Abstract
The goal is to elucidate the immune modulating activity of an adenovirus (Adv) vector which showed therapeutic activity in human clinical trials. The oncolytic adenovirus (Adv/CD-TK) expressing two suicide genes was tested in two HER2/neu positive BALB/c mouse mammary tumor systems: rat neu-induced TUBO and human HER2-transfected D2F2/E2. Intra-tumoral (i.t.) Adv/CD-TK injection of TUBO tumor plus systemic prodrug therapy showed limited antitumor activity, not exceeding that by the virus itself. Antibody (Ab) to the virus was induced in Adv-/Luc-treated mice, to coincide with the loss of transgene expression. Low replication activity of adenoviruses in rodent cells may limit viral persistence. Host immunity against Adv or Adv-infected cells further mutes suicide gene activity. Treatment of TUBO tumors with Adv/CD-TK alone, however, induced neu-specific Ab responses. Treatment with Adv/CD-TK/GM (Adv/GM) that also expressed mouse granulocyte macrophage colony stimulating factor (GM-CSF), but without prodrug treatment, delayed tumor growth, enhanced anti-neu Ab production and conferred complete protection against secondary tumor challenge. D2F2/E2 tumor-bearing mice showed decreased tumor growth following i.t. Adv/GM treatment and they generated greater HER2-specific T-cell responses. These data suggest that i.t. injection of Adv itself induces immune reactivity to tumor-associated antigens and the encoded cytokine, GM-CSF, amplifies that immune response, resulting in tumor growth inhibition. Incorporation of suicide gene therapy did not improve the efficacy of Adv therapy in this mouse mammary tumor system. Oncolytic adenoviral therapy may be streamlined and improved by substituting the suicide genes with immune modulating genes to exploit tumor immunity for therapeutic benefit.
Keywords: adenovirus, granulocyte macrophage colony stimulating factor, HER2/neu, immunotherapy, mouse mammary tumor, suicide gene
Abbreviations: Ab, antibody; Adv, adenovirus; CD, cytosine deaminase; 5-FC, 5-fluorocytosine; 5-FU, 5-fluorouracil; GCV, ganciclovir; GCV-MP, ganciclovir monophosphate; GFP, green fluorescent protein; GM-CSF, granulocyte macrophage colony stimulating factor; HSV-1, herpes simplex virus 1; IFNγ, interferon gamma; IgG, immunoglobulin; IL-12, interleukin 12; i.p., intra-peritoneal; i.t., intra-tumoral; mAb, monoclonal antibody; MOI, multiplicity of infection; PFU, plaque-forming unit; PSA, prostate-specific antigen; s.c., subcutaneous; SC, splenocytes; SFU, spot forming units; TK, thymidine kinase
Introduction
Oncolytic viral therapy is an investigational cancer treatment that has generated considerable interest although its efficacy as a monotherapy may be limited.1 To further enhance the efficacy of this treatment modality, a multimodal strategy has been developed that utilizes an oncolytic adenovirus containing two cytotoxic genes (Adv/CD-TK).1-4 One gene, cytosine deaminase (CD), converts the prodrug 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU), and the other, herpes simplex virus thymidine kinase (HSV-1 TK), converts ganciclovir (GCV) into ganciclovir monophosphate (GCV-MP). Each cytotoxic gene system is expected to generate a local chemotherapeutic effect and sensitize cells to radiation. The toxicity and efficacy of this multimodal approach has been evaluated in five phase I/II trials of non-metastatic prostate cancer.5-12 The investigational therapy was well-tolerated and evidence of antitumor activity has emerged. Long-term monitoring of prostate-specific antigen (PSA) kinetics suggests that some patients may develop antitumor immunity,7 however, the immune mechanism has not been defined. More recent studies have incorporated interleukin 12 (IL-12) in the therapeutic platform.11 The addition of IL-12 improved both local and metastatic tumor control in a syngeneic prostate cancer model which was accompanied by natural killer and cytotoxic T-cell activation, supporting a role of immune activation.
Here, we investigate in two immunologically defined mouse mammary tumor model systems whether the cytokine genes are required for the immune modulating effect and attempt to better define the role of adaptive immunity. GM-CSF, rather than IL-12, was evaluated because IL-12 may have direct cytolytic or anti-angiogenic effects to confound the analysis.
The BALB/c mouse mammary tumor line TUBO was derived from a spontaneous lobular carcinoma from a BALB NeuT female that expressed transforming rat neu, a homolog of human HER2.13,14 TUBO cells express modest levels of rat neu, and form progressively growing tumors in wild type BALB/c mice after subcutaneous (s.c.) or intra-fat pad inoculation, and induce only trace levels of anti-neu antibodies.15,16 TUBO is highly sensitive to anti-neu Ab, which causes tumor regression.16,17 Another BALB/c mammary tumor line, D2F2, was cloned from a prolactin-induced spontaneous mammary tumor. Stable transfection of D2F2 with wild-type human HER2 resulted in the cell line D2F2/E2. Similar to the TUBO cell line, D2F2/E2 produces progressively growing tumors in BALB/c mice.18 Unlike TUBO, growth of D2F2/E2 results in a high level of anti-human HER2 Ab response although the cells are relatively insensitive to the Ab. The predominant anti-HER2 Ab isotype in D2F2/E2 tumor-bearing mice is immunoglobulin G subclass 1 (IgG1), which is poor in mediating Ab -dependent cell-mediated cytotoxicity for tumor cell killing.19
By using these two HER2/neu-expressing mouse mammary carcinoma models in immune-competent mice, the induction of systemic anti-HER2/neu immunity was measured to correlate with tumor growth inhibition. We found that the antitumor effect elicited by Adv/CD-TK was modest and independent of suicide gene activity but that HER2/neu-specific adaptive immunity was induced and could be amplified by GM-CSF to render improved systemic protection. Oncolytic adenoviral therapy may be streamlined and enhanced by substituting the suicide genes with immune modulating genes to synergize with ionizing radiation or other conventional therapy.
