Abstract
Dab2 is an adapter protein involved in receptor-mediated signaling, endocytosis, cell adhesion, hematopoietic cell differentiation, and angiogenesis. It plays a pivotal role in controlling cellular homeostasis. In the immune system, the Dab2 is a Foxp3 target gene and is required for regulatory T (Treg) cell function. Dab2 expression and its biological function in dendritic cells (DCs) have not been described. In this study, we found that Dab2 was significantly induced during the development of mouse bone marrow (BM)-derived DCs (BMDCs) and human monocyte-derived DCs (MoDCs). Even in a steady state, Dab2 was expressed in mouse splenic DCs (spDCs). STAT5 activation, Foxp3 expression, and hnRNPE1 activation mediated by PI3K/Akt signaling were required for Dab2 expression during GM-CSF-derived BMDC development regardless of TGF-β signaling. Dab2-silencing was accompanied by enhanced IL-12 and IL-6 expression, and an improved capacity of DC for antigen uptake, migration and T cell stimulation, which generated strong CTL in vaccinated mice. Vaccination with Dab2-silenced DCs inhibited tumor growth more effectively than did vaccination with wild type DCs. Dab2-overexpression abrogated the efficacy of the DC vaccine in DC-based tumor immunotherapy. These data strongly suggest that Dab2 might be an intrinsic negative regulator of the immunogenicity of DCs, thus might be an attractive molecular target to improve DC vaccine efficacy.
Keywords: Dab2, dendritic cells, immunogenicity, molecular target
Abbreviations: BAT, blocking the TGF-β-activated translation element; BM, bone marrow; CFSE, 5, 6-carboxyfluorescein succinimidyl ester; CTL, cytotoxic T lymphocyte; Dab2KD, Dab2-knockdown; Dab2, disabled-2 adaptor protein; DCs, dendritic cells; Foxp3, forkhead box P3; GM-CSF, granulocyte-macrophage colony stimulating factor; hMoDC, human monocyte-derived dendritic cell; hnRNP E1, heterogeneous nuclear ribonucleoprotein E1; imDC, immature DC; mDC, mature DC; OT-1 and OT-2 mice, OVA257–264 and OVA323–339-peptide-specific T cell receptor transgenic mice; OVA, ovalbumin; PI3K, phosphoinositide-3 kinase; STAT5, transducer and activator of transcription 5; TGF-β, transforming growth factor-β; Treg, regulatory T; WT, wild type
Introduction
DCs are professional antigen presenting cells (APCs) that play a crucial role in activating T cell-mediated, antigen-specific adaptive immune responses.1 DCs have heterogeneous origins, morphologies, phenotypes, and immunological functions.2,3 Granulocyte-macrophage colony stimulating factor (GM-CSF) with or without interleukin (IL)-4 lead mouse BM cells and human monocytes to differentiate into DCs in vitro.4 However, the molecular mechanism underlying DC differentiation and maintenance from BM cells or monocytes is not well understood in normal or disease conditions. Environmental and genetic factors are expected to be critical for DC differentiation and immunogenicity, but the detailed molecular mechanisms remain widely unknown.
A microarray analysis and qRT-PCR evaluation of mRNAs extracted from DCs differentiated from BM stem cells with GM-CSF revealed that the disabled-2 (Dab2) adaptor protein is significantly induced during DC development. Most studies of Dab2 expression and functions have been performed with transforming growth factor-β (TGF-β) signaling in Treg cells, tumor cells, or nonlymphoid epithelial cells. However, we focused on the GM-CSF-mediated Dab2 expression during DC development and its role in DC differentiation and immunogenicity.
