (vs. control), †p <0.05 (vs. UDCA).
CD3/CD28 activation of CD4+ primary mouse T-lymphocytes led to a strong proliferation in controls and UDCA-treated cells, demonstrated by BrdU incorporation, while norUDCA completely repressed proliferation of CD3/CD28 (receptor dependent) as well as PMA/ionomycin (receptor independent) activated CD4+ T-lymphocytes (Fig. 5B). This has been verified by reduced intracellular CFSE concentration due to cell division activity (Supplementary Fig. 2). Moreover, norUDCA treated T-lymphocytes did not enter interphase of cell cycle compared to control and UDCA (Fig. 5B). To investigate potential apoptotic effects of norUDCA, annexin-V/7-AAD staining was performed. Neither UDCA nor norUDCA induced programmed cell death (Fig. 5C).
Taken together, these data indicate that norUDCA, in contrast to UDCA, directly impairs antigen presentation of APCs, by CIITA regulated suppression of MHC II expression [20] in vitro, and further indicate anti-proliferative properties of norUDCA in regard to inhibited activation (proliferation) of T-lymphocytes.
Discussion
This study aimed at investigating the therapeutic properties and potential direct anti-inflammatory and anti-fibrotic mechanisms of the novel bile acid norUDCA, in an inflammation-mediated model of liver fibrosis without cholestasis [21]. We have chosen its chemical mother compound UDCA as a clinical comparator.
Previous experiments, using cholestatic Abcb4−/− mice with sclerosing cholangitis and biliary type of liver fibrosis, have demonstrated anti-fibrotic effects of norUDCA [6] and, to a lesser degree, UDCA; the latter even aggravated liver injury at high doses [22]. Since both drugs are anti-cholestatic and previously observed anti-fibrotic effects may at least in part be related to these mechanisms, we searched for an alternative – preferentially non-cholestatic – model for liver fibrosis, to further discriminate the differential effects of both interesting therapeutic bile acids. S. mansoni infected NMRI mice develop a robust and sustained hepatic fibrosis without significantly elevated serum parameters for hepatocellular injury [21]. Proliferation of bile ducts was observed in areas of hepatic granulomas, while “granuloma free” area was completely unremarkable. Notably, the local ductular reaction in response to granulomas was even enhanced after UDCA treatment but declined after norUDCA treatment. In areas of hepatic granulomas, where pre-existing bile ducts may be compressed, choleretic UDCA could provoke a local aggravation of the biliary reaction, as observed under more pronounced cholestatic conditions (e.g., Abcb4/Mdr2−/− mice and bile duct ligation), by enhancing biliary pressure and subsequent bile infarcts while norUDCA was beneficial [22], [23]. However, in the current S. mansoni mouse model, UDCA did not aggravate hepatic injury or inflammation compared to untreated control, as reflected by unchanged ALT levels and even improved inflammatory markers. Although, norUDCA is also a potent choleretic, the associated induction of bicarbonate rich choleresis as a result of cholehepatic shunting [23], and its antiproliferative properties [24], may explain its beneficial effects on ductular reaction. In this regard, it is also important to note that elevated serum bile acid levels, as observed in the current study in norUDCA-fed S. mansoni-infected mice, may be attributed to the high bioavailability of cholehepatic-shunting norUDCA, rather than reflecting a cholestatic condition. This is related to the relative conjugation resistance of norUDCA [13], [15]. As such, the reabsorption of secreted norUDCA by cholangiocytes leads to high serum and intrahepatic norUDCA concentrations [6], [25]. As a possible consequence, the observed beneficial anti-fibrotic and anti-inflammatory effects of norUDCA in vivo could, at least in part, be related to direct antihelminthic actions of norUDCA. However, the reduction of granuloma size in the presence of unchanged absolute granuloma numbers and unchanged survival of isolated worms and eggs in vitro argues against this interesting option.