Results
Adenoviral uptake and transgene expression by mouse mammary tumor cells
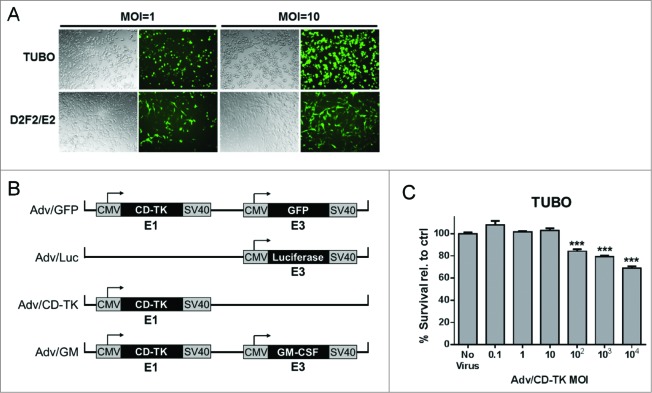
It was first necessary to test whether mouse mammary tumor cells can be infected by Adv to express the encoded transgene. TUBO and D2F2/E2 cells were co-cultured with Adv/GFP that encodes CD/TK and green fluorescent protein (GFP), at a multiplicity of infection (MOI) of 1 or 10. Dose-dependent expression of GFP was observed in both TUBO and D2F2/E2 cell lines in 24 h (Fig. 1A), showing uptake of the adenovirus and GFP expression in ∼40% (MOI = 1) to ∼90% of (MOI = 10) cells.
Figure 1.
Adenoviral infection of mouse mammary tumor cells TUBO and D2F2/E2. (A) TUBO and D2F2/E2 cells were infected with Adv/GFP at an MOI of 1 (left) or 10 (right) and cultured for 48 h prior to GFP detection. (B) Schematic of the adenovirus constructs. (C) Toxicity of Adv/CD-TK in vitro. TUBO cells were infected in quintuplicate at the indicated MOI and cultured 48 h prior to viability analysis by Alamar Blue assay. Results are presented as percent survival relative to the no virus control sample (***p < 0.001 relative to control)
To determine the appropriate MOI for in vitro analysis, TUBO cells were co-cultured with Adv/CD-TK at MOIs ranging from 0.1 to 104. At MOIs exceeding 10, increased cell death was observed in both TUBO (Fig. 1C) and D2F2/E2 (not shown) cells, indicating a cytotoxic effect with virus overload. Thus, further in vitro studies to evaluate the role of CD/TK were performed using an MOI of 1.
Sensitivity of mouse mammary tumors treated with Adv/CD-TK and 5-FC/GCV
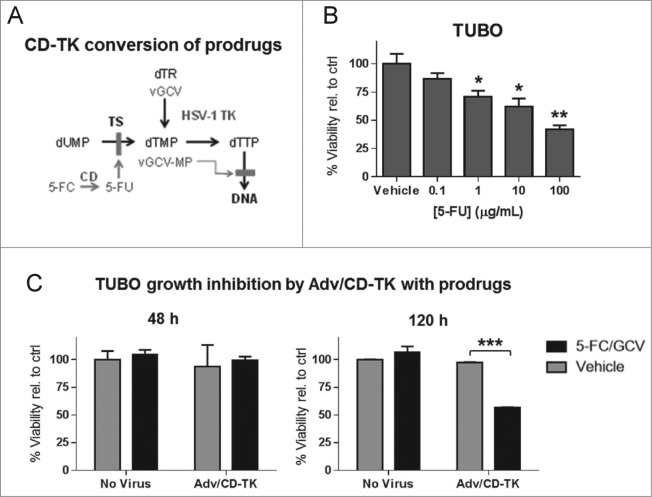
Adv/CD-TK encodes two suicide genes that convert non-toxic prodrugs, 5-FC and GCV, to their active forms, 5-FU and GCV-MP respectively, to inhibit DNA synthesis and induce apoptosis (Fig. 2A). The sensitivity of TUBO cells to 5-FU was tested by culturing TUBO cells with 5-FU at 0.1–100 μg/mL (Fig. 2B). Within 48 h, cell viability was reduced in a dose-dependent manner at concentrations above 1 μg/mL. Similar results were obtained in D2F2/E2 cells (not shown), indicating that both tumor lines are sensitive to the CD conversion product, 5-FU. The active metabolite of GCV, GCV-MP, is not commercially available in a stable form to allow similar in vitro testing.
Figure 2.
TUBO cell sensitivity to CD/TK conversion of prodrugs 5-FC and GCV. (A) Schematic depicting the conversion of the prodrugs 5-FC and GCV into their active forms and the resulting inhibition of DNA synthesis. (B) The percent growth inhibition of TUBO cells treated in quintuplicate for 48 h with the indicated concentration of 5-FU relative to untreated control was measured by Alamar Blue assay (*p < 0.05, **p < 0.01 relative to control). (C) TUBO cells were infected at an MOI of 1 with Adv/CD-TK followed by treatment with 5-FC and GCV or vehicle control for 48 h (left) or 120 h (right). The percent growth inhibition relative to control was measured by Alamar Blue assay for quintuplicate samples (***p < 0.001).
To test if Adv/CD-TK infection of mammary tumor cells enables the conversion of prodrugs 5-FC and GCV to their active, toxic forms, TUBO cells were infected with Adv/CD-TK at an MOI of 1 and incubated with 5-FC and GCV for 48 or 120 h, as previously reported (Fig. 2C).2,3 After 120 h, a 50% reduction in viability was observed in cells treated with Adv/CD-TK and prodrugs, while Adv/CD-TK alone, 5-FC/GCV alone, and vehicle control treated cells remained the same. No significant change was observed after 48 h. These results are consistent with previous observations where cell survival and viability were assayed 5–14 d after treatment to allow for transgene expression, prodrug conversion, and the manifestation of toxicity.20,3 A parallel experiment with D2F2/E2 cells yielded similar results (not shown). These results established the sensitivity of mammary tumor cells to this suicide gene therapy in vitro.