Dab2 is a tumor suppressor/endocytic adaptor protein that is involved in receptor-mediated endocytosis/trafficking and TGF-β signaling.5-7 The Dab2 gene encodes two isoforms: p96 and p67.8 p96 is predominantly expressed in adults, while p67 is mainly present during embryogenesis.6 Dab2 inhibits cell growth and proliferation in many cell types9,10 and is significantly down-regulated in various tumors9,11,12 Dab2-deficient breast cancer cells had impaired ability to deplete TGF-β receptors through endocytosis, leading to TGF-β accumulation in the tumor microenvironment and immune tolerance.13 Expression of Dab2, a forkhead box P3 (Foxp3) target gene, is restricted to CD4+ Treg cells in peripheral lymphocytes, and Treg cells lacking Dab2 are functionally impaired.14 Foxp3 expression and Treg function require TGF-β signaling.15 Dab2 is also broadly expressed in many nonlymphoid cells and organs16-18 and is critical for embryogenesis and transformations, such as epithelial to mesenchymal transitions (EMT) induced by TGF-β/Smad signaling.19,20 Heterogeneous nuclear ribonucleoprotein E1 (hnRNP E1) inhibits Dab2 translation under normal conditions by blocking the TGF-β-activated translation (BAT) element in the 3′-untranslated region of Dab2 mRNA transcripts.21 TGF-β activation leads to hnRNP E1 phosphorylation by protein kinase B (PKB)-β/Akt2, inducing its release from the BAT element and translation of Dab2 mRNAs.21,22 Dab2 contains an amino-terminal phosphotyrosine binding (PTB) domain, which interacts with transmembrane region of TGF-β receptors.19 Dab2 negatively regulates TGF-β-induced activation of c-Jun N-terminal kinase (JNK) without influencing the Smad pathway, suggesting that Dab2 controls TGF-β signaling by balancing the Smad and JNK pathways.23 Additionally, Dab2 contains a C-terminal proline-rich domain (PRD) that interacts with SH3 domains in proteins such as Grb2.24 Dab2 competes with SOS for binding to Grb2, which modulates the growth factor receptor/Ras signaling pathways.10
GM-CSF is a critical regulator of DC development through intracellular signaling pathways, including the Janus kinase (JAK)/signal transducer and activator of transcription 5 (STAT5), mitogen-activated protein kinase (MAPK), phosphoinositide-3 kinase (PI3K), and canonical NF-kB pathways.2 STAT5 signaling is essential for GM-CSF-dependent DC development.25,26 STAT5 signaling is also needed for Foxp3 expression in Treg cells27,28 and activates the PI3K/Akt and Ras/MAPK pathways.29 Active protein kinase B (PKB)/Akt interacts with Dab2 to facilitate Dab2-mediated albumin endocytosis and control the albumin overload-induced proximal tubule injury.30
Despite the important functions of Dab2 in Treg and other nonlymphoid cell types, Dab2 has not been addressed in association with GM-CSF signaling, DC development, or DC immunogenicity. In the present study, we found that Dab2 was significantly expressed during GM-CSF-mediated BMDC development. Dab2 was also found to be expressed in steady-state mouse spDCs and human MoDCs as well. GM-CSF-mediated Dab2 expression required STAT5 signaling, Foxp3 expression, and PI3K/AKT-mediated hnRNP activation as shown by Dab2 expression during the TGF-β-mediated EMT transition. However, Dab2 expression during GM-CSF-derived BMDC development was found to have no relationship to TGF-β signaling. Dab2-silencing up-regulated the expression of surface immune-related molecules, antigen uptake capacity, DC migration and Th1 cytokine secretion. Dab2-silencing enhanced the DC vaccine efficacy in tumor immunotherapy. Finally, Dab2 over-expression abrogated the efficacy of a DC vaccine. Our findings suggest that Dab2 in DCs plays an important role as an intrinsic negative regulator in controlling DC immunogenicity. Dab2 might be an attractive molecular target to improve DC vaccine efficacy.
Results
Dab2 was significantly induced during DC development
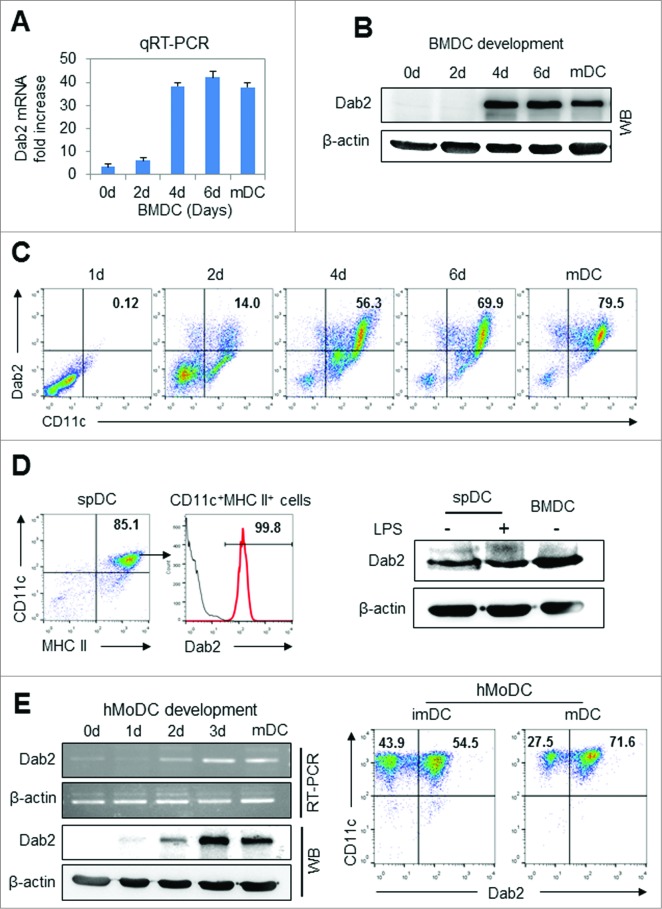
Dab2 expression was significantly induced at a later stage of DC development from mouse BM cells at both the mRNA (Fig. 1A) and protein (Fig. 1B) levels. Intracellular Dab2 expression increased in parallel with CD11c expression during BMDC differentiation (Fig. 1C). Dab2 was also highly expressed in primary major histocompatability complex class (MHC) IIhigh CD11c+ spDCs isolated from normal mice as was shown in BMDCs (Fig. 1D). Dab2 was also found to be expressed in human MoDCs (Fig. 1E). These results suggest that Dab2 may play an important role in DC differentiation, immunogenicity, or both in vivo.
Figure 1.