The severity of S. mansoni pathology depends at least on the balance of TH1 and TH2 mediated responses, and is mostly related to a dysbalance of matrix degradation and its inhibition [26]. Herein we demonstrate a significantly shifted ratio of MMP-2 and TIMP-1 towards MMP-2 after norUDCA treatment, and markedly reduced inflammatory response in S. mansoni infected mice, after UDCA and norUDCA feeding. However, only norUDCA treatment led to a reduced granuloma size with secondary beneficial effects on TH2-cytokine (IL-13 and -4) driven hepatic fibrosis. In hepatic schistosomiasis, inflammation (TH1) and fibrosis (TH2) are differentially regulated since it is known that IL-13 affects fibrosis directly in a TGF-beta independent manner [27], and IL-13−/− mice failed to develop an adequate fibrotic response to S. mansoni egg antigens [28]. In our study, we demonstrated comparable effects of UDCA and norUDCA on proinflammatory cytokines, but without any beneficial effects on hepatic fibrosis in UDCA treated mice, possibly indicating a minor relevance of these markers for S. mansoni related disease progression. The specific anti-fibrotic effect of norUDCA in this model may be linked to mechanisms directed against TH2 response. This concept is further supported by norUDCA effect on Retnla, presenting a marker for alternatively activated macrophages and negative regulator of TH2-response [29], [30]. Notably, we observed a significant reduction of Retnla expression after norUDCA treatment, comparable to Retnla levels of naive mice. Unfortunately, little is known about Retnla function in TH2 immunity and affected cell types, but its regulation is controlled by IL-4/IL-13 and STAT6 [31], [32]. We demonstrated reduced serum levels of both TH2 cytokines (IL-13 and -4) accompanied by reduced numbers of CD3+ and Ki-67+ cells in granulomas of the norUDCA (but not UDCA) group. The relevance of local expansion of activated T-lymphocytes for granuloma formation and controlling of inflammatory processes is still unclear [33]. However, our analysis revealed a significantly changed percentage distribution of cellular composition of hepatic granulomas after norUDCA treatment accompanied by reduced granuloma size, declined formation of fibrotic septa, and reduced hepatic fibrosis. In addition, we could demonstrate a reduction of Ki-67+ cells at the edges of granulomas, where CD3+ cells are located; suggesting that local proliferation of T-lymphocytes may be restrained after norUDCA treatment. This is in line with our in vitro settings of restimulated T-lymphocytes with anti-CD3/CD28 coupled beads and receptor independent stimulation with PMA/Ionomycin that was completely detained after norUDCA incubation without inducing apoptosis in these cells.
In addition, a strongly reduced expression of MHC II molecules on BMDMs as well as BMDCs after incubation with norUDCA could in turn provide an explanation for a reduced antigen presentation and subsequent T-cell activation observed with norUDCA. Noteworthy, in our setting UDCA did not show any effects on maturation of APCs and MHC II expression. However, UDCA is able to directly reduce MHC II expression on biliary epithelium and hepatocytes by activating the glucocorticoid receptor, followed by an inhibited IFN-γ-mediated MHC II activation [34]. Together with our observation on alternatively activated macrophages, we suggest that the UDCA effect might be restricted to the classical activation cascade of macrophages and is lost upon alternatively IL-4/IL-13 activated macrophages, within a TH2 milieu [35], [36]. Further characterization of the APC/T-cell interplay is clearly required to obtain additional insights into the anti-inflammatory and immune modulatory mechanisms of norUDCA activity and the interactions with the complex inflammation process of S. mansoni infection.
In summary, this study demonstrates: (i) beneficial effects of norUDCA on granuloma size and hepatic fibrosis, (ii) anti-inflammatory properties of norUDCA directed to MHC class II protein expression on dendritic cells and macrophages, and (iii) direct anti-fibrotic effects of norUDCA by reduced T-lymphocyte proliferation and finally reduced serum levels of IL-13 and IL-4. This potentiality may qualify norUDCA as a promising drug for non-cholestatic, inflammation-driven liver fibrosis.
Financial support
The study was supported by grants P-19118 and F3517-B20 from the Austrian Science Foundation and a GEN-AU project grant from the Austrian Ministry for Science to Michael Trauner. Martina Sombetzki was supported by a Ph.D. scholarship (Landesgraduiertenförderung des Landes Mecklenburg-Vorpommern, Germany).
Conflict of interest
PF and MT received a research grant from the Dr. Falk Pharma Gmbh, Freiburg, Germany and the authors received norUDCA from Falk for this study. The Medical University of Graz has filed patents (WO 2006/119803 A1 and WO 2009/013334) on the medical use of norUDCA and PF and MT are listed as co-inventors.
Authors’ contributions
Substantial contributions to the conception and design; or the acquisition, analysis, or interpretation of the data: MS, CDF, PF, RE, DS, NDG, CO, MM, TC, ES; MT.
Drafting of the article or critical revision for important intellectual content: MS, MT, PF, ML, ECR, CL.
Final approval of the version to be published: MT, ECR, BMH, CS.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved: MT, ECR.
Acknowledgements
The skillful technical support of Judith Gumhold, Dagmar Silbert, Anita Krnjic, Katharina Kinslechner, Nicole Deinet, Nadja Leditznig and Daniel Wolters are gratefully acknowledged. Liver tests were thankworthy performed by Dr. W. Erwa and colleagues (Graz).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2014.11.020.