Intra-tumoral adenovirus transgene expression and anti-Adv immunity
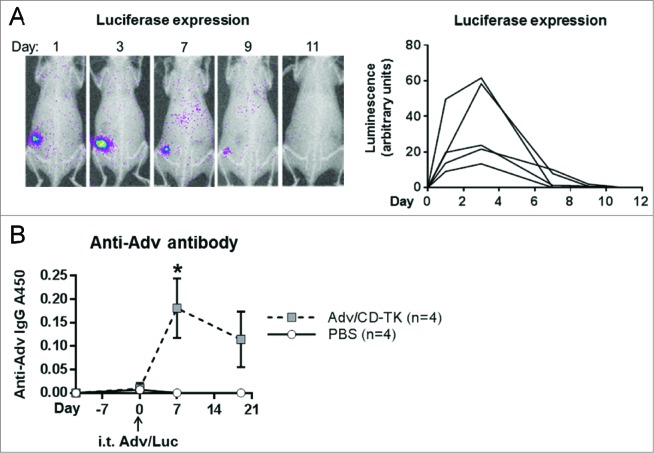
To test the uptake and transgene expression of adenoviral vector in vivo, TUBO tumors grown s.c. in BALB/c mice were injected i.t. with 108 plaque-forming units (PFU) of Adv/Luc (luciferase). Luciferase expression is demonstrated in sequential images of a representative animal on days 1, 3, 7, 9, and 11 (Fig. 3A, left panel). Of the five mice receiving a single treatment of Adv/Luc, luciferase activity lasted 7 d in three mice while the remaining two sustained expression until day 11 (Fig. 3A, right panel). Maximal luciferase expression in all test mice was observed on day 3. The transient nature of Adv/Luc expression may suggest a short term persistence of Adv in rodent cells21-23 and/or a role of anti-viral immunity that clears virus infected cells within 7–11 d.
Figure 3.
In vivo Adv transgene persistence and induction of Adv antibodies. (A) Viral transgene persistence was measured by i.t. injection of 108 PFU Adv/Luc (n = 5) followed by in vivo luciferase imaging (left, representative animal) at the indicated time points. Density analysis of luminescence was calculated to determine the luciferase expression kinetics (right). (B) ELISA was used to measure anti-Adv antibody in serum from mice treated with i.t. Adv/CD-TK (n = 4) or PBS control (n = 4) as in (A) at the indicated time points. Results are presented as A450 readings of the colorimetric assay (*p < 0.05)
To test the induction of anti-Adv immunity, mice inoculated with TUBO tumors were injected i.t. with 108 PFU Adv/CD-TK or PBS. Serum was collected prior to tumor inoculation, at time of treatment (day 12), and then 1 and 3 weeks after i.t. injection. Adv-specific antibodies in mouse serum were measured by ELISA (Fig. 3B). Within 1 week of Adv/CD-TK injection, Adv-specific Ab was significantly elevated compared to controls (p = 0.038) and it persisted beyond the time when Adv/Luc was detectable by live imaging. Therefore, one time infection of naïve mice with Adv/CD-TK promptly induced Adv immunity which may contribute to transient Adv persistence in mice.
Intra-tumoral adenovirus alone primes IgG1 antibody to tumor-associated antigen
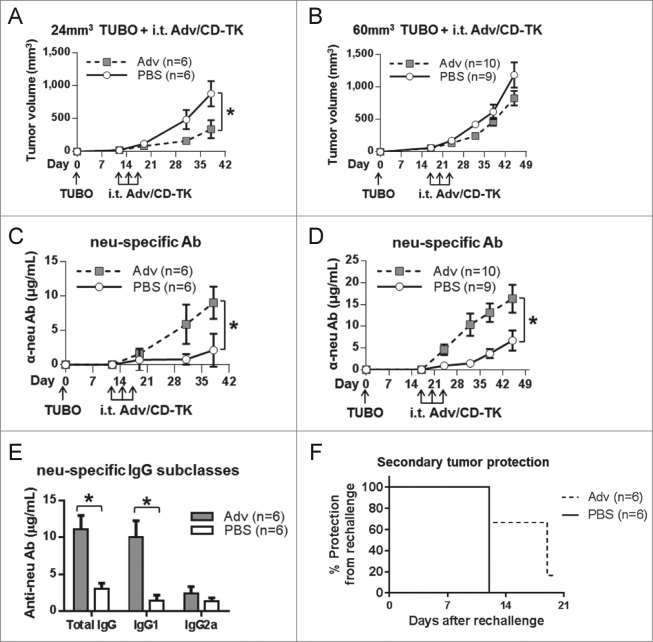
The ability of Adv/CD-TK itself, without the administration of prodrugs, to induce antitumor activity in TUBO tumor-bearing mice was assessed. BALB/c mice were inoculated with TUBO cells s.c. and when the tumors were approximately 24 mm3 or 60 mm3 in size, mice were injected i.t. three times, every other day with Adv/CD-TK at 108 PFU (an estimated MOI of 1 based on tumor volume), without prodrugs. Tumor volume (Figs. 4A, B) and induction of antibodies to the tumor-associated antigen rat neu (Figs. 4C, D) were monitored. A significant reduction in tumor growth rate was observed when 24 mm3 tumors were treated with Adv/CD-TK alone, but not when Adv/CD-TK was administered to 60 mm3 tumors. Antibodies to tumor-associated neu were elevated compared to the PBS control group, whether in the context of 24 or 60mm3 tumors (p < 0.05). Anti-neu IgG subclass analysis showed IgG1 as the predominant isotype (Fig. 4E), consistent with Th2 activation, which is commonly observed with whole cells or protein antigen.19,24
Figure 4.
Induction of anti-tumor IgG1 and TUBO tumor growth inhibition. TUBO was inoculated s.c. and permitted to grow for (A) 12 d or (B) 17 d until tumors reached an average of 24 or 60 mm3, respectively. (C/D) Adv alone or PBS control was injected i.t. every other day for a total of three times and tumor growth was monitored (*p < 0.05). Tumor-specific anti-neu antibody was measured in serum (*p < 0.05). (E) IgG subclasses were measured in serum from mice in (A) on day 38 using subclass-specific secondary antibodies (*p < 0.05). (F) Mice treated as in (A) were given a secondary TUBO inoculation on the contralateral flank on day 19 and monitored for an additional 20 d.