Dab2 expression in BMDC, splenic DC and human monocyte-derived DC (A and B) Dab2 expression was assessed during BM (C57 BL/6) cell-derived DC (BMDC) development. Cells developing into BMDCs were harvested on days 0, 2, 4, and 6, and Dab2 mRNA and protein levels were assessed by quantitative real-time (qRT)-PCR with Fast SYBR® Green Master Mix kit (Life Technologies) and by Western blot with rabbit anti-mouse Dab2 polyclonal antibody (Protein Tech), respectively. qRT-PCR data are shown as mean ± SD of nine samples pooled from three independent experiments. (C) Cells were harvested on days 0, 2, 4, and 6 during DC development, and assessed by flow cytometry after staining with FITC-labeled CD11c and PE-labeled intracellular Dab2 antibodies. (D) Splenic DCs were isolated from mouse (C57BL/6) spleen using CD11c+ isolation kit (Miltenyi Biotech) and treated with or without LPS (100 ng/mL) for 24 h. Intracellular Dab2 expression in splenic DCs was assessed by FACS (left) and Western blot assay (right). (E) Dab2 mRNA and protein expression were assessed by real-time (RT)-PCR and by Western blot with rabbit anti-mouse Dab2 polyclonal antibody (Protein Tech), respectively, during human monocyte-derived DC (hMoDC) development (left) as described in Materials and Methods . Intracellular Dab2 expression in MoDCs was also assessed by FACS (right).
Dab2 expression in DCs requires STAT5 signaling, Foxp3, and hnRNP E1 activation
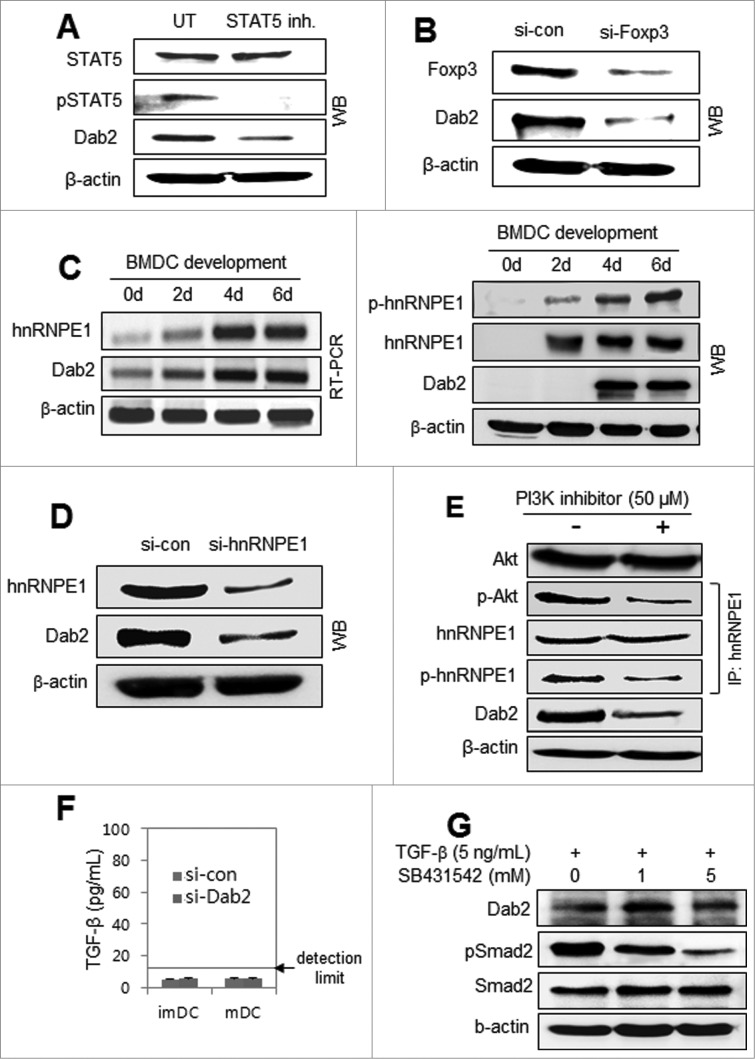
Most studies on Dab2 have reported its expression in association with TGF-β or TGF-β related signaling.13-16, 19-23 None of the papers addressed Dab2 expression with GM-CSF signaling. We examined whether TGF-β signaling pathways were involved in the Dab2 expression in DCs induced by GM-CSF signaling. STAT5 signaling, which is essential for GM-CSF-mediated DC differentiation,26 has not been examined in relation to Dab2 expression. Treating DC precursor cells with STAT5 inhibitor downregulated GM-CSF-induced Dab2 expression (Fig. 2A), suggesting that GM-CSF-mediated STAT5 activation is required for Dab2 expression in DCs. Silencing Foxp3 in DC precursor cells also downregulated Dab2 expression in GM-CSF-derived DCs (Fig. 2B). hnRNP E1 is involved in controlling Dab2 translation by blocking the BAT element of Dab2 mRNA. Once hnRNP E1 is phosphorylated by the PI3K/PKB/Akt pathway under TGF-β signaling, hnRNP E1 is released from the BAT element and Dab2 is expressed.21,22 The hnRNP E1 was significantly induced during GM-CSF-derived DC development at both the mRNA (left) and protein (right) levels and was activated in parallel with Dab2 expression (Fig. 2C). Silencing hnRNPE1 decreased Dab2 expression in DCs (Fig. 2D). A PI3K inhibitor blocked the GM-CSF-mediated activation of Akt/PKB, which was accompanied by impaired hnRNPE1 phosphorylation, resulting in significantly downregulated Dab2 expression in a short time (Fig. 2E). TGF-β was undetectable in the culture supernatants of imDCs and mDCs (Fig. 2F). Dab2 expression in BMDCs was not affected by TGF-β and/or by TGF-β receptor inhibitor (Fig. 1G). These data imply that GM-CSF-mediated Dab2 expression during DC development from BM precursor cells is not associated with TGF-β signaling.