Supplementary data
References
- 1.Friedman S.L. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 2.Brenner D.A. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves H.L., Friedman S.L. Activation of hepatic stellate cells–a key issue in liver fibrosis. Front Biosci. 2002;7:d808–d826. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- 4.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 5.Andrade Z.A. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009;31:656–663. doi: 10.1111/j.1365-3024.2009.01157.x. [DOI] [PubMed] [Google Scholar]
- 6.Fickert P., Wagner M., Marschall H.U., Fuchsbichler A., Zollner G., Tsybrovskyy O., et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe S., Yaginuma R., Ikejima K., Miyazaki A. Liver diseases and metabolic syndrome. J Gastroenterol. 2008;43:509–518. doi: 10.1007/s00535-008-2193-6. [DOI] [PubMed] [Google Scholar]
- 8.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 10.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 11.Trauner M., Claudel T., Fickert P., Moustafa T., Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220–224. doi: 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- 12.Trauner M., Fickert P., Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 13.Halilbasic E., Fiorotto R., Fickert P., Marschall H.U., Moustafa T., Spirli C., et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology. 2009;49:1972–1981. doi: 10.1002/hep.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon Y.B., Hagey L.R., Hofmann A.F., Gurantz D., Michelotti E.L., Steinbach J.H. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann A.F., Zakko S.F., Lira M., Clerici C., Hagey L.R., Lambert K.K., et al. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. Hepatology. 2005;42:1391–1398. doi: 10.1002/hep.20943. [DOI] [PubMed] [Google Scholar]
- 16.Brunet L.R., Dunne D.W., Pearce E.J. Cytokine interaction and immune responses during Schistosoma mansoni infection. Parasitol Today. 1998;14:422–427. doi: 10.1016/s0169-4758(98)01317-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann K.F., Cheever A.W., Wynn T.A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 18.Wilson M.S., Mentink-Kane M.M., Pesce J.T., Ramalingam T.R., Thompson R., Wynn T.A. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadecker M.J., Asahi H., Finger E., Hernandez H.J., Rutitzky L.I., Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–179. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 20.Otten L.A., Steimle V., Bontron S., Mach B. Quantitative control of MHC class II expression by the transactivator CIITA. Eur J Immunol. 1998;28:473. doi: 10.1002/(SICI)1521-4141(199802)28:02<473::AID-IMMU473>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Loebermann M., Sombetzki M., Langner C., Fuchsbichler A., Gumhold J., Silbert D., et al. Imbalance of pro- and antifibrogenic genes and bile duct injury in murine Schistosoma mansoni infection-induced liver fibrosis. Trop Med Int Health. 2009;14:1418–1425. doi: 10.1111/j.1365-3156.2009.02387.x. [DOI] [PubMed] [Google Scholar]
- 22.Fickert P., Zollner G., Fuchsbichler A., Stumptner C., Weiglein A.H., Lammert F., et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 23.Fickert P., Pollheimer M.J., Silbert D., Moustafa T., Halilbasic E., Krones E., et al. Differential effects of norUDCA and UDCA in obstructive cholestasis in mice. J Hepatol. 2013;58:1201. doi: 10.1016/j.jhep.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moustafa T., Fickert P., Magnes C., Guelly C., Thueringer A., Frank S., et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology. 2012;142:140–151. doi: 10.1053/j.gastro.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann A.F. Current concepts of biliary secretion. Dig Dis Sci. 1989;34:16S–20S. doi: 10.1007/BF01536657. [DOI] [PubMed] [Google Scholar]
- 26.Baker A.H., Edwards D.R., Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 27.Kaviratne M., Hesse M., Leusink M., Cheever A.W., Davies S.J., McKerrow J.H., et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie G.J., Fallon P.G., Emson C.L., Grencis R.K., McKenzie A.N. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesce J.T., Ramalingam T.R., Wilson M.S., Mentink-Kane M.M., Thompson R.W., Cheever A.W., et al. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stütz A.M., Pickart L.A., Trifilieff A., Baumruker T., Prieschl-Strassmayr E., Woisetschläger M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol. 2003;170:1789–1796. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 31.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 32.Pesce J.T., Ramalingam T.R., Mentink-Kane M.M., Wilson M.S., El Kasmi K.C., Smith A.M., et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan L.H., Wang M., Suresh M., Co D.O., Weinstock J.V., Sandor M. CD4+ TCR repertoire heterogeneity in Schistosoma mansoni-induced granulomas. J Immunol. 2002;169:6386–6393. doi: 10.4049/jimmunol.169.11.6386. [DOI] [PubMed] [Google Scholar]
- 34.Calmus Y., Gane P., Rouger P., Poupon R. Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1990;11:12–15. doi: 10.1002/hep.1840110104. [DOI] [PubMed] [Google Scholar]
- 35.Herbert D.R., Holscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M., et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 36.Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.