Secondary tumor challenge was undertaken in the 12-treated mice with 24 mm3 tumors (Fig. 4A). Mice were inoculated with TUBO tumors on the contralateral flank on day 19 and monitored for an additional 20 d. Development of the secondary tumor was delayed in the Adv/CD-TK-treated mice, suggesting the induction of a systemic antitumor immune response (Fig. 4F). This in vivo experiment demonstrates modest efficacy of Adv alone in both primary and secondary tumor growth inhibition, which may be mediated by the induced anti-neu Ab.
Prodrug treatment following Adv/CD-TK infection rendered no additional benefit
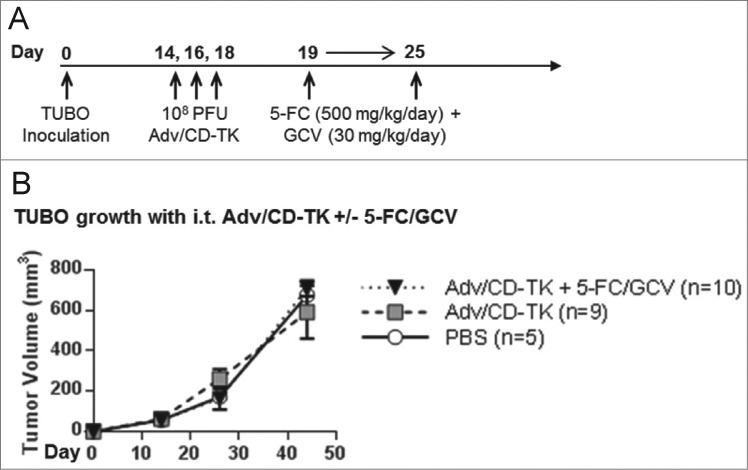
To test if the generation of cytotoxic agents via CD-TK further impacts tumor growth or alters immunity, TUBO tumors (∼60 mm3) were treated with three i.t. injections of PBS or Adv/CD-TK at 108 PFU. Prior data supports the use of three i.t. injections of Adv with the following drug dosing regimen.2-4 Pro-drugs 5-FC (500 mg/kg/day) and GCV (30 mg/kg/day) were injected intra-peritoneally (i.p.) in 1 mL PBS daily for one week starting the day after the final Adv injection (Fig. 5A). There was no significant difference between the groups in their tumor volume (Fig. 5B). Thus, addition of prodrug therapy did not reduce tumor growth.
Figure 5.
In vivo treatment with Adv/CD-TK and 5-FC/GCV prodrugs. (A) Diagram of treatment regimen. Mice were inoculated with TUBO s.c. and injected i.t. with 108 PFU Adv/CD-TK or equal volume PBS (n = 5) on days 14, 16, and 18. Mice receiving Adv/CD-TK were then treated with the indicated dose of 5-FC and GCV (n = 10) or PBS (n = 9) i.p. daily for 1 week. (B) Tumor growth was monitored in mice treated as indicated in (A).
The rapid clearance of the Adv/Luc which correlates with the induction of anti-adenoviral immunity is consistent with the limited therapeutic response with or without prodrug therapy. Because utilization of prodrug was not beneficial in the mammary tumor models in immune-competent mice, the clinical benefit observed in prostate cancer patients may be attributed to Adv-induced tumor immunity. Adv/CD-TK/mGM-CSF (Adv/GM) encoding mouse GM-CSF was generated to test if the observed antitumor adaptive immunity can be enhanced.
Adenovirus encoding GM-CSF promotes antitumor immunity in TUBO tumor-bearing mice
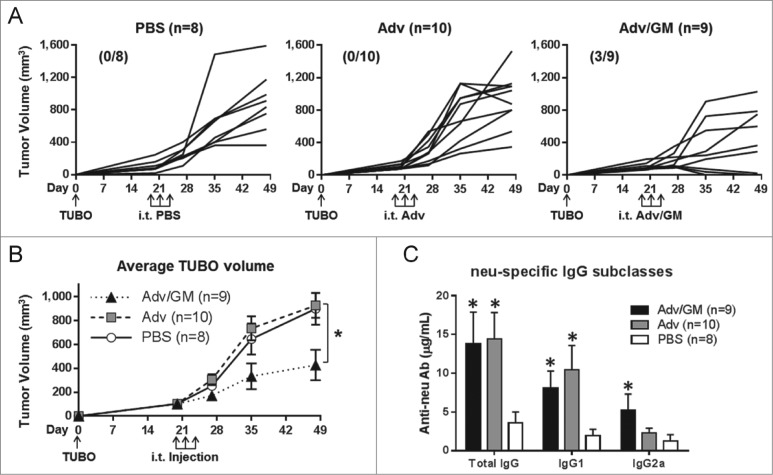
Mice bearing TUBO tumors of approximately 100 mm3 were injected i.t. three times with PBS, Adv or Adv/GM without prodrug treatment. Tumor growth was monitored for four weeks (Fig. 6A) and serum was collected at the termination of the experiment. Treatment with Adv/GM significantly reduced tumor growth rate compared to PBS control and rendered full regression in 3 of 9 mice (p < 0.05, Fig. 6B). Neu-specific IgG was elevated in both groups of Adv-treated mice compared to PBS controls (Fig. 6C). Isotype analysis showed a significant increase in IgG2a subclass following Adv/GM treatment, suggesting the emergence of a Th1 response in this group (Fig. 6C). Since TUBO cells are highly sensitive to anti-neu Ab 17 induction of neu-specific Ab by i.t. Adv treatment can be expected to render growth inhibition.
Figure 6.