Figure 2 (See previous page).
Dab2 expression in DCs requires STAT5 signaling, Foxp3 expression, and hnRNPE1 activation but has no association with TGF-β signaling. (A) Mouse (C57BL/6) BMDCs were treated with 100 μM STAT5 inhibitor for 4 h before harvest. Harvested cells were subjected to Western blot analysis of STAT5 and Dab2 expression with STAT5, phospho-STAT5, and Dab2 antibodies. (B) DC precursor cells on day 4 during BMDC development were transfected with control and Foxp3 siRNAs and then harvested after 48 h. Dab2 expression was assessed by Western blot with Dab2 and Foxp3 antibodies. (C) mRNA (left) and protein (right) expressions of hnRNPE1 and Dab2, together with hnRNPE1 phosphorylation, were assessed during BMDC ←development by RT-PCR and Western blot analysis. (D) BMDC precursor cells on day 4 were transfected with si-con and si-hnRNPE1 and harvested after 48 h. Dab2 and hnRNPE1 expression was assessed by Western blot with Dab2 and hnRNPE1 antibodies. (E) BMDCs were treated with 50 μM PI3K inhibitor (Calbiochem) for 20 min before harvest and subjected to Western blot analysis of Akt and Dab2. hnRNPE1 was immunoprecipitated from BMDC lysates with anti-hnRNPE1 antibody, followed by immunoblot analysis with phospho-hnRNPE1 (phospho-serine) and phospho-Akt antibodies. (F) BMDC precursor cells on day 4 were transfected with Dab2-specific siRNAs (si-Dab2) or control siRNA (si-con). After 48 h, the level of TGF-β in culture supernatant was examined using TGF-β ELISA kit (BioLegend). (G) BMDCs were treated or untreated with 5 ng/mL TGF-β for 24 h in the presence or absence of SB431524 (TGFβRI). Dab2 and Smad2 expressions, and phospho-Smad2 were assessed by Western blot.
Dab2 controls the expression of MHC and co-stimulatory molecules during DC development
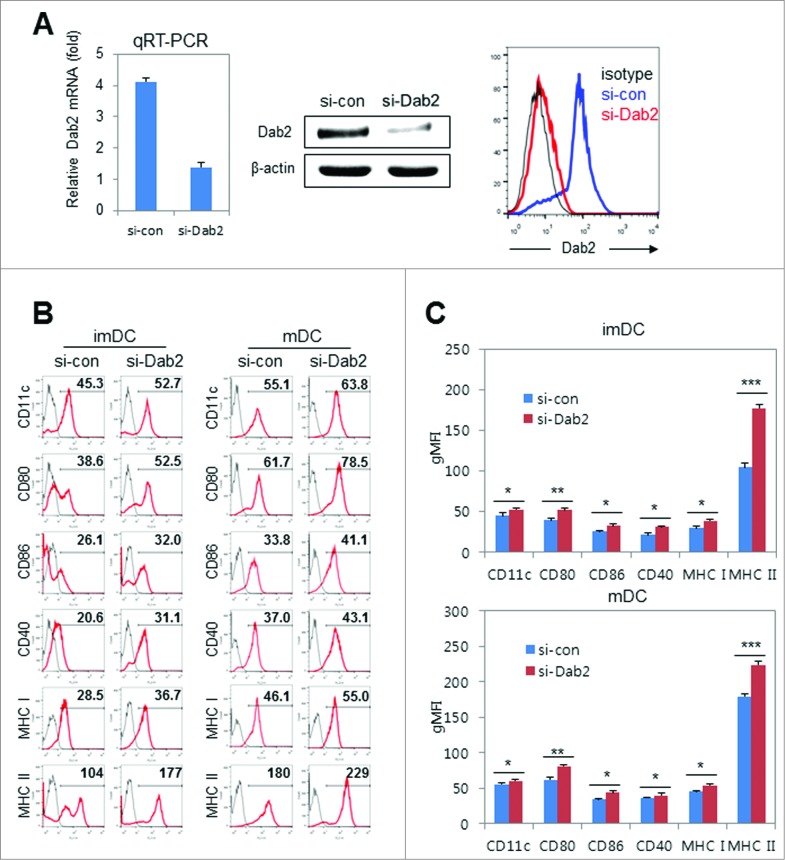
Next, we investigated the surface phenotypes of BMDCs in the absence of Dab2. Dab2-specific siRNA (si-Dab2) was used to block Dab2 expression by over 80% at both the mRNA (qRT-PCR) and protein levels in Western blot and flow cytometry (Fig. 3A). In Dab2-knockdown (Dab2KD) DCs, the expression of MHC I and MHC II molecules and co-stimulatory molecules CD40 and CD80 increased in both imDCs and mDCs (Fig. 3B). These phenotypic changes by Dab2-silencing in both imDCs and mDCs were statistically significant (Fig. 3C). These data imply that Dab2 expression during BMDC differentiation is likely involved in the negative regulation of DC immunogenicity.
Figure 3.
Effects of Dab2-silencing on BMDC surface phenotypes. (A) BMDC precursor cells on day 4 were transfected with si-Dab2 or si-con and harvested on day 6 as WT or Dab2KD BMDCs. Dab2 silencing was measured by qRT-PCR (left), Western blot (middle) and flow cytometry (right). qRT-PCR data are shown as mean ± SD of n = 9 samples pooled from three independent experiments. (B) Representative flow cytometry data showing surface phenotypes of Dab2KD (si-Dab2) and WT (si-con) immature (imDC) and mature (mDC) BMDCs. (C) Geometric mean fluoescence intensities (gMFI) of each BMDC surface molecule shown in flow cytometry (B) are presented as mean ± SD of nine samples pooled from three individual experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with control WT DCs, Student's t-test.
Dab2 inhibits the antigen-uptake and migration capacities of BMDCs
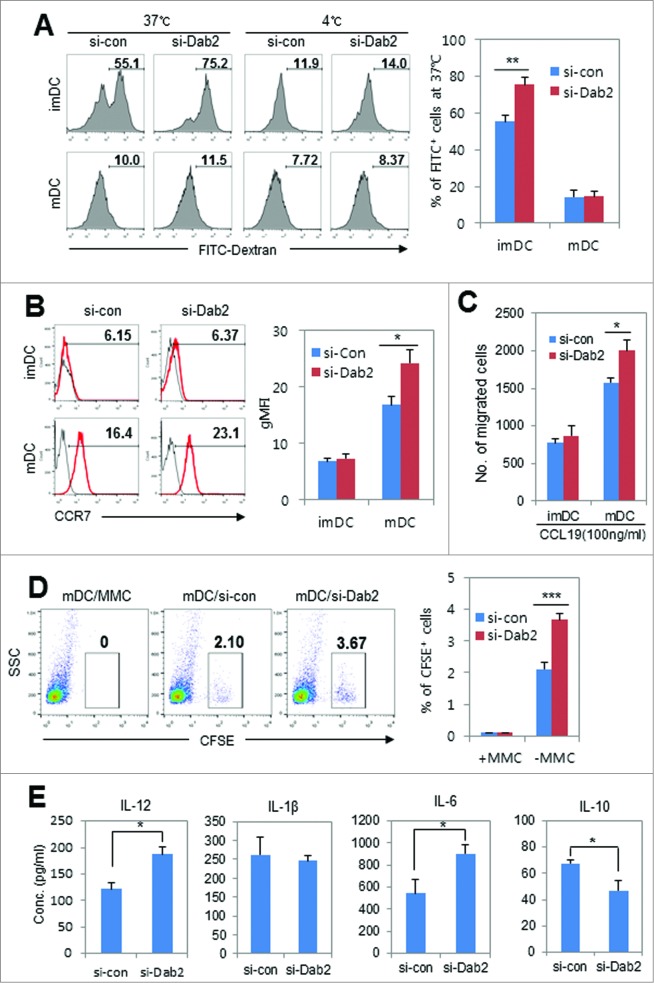
Immature DCs (imDCs) take up antigens through receptor-mediated endocytosis and receptor-independent macropinocytosis.31 Dab2 is well established as an endocytic adaptor protein in endoderm cells.6 To examine the role of Dab2 in antigen uptake by imDCs, we explored the antigen uptake capacity of Dab2KD DCs. Unexpectedly, Dab2-sliencing significantly increased antigen uptake by imDCs (Fig. 4A), suggesting that Dab2 in DCs, unlike in other cell types, may be a negative regulator for antigen uptake by imDCs. It was reported that, differing from Dab2, TGF-β-induced protein (βIg-h3) selectively upregulated in imDCs stimulates antigen uptake by DCs through endocytosis.32 Detailed molecular mechanisms underlying Dab2-mediated inhibition of antigen uptake of imDCs remain to be established. Next, we examined the migration of Dab2KD DCs. We analyzed CCR7 expression on Dab2KD imDCs and mDCs. CCR7 expression was significantly enhanced by Dab2-silencing in mDCs while the expression was not affected by Dab2-silencing in imDCs (Fig. 4B). mDC migration toward CCL19 in a transwell plate increased by about 25% after silencing Dab2 as compared with WT mDCs (Fig. 4C). The number of DCs that migrated from the injection site to the regional lymph node in mice also significantly increased by Dab2-silencing (Fig. 4D). We next analyzed cytokine secretion from WT and Dab2KD DCs. IL-12 and IL-6 secretions were significantly enhanced by silencing Dab2 in mDCs while IL-10 secretion was significantly reduced by silencing Dab2 as compared with control DCs (Fig. 4E). Other pro-inflammatory cytokines, such as TNF-α and IL-1β, were not affected by Dab2-silencing. These data suggest that Dab2KD DCs would be more effective than WT mDCs in activating antigen-specific T cell responses.
Figure 4 (See previous page).