Adv/GM-CSF induces anti-tumor immunity associated with induction of neu-specific IgG2a antibody. Wild-type BALB/c mice were inoculated s.c. with TUBO and were treated i.t. three times with PBS alone or 108 PFU Adv/GM-CSF in 10 μL PBS every other day as indicated beginning on day 20 when tumors were ∼100 mm3. (A) Tumor growth was monitored for individual mice in each group with (complete regressions/total) indicated and (B) average TUBO tumor volumes (*p < 0.05). (C) Neu-specific total IgG, IgG1, and IgG2a antibody were measured on day 48 (*p < 0.05 relative to PBS control)
In a repeated experiment of Adv/GM, TUBO-bearing mice were treated with PBS or Adv/GM when tumors were ∼35 mm3 ( Fig. S1A). Tumor growth was significantly lower in the Adv/GM group (Fig. S1B). Additionally, 4/10 mice in the treatment group experienced complete tumor regression compared to 0/9 in the control group. Anti-neu IgG levels were again higher in the Adv/GM group with a significant elevation in both IgG1 and IgG2a subclasses (Fig. S1C, D). To examine the potency of systemic antitumor immunity induced by i.t. Adv treatment, one week after treatment with PBS or Adv/GM, three mice each from the Adv- and PBS-treated groups were given a second, contralateral inoculation of TUBO cells. Control mice all developed palpable secondary tumors within 15 d of inoculation. All mice receiving Adv/GM were free from secondary tumor growth throughout the entire experiment (six weeks) (Fig. S1E). Thus, antitumor immunity induced by i.t. Adv can be amplified by GM-CSF to favor potent systemic protection.
Adenovirus encoding GM-CSF promotes anti-tumor immunity in antibody-resistant D2F2/E2 tumor-bearing mice
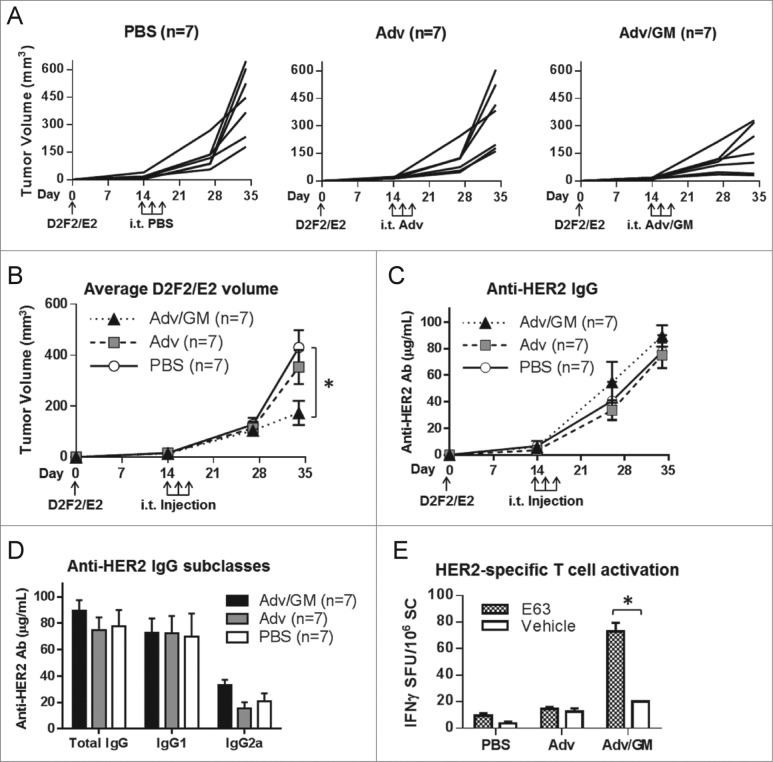
Intra-tumoral adenoviral therapy was further tested in the mouse mammary tumor cell line D2F2/E2. Un-manipulated growth of D2F2/E2 in BALB/c mice results in HER2-specific Ab production, which does not impede tumor growth. We showed previously that CD8 T cells are required to control D2F2/E2 tumor growth. 24,25 BALB/c mice were inoculated with D2F2/E2 tumor cells. Adv, Adv/GM, or PBS was administered when tumors were at an average of 24 mm3. Tumor growth was again significantly reduced in the Adv/GM-treated group compared to the PBS-treated group (Figs. 7A, B). Overall anti-HER2 Ab production and Ab subclasses were similar in all groups, regardless of treatment (Figs. 7C, D). To analyze T-cell activation following treatment, splenocytes were harvested for all mice and were subsequently incubated with E63, the dominant HER2 peptide presented by Kd in BALB/c mice, or with vehicle control. Interferon gamma (IFNγ) production, indicating HER2-specific T-cell activation, was significantly elevated in splenocytes from mice treated with Adv/GM after stimulation with E63 peptide vs. vehicle control (Fig. 7E). Treatment with Adv/GM elicited significant HER2-specific T-cell responses corresponding to decreased D2F2/E2 tumor growth.
Figure 7.
i.t. injection of Adv/GM-CSF enhances Her-2-specific T cell responses and decreased D2F2/E2 tumor growth. BALB/c mice were inoculated s.c. with D2F2/E2 on day 0. Beginning on day 14 when tumors reached 24mm3, i.t. injections were given every other day for a total of three times with PBS alone or 108 PFU Adv alone or Adv/GM-CSF in 10 μL PBS as indicated. Tumor growth of (A) individual animals and (B) averages of each group were monitored (*p < 0.05). (C) HER2-specific IgG was measured in serum during the course of the experiment and (D) HER2 IgG subclasses were evaluated in serum samples at day 34. (E) At day 34, mice were euthanized and splenic HER2-specific IFNγ-producing T cells were analyzed by ELISPOT. Splenocytes (SC) were cultured with either 20 μg/mL of the dominant HER2-specific CD8 T-cell peptide E63 or vehicle alone. Results are presented as IFNγ spot forming units (SFU) per 106 SC (*p < 0.05)
Discussion
To better understand the process and improve the outcomes of adenoviral immunotherapy which delayed disease recurrence in human trials, the current study sought to characterize the systemic antitumor immunity induced by i.t. Adv/CD-TK therapy using two tumor models, and test the efficacy with GM-CSF gene expression. While in vitro data suggested therapeutic activity of the Adv/CD-TK with prodrugs, in vivo results showed limited anti-tumor activity that did not exceed the effect of the virus itself. This is consistent with poor Adv replication in rodent cells.21-23 The resulting transcription of suicide genes may therefore be at a suboptimal level in vivo, thus limiting the effects of prodrug therapy. Neu-specific Ab was significantly elevated in TUBO tumor bearing mice that received Adv/CD-TK with and without prodrug therapy. The delivery of the GM-CSF gene by Adv serves as an effective adjuvant. TUBO tumor-bearing mice treated with Adv/GM showed significantly delayed tumor growth compared to control group mice. Furthermore, Adv/GM therapy conferred complete protection against secondary tumor challenge. D2F2/E2 tumor-bearing mice also showed decreased tumor growth following Adv/GM treatment and they generated greater HER2-specific T-cell responses. Therefore, in the present mammary tumor models, the i.t. injection of Adv itself induces immune reactivity to tumor-associated antigens and the encoded cytokine, GM-CSF, amplifies that immune response, resulting in tumor growth inhibition.