Effects of Dab2-silencing on antigen uptake, migration, and cytokine secretion of BMDCs. (A) WT or Dab2KD imDCs and LPS-stimulated (100 ng/mL for 24 h) mDCs (2 × 105 cells) were equilibrated with 1 mg/mL FITC-conjugated dextran for 1 h at 37°C or 4°C, respectively. Cells were washed and analyzed by flow cytometry. Representative flow cytometry of FITC+ cell populations is shown (left) with the mean ± SD of 9 samples pooled from three independent experiments (right). **p < 0.01, *p < 0.05 compared with control imDCs and mDCs. Student's t-test. (B) WT or Dab2KD imDCs and LPS-stimulated mDCs were stained with PE-conjugated anti-CCR7 mAb followed by FACS analysis. Representative flow cytometry analysis of the CCR7+ cell populations (%) in imDCs and mDCs (left) and accumulated statistical data (right) are shown with the mean ± SD of 9 samples pooled from three independent experiments. **p < 0.01, Student's t-test. (C) The migration of WT and Dab2KD imDCs and mDCs was assessed by an in vitro chemotaxis assay as described in the materials and methods. The data are shown as mean ± SD of nine samples pooled from three independent experiments. *p < 0.05, Student's t-test. (D) In vivo migration assay with Dab2KD BMDC was performed as described in materials and methods. CFSE-labeled Dab2KD or WT mature DCs (1 × 106 cells) were inoculated s.c in the right flank region of C57BL/6 mice. After 24 h, CFSE-labeled DCs in the inguinal lymph nodes were examined by flow cytometry. Mitomycine C (MMC)-treated (50 μg/mL for 30 min) CFSE-labeled BMDCs were used as a negative control. Representative FACS data (left) and statistical data from three independent experiments are shown as mean ± SD (right). ***p < 0.001. (E) Cytokine production was assessed by ELISA of WT and Dab2KD BMDC culture supernatants after stimulating with LPS (100 ng/mL) for 24 h. The data are shown as mean ± SD of nine samples pooled from three independent experiments. *p < 0.05 compared with control mDCs, Student's t-test.
Dab2 expression inhibits T cell stimulation by DCs
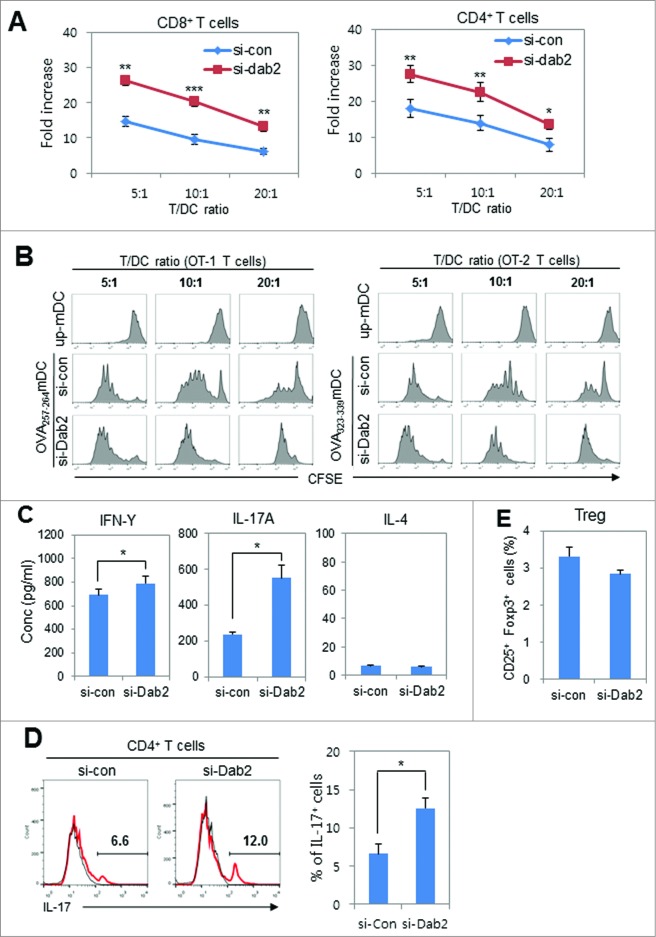
Next, we assessed the ability of Dab2KD DCs to stimulate T-cell proliferation by using ovalbumin (OVA)-specific T cells obtained from C57BL/6-originated OVA-specific T cell receptor transgenic OT-1 and OT-2 mice. Dab2KD BMDCs were pulsed with OVA peptide (OVA257–264 and OVA323–339) and co-cultured with 5,6-carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve OT-1 and OT-2 T cells, respectively. Dab2KD DCs were much more effective in stimulating OT-1 and OT-2 T cell proliferations as compared with WT DCs (Fig. 5A). The proliferation profiles of OT-1 and OT-2 T cells in DC/T cell co-cultures at three different ratios (Fig. 5B) revealed that the T (both CD4+ and CD8+) cell stimulation capacity of DCs was significantly improved by Dab2-silencing. To determine the major subset(s) of CD4+ T cells that respond to Dab2-silencing of DCs, Th1, Th2, and Th17 cytokines were assessed in the supernatants of Dab2KD DCs/OT-2 T cell co-cultures. Among the cytokines in the supernatants of Dab2KD DC/T cell co-cultures, the IL-17A (Th17 cytokine) level was significantly enhanced by Dab2-silencing while the γ-interferon (IFNγ) level (Th1 cytokine) was much less enhanced and the IL-4 (Th2 cytokine) level did not change (Fig. 5C). In good agreement with the cytokine analysis, intracellular cytokine staining also showed that the Th17 population significantly increased in DC/T cell co-culture experiments after Dab2-silencing (Fig. 5D). Dab2-silencing slightly reduced the population of CD4+CD25+Foxp3+ Treg cells in DC/OT-2 T cell co-cultures, but the reduction was not statistically significant (Fig. 5E). These results suggest that Dab2 expression in DCs regulates the DC-mediated Th1 and Th17 immunity without affecting Th2 and Treg responses.
Figure 5 (See previous page).
The effects of Dab2-silencing on the ability of BMDCs to stimulate T cell proliferation. (A) WT and Dab2KD mDCs (from C57BL/6 mice) that were pulsed with OVA257–264 or OVA323–329 peptides were co-cultured with CFSE-labeled OT-1 or OT-2 T cells, respectively, for 4 d at three different ratios. CFSE-positive proliferated (CFSE-diluted) T cells were gated, calculated, and represented by fold increases as described in materials and methods. Data are shown as mean ± SD of six samples pooled from three independent experiments. *p < 0.05 and **p < 0.01, Student t-test. (B) OVA peptide (OVA257–264 or OVA323–329)-pulsed WT and Dab2KD mDCs were co-cultured with CFSE-labeled OT-1 or OT-2 T cells, respectively, for 4 d at different T:DC cell ratios. CFSE-labeled proliferating T cells were gated and represented by histogram. (C) Th1 (IFNγ), Th17 (IL-17), and Th2 (IL-4) cytokines in the supernatant of DC/T cell co-cultures at a 1:10 ratio were assessed by ELISA at day 3. The data are shown as mean ± SD of six samples pooled from three independent experiments. *p < 0.05 compared with WT (si-con) DC, student t-test. (D) Treg cell populations were assessed by intracellular Foxp3 staining and CD25 surface staining from co-cultures of mDCs (C57BL/6) pulsed with OVA323–339 peptide and OT-2 T cells at a ratio 1:10 for 5 d. CD25+ Foxp3+ Treg cells were assessed by flow cytometry and are shown as mean ± SD of six samples pooled from three independent experiments. (E) OVA323–329 peptide-pulsed WT and Dab2KD mDCs were co-cultured with OT-2 T cells for 3 d, and Th17 cells among the OT-2 cells were assessed by FACS after intracellular staining with anti-IL-17 antibody (left). Statistical data (right) are shown as mean ± SD of six samples pooled from three independent experiments. *p < 0.05, Student t-test.
Dab2-silencing enhanced the DC vaccine efficacy in tumor immunotherapy
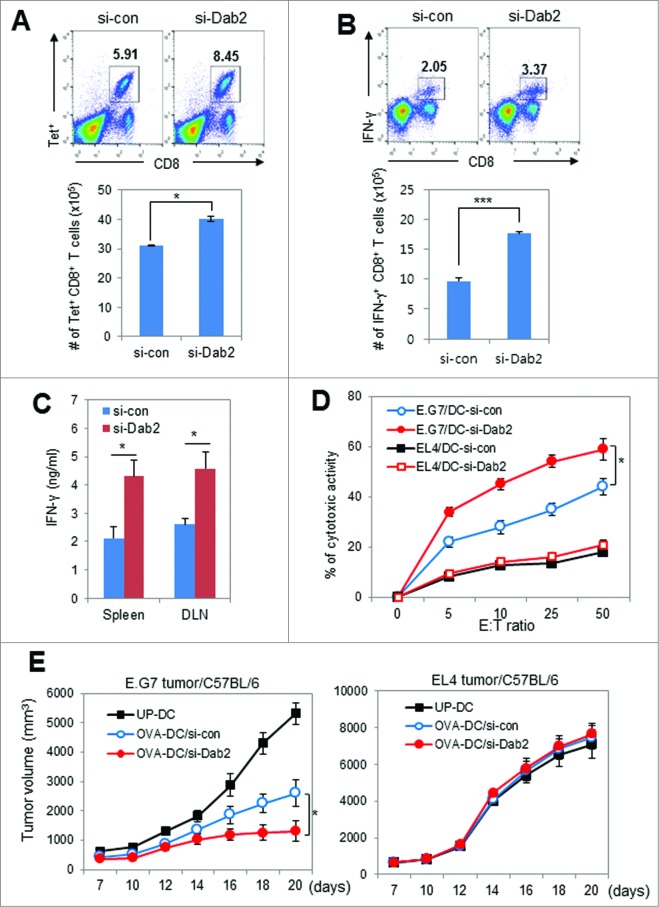
To elucidate the effects of Dab2-silencing on DC-mediated effector T cell proliferation in vivo, OT-1 T cell-transfused mice were injected with OVA257–264-pulsed Dab2KD or WT mDCs. Thereafter, the OVA-specific CD8+ T cells in the spleen were assessed by tetramer assay and intracellular IFNγ staining. Both populations of tetramer-positive (Fig. 6A) and IFNγ-expressing (Fig. 6B) CD8+ effector T cells were significantly increased in mice injected with Dab2KD DCs as compared with WT DCs. We examined CTL activity in mice vaccinated with Dab2KD or WT DCs both pulsed with OVA257–264 peptide. The IFNγ levels in culture supernatants of T cells isolated from the spleen and the draining lymph node (DLN) of mice vaccinated with Dab2KD DCs were much higher than those assessed in mice vaccinated with WT DCs (Fig. 6C). In addition, the CTL activity in the DLNs of mice vaccinated with Dab2KD DCs was significantly enhanced as compared with those in mice vaccinated with WT DCs (Fig. 6D). These data indicate that Dab2 expression in DCs plays an important role in controlling DC-mediated Th1 immunity and CTL activity. To assess Dab2 function in DC-based tumor immunotherapy, C57BL/6 tumor-bearing mice were vaccinated twice with OVA peptide (OVA257–264 and OVA323–339)-pulsed Dab2KD or WT DCs vaccine. The Dab2KD DC vaccine inhibited E.G7 tumor growth more significantly than did WT DCs. EL4 tumors did not change, even when vaccinated with Dab2KD DCs (Fig. 6E). These results suggest that Dab2-silencing made the DC vaccine more effective for tumor immunotherapy by strengthening specific antitumor immunity.