In human trials, a synergistic effect between adenoviral therapy and ionizing radiation has been reported.26,27 When Adv/CD-TK was used in a phase I study of prostate cancer, the treated patients were sensitized to radiation with extended time to disease progression when compared to radiation alone.5,7 Several differences between the human trials and mouse mammary tumor studies may contribute to the outcome disparity. Adv/CD-TK is replication competent in human cells, but does not produce a significant viral burst in mice, although replication of Adv DNA is supported.28 The infectivity and subsequent oncolytic effect of Adv may be more substantial in humans to slow tumor growth. Murine imaging and kinetics studies, however, demonstrate a rapid induction of anti-Adv immunity in Adv/CD-TK-treated naïve mice and loss of transgene expression by 11 d after administration (Figs. 3A, B). The presence or induction of viral immunity may promptly contain the virus or infected cells. It may be reasonable to replace suicide genes with immune-modulating molecules, such as GM-CSF or IL-12 to amplify long-term immune protection while streamlining the treatment protocol.
To determine if tumor immunity can be further enhanced, Adv/CD-TK expressing IL-12 was tested in a preclinical model of prostate cancer.11 Addition of IL-12 reduced s.c. and orthotopic tumor growth with enhancement of natural killer cell and cytotoxic T lymphocyte activity. In immunohistochemistry specimens, both CD4+ and CD8+ infiltrates were increased with IL-12. Our previous report of IL-12 gene electrotransfer into the mouse mammary tumors also showed the induction of antitumor immunity and tumor rejection.29 In the current study, GM-CSF was used to resolve the role of immune modulation because IL-12 may have direct antitumor activity or angiogenesis inhibitory effect. A more pronounced IgG2a response, indicative of Th1 activation was observed in those mice receiving Adv/GM. D2F2/E2 data further demonstrate augmented HER2 specific T-cell activation in Adv/GM-treated mice, with higher numbers of IFNγ producing cells seen in ELISPOT (Figs. 7D, E). Thus, tumor immunity induced by Adv is enhanced and tilted toward Th1 by GM-CSF to support the strategy of incorporating immune-modulating genes in Adv vectors.
The present study provides an improved understanding of the immune response to i.t. injections of Adv in a breast cancer model. The introduction of GM-CSF via the Adv vector promoted HER2/neu immunity and protected mice from secondary tumor challenge. Therefore, future studies are warranted to elucidate the potential clinical applications of various immune modulators in adenoviral therapy.
Materials and Methods
Mice
BALB/c mice were purchased from the NIH for all in vivo studies which were conducted when mice reached 6–8 weeks of age. All animal procedures were approved by the Wayne State University Institutional Animal Care and Use Committee and detailed in the accepted protocol.
Tumor cell lines
TUBO and D2F2/E2 cell lines were maintained in high-glucose DMEM media supplemented with 5% cosmic calf serum (HyClone, AXA30096), 5% fetal bovine serum (Sigma–Aldrich, F4135), 10% NCTC-109 medium (Invitrogen, 21340), 2 mM l-glutamine (Invitrogen, 25030), 0.1 mM MEM nonessential amino acids (Invitrogen, 11140), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, 15140), with 0.8 mg/mL Geneticin® (Invitrogen, 11811) included for D2F2/E2.
Adenoviral constructs
The replication-competent adenovirus Ad5yCD/mutTKSR39rep (Adv/CD-TK) contains the fusion gene yeast CD and the SR39 mutant form of HSV-1 TK.3 These genes are under control of the CMV promoter and exist in the E1 portion of the adenoviral vector (Fig. 1B). Additional adenoviral constructs containing the Adv/CD-TK with GFP (Adv/GFP) and GM-CSF (Adv/GM) have been created with GFP or GM-CSF introduced in the E3 portion of the genome. An adenovirus expressing luciferase (Adv/Luc) contains the transgene in the E3 region.
In Vitro assays
GFP infection to assess adenoviral infectivity was performed with Adv/GFP. Visualization with microscopy was conducted at each time point to assess infectivity of the virus.
Cell proliferation and adenoviral toxicity was measured using Alamar Blue fluorescence (Invitrogen, DAL1025). Cells were plated in quintuplicate wells and plates were read with the Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT). Statistical evaluation was completed using the unpaired Student's t test.
Tumor challenge, adenovirus injection, and prodrug treatment
All tumor inoculations were s.c. with 2.5 × 105 tumor cells per mouse. At designated tumor volumes, TUBO and D2F2/E2 tumors were injected three times every other day with 10 μL of either PBS or the specified Adv at 108 PFU.30 Viral particles were purified with the Adenopure system (Puresyn, 70100) per manufacturer instruction and viral PFU were determined by ELISA (Cell Biolabs, VPK-110). Prodrugs were administered as 1 mL PBS i.p. containing 5-FC (Sigma–Aldrich, F7129) at 500 mg/kg/day and GCV (Sigma–Aldrich, G2536) at 30 mg/kg/day or 1mL PBS, at the designated time points based on prior investigation.3,4 Tumor volumes were analyzed by one-way ANOVA with Tukey's posttest. Protection from contralateral tumor challenge was analyzed by log-rank test.
Luciferase imaging
BALB/c mice bearing ∼50 mm3 TUBO tumors were treated with 108 PFU of Adv/Luc. Expression of luciferase is measured by live animal luminescence imaging using the In Vivo MS FX Pro (Carestream, Woodbridge, CT) after 150 mg/kg i.p. injection of the substrate D-luciferin (Caliper Life Sciences, 119222).
ELISA Measurement of anti-adenoviral antibody
Anti-adenoviral IgG was compared between groups using an ELISA kit (MyBioSource MBS702268) per manufacturer instructions. Briefly, serum sample was added to the pre-coated assay plate bound with type 5 adenovirus and incubated for 30 min at 37°C. Wells were washed prior to addition of horse radish peroxidase (HRP)-conjugated anti-mouse secondary Ab and further incubated for 20 min. Wells were again washed prior to addition of substrate for 10 min at 37°C in the dark and 50 μL of stop solution was added to each well. The optical density was measured using the Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT) set to 450 nm wavelength.
Flow cytometry with measurement of anti-HER2/neu antibody
Anti-neu Ab was measured using 3T3/NKB cells. Anti-HER2 Ab was measured using 3T3-EKB cells. These cell lines were maintained using DMEM media supplemented similar to the TUBO cell line with the addition of 0.8 mg/mL Geneticin® and 0.8 mg/mL Zeocin™. The neu (Clone 7.16.4, Calbiochem, OP16) or HER2 (Clone TA-1, Calbiochem, OP39) mAb which recognize the extracellular portion of the rat neu or HER2 proteins, respectively, were used to create standard curves.31 A phycoerythrin-conjugated goat-anti-mouse total IgG (Jackson ImmunoResearch Laboratories, 115-116-071), IgG1 (Jackson ImmunoResearch Laboratories, 115-115-205), or IgG2a (Jackson ImmunoResearch Laboratories, 115-115-206) secondary Ab was used to detect bound serum IgG. All flow cytometry was performed on the BD FACSCanto™ II (BD Biosciences, San Jose, CA) system. Anti-HER2/neu Ab levels were analyzed using the Mann–Whitney test.
ELISPOT measurement of T cell response
Spleens of D2F2/E2 mice were harvested and treated as previously described.32,33 Splenocytes were suspended in RPMI supplemented with 10% FBS, 2 mmol/L L-glutamine, and 100 units/mL penicillin and 100 μg/mL streptomycin. 2 × 105 splenocytes were added to each well of a 96-well HTS IP plate (Millipore, MSIPS4W10), precoated with rat anti-mouse IFNγ (Clone AN-18, eBioscience, 16-7313-85) and stimulated with 20 μg/mL HER2 E63 peptide (TYLPTNASL, Invitrogen) resuspended in DMSO for 48 h at 37°C in 5% CO2.
After incubation, cells were removed and 2 μg/mL biotinylated rat anti-mouse-IFNγ Clone R4-6A2, eBioscience, 13-7312-85) was added. Plates were incubated for 12 h at 4°C and washed to remove unbound Ab. Bound Ab was detected by incubation with 0.5 μg/mL UltraAvidin™-HRP (Leinco, A106) for 2 h at room temperature. The substrate 3-amino-9-ethylcarbazole (AEC; BD Biosciences, 551951) was added and the plate was incubated for 3–5 min. AEC solution was then discarded and the plates were washed six times with water. Visualized cytokine spots were enumerated using the ImmunoSpot© analyzer (CTL, Shaker Heights, OH) and expressed as the number of cytokine-producing cells per 106 splenocytes. Results were analyzed by Mann–Whitney test.
Supplementary Material
Acknowledgments
The authors wish to thank Joyce Reyes for her expert technical assistance and the microscopy, imaging, and cytometry resources core.
Disclosure of Potential Conflicts of Interest
The authors disclose no potential conflicts of interest.
Funding
The study was supported by NIH RO1 CA76340 (W.Z. Wei), NIH RO1 CA160289 (S. Freytag), and Young Family Foundation.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther 1998; 9:1323-33; PMID:; http://dx.doi.org/ 10.1089/hum.1998.9.9-1323 [DOI] [PubMed] [Google Scholar]
- 2. Rogulski KR, Wing MS, Paielli DL, Gilbert JD, Kim JH, Freytag SO. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther 2000; 11:67-76; PMID:; http://dx.doi.org/ 10.1089/10430340050016166 [DOI] [PubMed] [Google Scholar]
- 3. Freytag SO, Paielli D, Wing M, Rogulski K, Brown S, Kolozsvary A, Seely J, Barton K, Dragovic A, Kim JH. Efficacy and toxicity of replication-competent adenovirus-mediated double suicide gene therapy in combination with radiation therapy in an orthotopic mouse prostate cancer model. Int J Radiat Oncol Biol Phys 2002; 54:873-85; PMID:; http://dx.doi.org/ 10.1016/S0360-3016(02)03005-5 [DOI] [PubMed] [Google Scholar]
- 4. Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M, Seely J, Kim JH, Freytag SO. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther 2006; 13:347-6; PMID:; http://dx.doi.org/ 10.1016/j.ymthe.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, DePeralta-Venturina M, Xia X, Brown S, Lu M, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res 2003; 63:7497-506; PMID: [PubMed] [Google Scholar]
- 6. Freytag SO, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, Barton KN, Brown SL, Lu M, Kim JH. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther 2007; 15:636-42; PMID:; http://dx.doi.org/ 10.1038/sj.mt.6300068 [DOI] [PubMed] [Google Scholar]
- 7. Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, Zhang Y, Barton KN, Brown SL, Lu M, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther 2007; 15:1016-23; PMID:; http://dx.doi.org/ 10.1038/mt.sj.6300120 [DOI] [PubMed] [Google Scholar]
- 8. Barton KN, Stricker H, Brown SL, Elshaikh M, Aref I, Lu M, Pegg J, Zhang Y, Karvelis KC, Siddiqui F, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther 2008; 16:1761-69; PMID:; http://dx.doi.org/ 10.1038/mt.2008.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barton KN, Stricker H, Elshaikh MA, Pegg J, Cheng J, Zhang Y, Karvelis KC, Lu M, Movsas B, Freytag SO. Feasibility of adenovirus-mediated hNIS gene transfer and 131I radioiodine therapy as a definitive treatment for localized prostate cancer. Mol Ther 2011; 19:1353-59; PMID:; http://dx.doi.org/ 10.1038/mt.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freytag SO, Stricker H, Lu M, Elshaikh M, Aref I, Pradhan D, Levin K, Kim JH, Peabody J, Siddiqui F, et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2014; 89:268-76; PMID:; http://dx.doi.org/ 10.1016/j.ijrobp.2014.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freytag SO, Barton KN, Zhang Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther 2013; 20:1131-39; PMID:; http://dx.doi.org/ 10.1038/gt.2013.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, Nafziger D, Pegg J, Paielli D, Brown S, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res 2002; 62:4968-76; PMID: [PubMed] [Google Scholar]
- 13. Rovero S, Amici A, Carlo ED, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol 2000; 165:5133-42; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.9.5133 [DOI] [PubMed] [Google Scholar]
- 14. Rovero S. BKCEAAQEPPMPFG. Insertion of the DNA for the 163-171 peptide of IL1beta enables a DNA vaccine encoding p185(neu) to inhibit mammary carcinogenesis in Her-2/neu transgenic BALB/c mice. Gene Therapy 2001; 8:447-52; PMID:; http://dx.doi.org/ 10.1038/sj.gt.3301416 [DOI] [PubMed] [Google Scholar]
- 15. Jacob JB, Kong YM, Nalbantoglu I, Snower DP, Wei WZ. Tumor regression following DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J Immunol 2009; 182:5873-5881; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0804074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JM, Terabe M, Sakai Y, Munasinghe J, Forni G, Morris JC, Berzofsky JA. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. J Immunol 2005; 174:4228-36; PMID:; http://dx.doi.org/ 10.4049/jimmunol.174.7.4228 [DOI] [PubMed] [Google Scholar]
- 17. Veenstra J, Gibson HM, Littrup PJ, Reyes JD, Cher ML, Takashima A, Wei WZ. Cryotherapy with concurrent CpG oligonucleotide treatment controls local tumor recurrence and modulates Her2/neu immunity. Cancer Res 2014; 74:5409-20; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer 1999; 81:748-54; PMID:; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19990531)81:5%3c748::AID-IJC14%3e3.0.CO;2-6 [DOI] [PubMed] [Google Scholar]
- 19. Pilon SA, Piechocki MP, Wei WZ. Vaccination with cytoplasmic ErbB-2 DNA protects mice from mammary tumor growth without anti-ErbB-2 antibody. J Immunol 2001; 167:3201-06; PMID:; http://dx.doi.org/ 10.4049/jimmunol.167.6.3201 [DOI] [PubMed] [Google Scholar]
- 20. Xie Y, Gilbert JD, Kim JH, Freytag SO. Efficacy of adenovirus-mediated CD/5-FC and HSV-1 thymidine kinase/ganciclovir suicide gene therapies concomitant with p53 gene therapy. Clin Cancer Res 1999; 5:4224-32; PMID: [PubMed] [Google Scholar]
- 21. Blair GE, Dixon SC, Griffiths SA, Zajdel ME. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res 1989; 14:339-46; PMID:; http://dx.doi.org/ 10.1016/0168-1702(89)90026-9 [DOI] [PubMed] [Google Scholar]
- 22. Jogler C, Hoffmann D, Theegarten D, Grunwald T, Uberla K, Wildner O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J Virol 2006; 80:3549-58; PMID:; http://dx.doi.org/ 10.1128/JVI.80.7.3549-3558.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R, Willcox HN. Infection of mouse liver by human adenovirus type 5. J Gen Virol 1978; 40:45-61; PMID:; http://dx.doi.org/ 10.1099/0022-1317-40-1-45 [DOI] [PubMed] [Google Scholar]
- 24. Whittington PJ, Radkevich-Brown O, Jacob JB, Jones RF, Weise AM, Wei WZ. Her-2 DNA versus cell vaccine: immunogenicity and anti-tumor activity. Cancer Immunol Immunother 2009; 58-759-67; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whittington PJ, Piechocki MP, Heng HH, Jacob JB, Jones RF, Back JB, Wei W-Z. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Res 2008; 68:7502-11; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Cook T, Alber S, Liu K, Kovesdi I, Watkins SK, Vodovotz Y, Billiar TR, Blumberg D. Adenoviral gene transfer of the human inducible nitric oxide synthase gene enhances the radiation response of human colorectal cancer associated with alterations in tumor vascularity. Cancer Res 2004; 64:1386-95; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-1307 [DOI] [PubMed] [Google Scholar]
- 27. Rhee JG, Li D, Suntharalingam M, Guo C, O'Malley BW, Jr., Carney JP. Radiosensitization of head/neck squamous cell carcinoma by adenovirus-mediated expression of the Nbs1 protein. Int J Radiat Oncol Biol Phys 2007; 67:273-8; PMID:; http://dx.doi.org/ 10.1016/j.ijrobp.2006.09.019 [DOI] [PubMed] [Google Scholar]
- 28. Paielli DL, Wing MS, Rogulski KR, Gilbert JD, Kolozsvary A, Kim JH, Hughes J, Schnell M, Thompson T, Freytag SO. Evaluation of the biodistribution, persistence, toxicity, and potential of germ-line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther 2000; 1:263-74; PMID:; http://dx.doi.org/ 10.1006/mthe.2000.0037 [DOI] [PubMed] [Google Scholar]
- 29. Radkevich-Brown O, Piechocki MP, Back JB, Weise AM, Pilon-Thomas S, Wei WZ. Intratumoral DNA electroporation induces anti-tumor immunity and tumor regression. Cancer Immunol Immunother 2010; 59:409-17; PMID:; http://dx.doi.org/ 10.1007/s00262-009-0760-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freytag SO, Barton KN, Brown SL, Narra V, Zhang Y, Tyson D, Nall C, Lu M, Ajlouni M, Movsas B, et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol Ther 2007; 15:1600-1606; PMID:; http://dx.doi.org/ 10.1038/sj.mt.6300212 [DOI] [PubMed] [Google Scholar]
- 31. Piechocki MP, Pilon SA, Wei WZ. Quantitative measurement of anti-ErbB-2 antibody by flow cytometry and ELISA. J Immunol Methods 2002; 259:33-42; PMID:; http://dx.doi.org/ 10.1016/S0022-1759(01)00487-2 [DOI] [PubMed] [Google Scholar]
- 32. Radkevich-Brown O, Jacob JB, Kershaw MH, Wei WZ. Genetic regulation of the response to Her-2 DNA vaccination in human Her-2 transgenic mice. Cancer Res 2009; 69:212-8; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei WZ, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YM. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Res 2005; 65:8471-78; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-0934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.