Figure 6 (See previous page).
Dab2KD DC vaccine was more effective than WT DC vaccine in the induction of antitumor immunity in tumor immunotherapy. (A) CD8+ T cells proliferation in vivo by WT or Dab2KD BMDCs was assessed by tetramer assay as described in materials and methods. Naive C57BL/6 mice were transferred i.v with OT-1 T cells together with OVA-pulsed Dab2KD or WT mDCs, and then boosted with OVA257–264 peptide on day 7. Three day later, splenocytes from the mice were stained with PE-labeled H-2Kb/OVA257–264 tetramer and FITC-anti-CD8 antibodies, and subsequently analyzed by flow cytometry. Shown is representative FACS data (upper) and the number of tetramer(Tet)+CD8+ T cells is represented as mean± SD of six samples from two mice (lower). *p < 0.05, Student t-test. (B) The splenocytes prepared for tetramer assay in (A) were activated with phorbol myristate acetate (PMA)/ionomycin (40 ng/mL each, Sigma–Aldrich) for 4 h. Cells were then examined by flow cytometry after surface and intracellular staining with FITC-anti-CD8 antibody and PerCP/Cy-anti-IFNγ antibody, respectively. Shown is representative FACS data (upper), and the number of IFNγ+CD8+ T cells is represented as mean ± SD of six samples from two mice (lower). ***p < 0.001, Student t-test. (C) OT-1 mice were immunized twice at a 1-week interval with 1 × 106 OVA peptide (OVA257–264)-pulsed Dab2KD or WT mBMDCs (C57BL/6). Cells from the spleen and DLN of vaccinated mice were harvested and cultured for 5–7 d in the presence of OVA257–264 peptide. The level of IFNγ in the culture supernatants on day 2 of culture was assessed by ELISA, and the data are shown as mean ± SD of six simples from two independent experiments. *p < 0.05, Student t-test. (D) CTL activity in the DLNs of vaccinated OT-1 mice was assessed by flow cytometry using CFSE-labeled E.G7 and EL4 as target cells. Quantitative CTL activities are shown as mean ± SD (n = 3). *p < 0.05, Student t-test. (E) C57BL/6 mice were inoculated s.c. with E.G7 and EL4 tumor cells (5 × 105) in the right flank and immunized twice on day 3 and day 10 with 1 × 106 Dab2KD (si-Dab2) or WT (si-con) mDCs (from C57BL/6 BM cells) that were pulsed with OVA peptide. Tumor growth was monitored and represented as mean ± SD of four mice from each of two experiments (bottom). *p < 0.05, Student t-test.
DC immunogenicity was significantly impaired by overexpression of Dab2
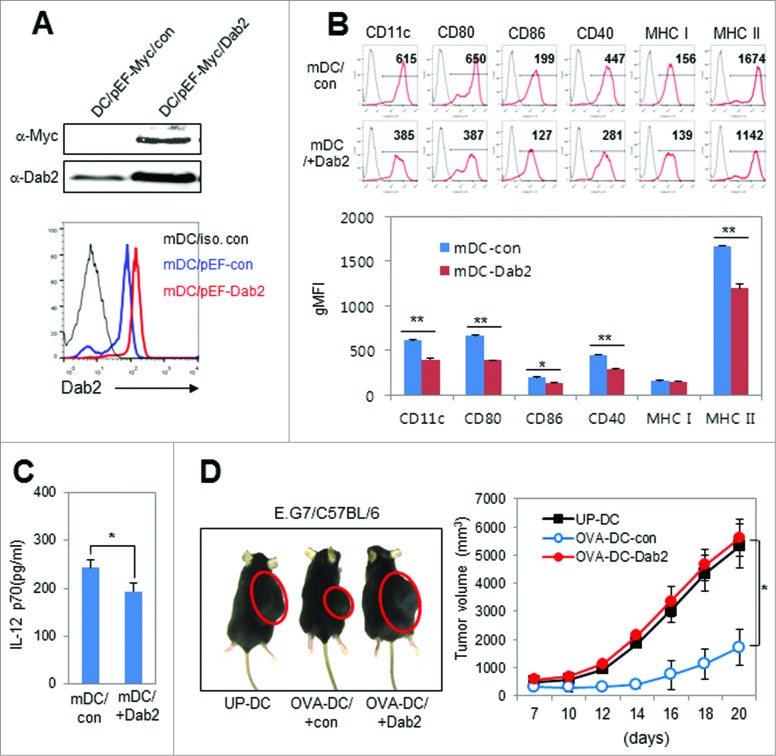
We next examined the effect of Dab2 over-expression during DC development on the DC phenotype and immunogenicity in vitro and in vivo. DC precursor cells were transfected with a plasmid expressing Dab2 (pEF-Myc/His-p96), and Dab2 overexpression in DCs was examined by Western blot and flow cytometry (Fig. 7A). Dab2 over-expression downregulated the expression of costimulatory molecules CD80, CD86, and MHC I and MHC II on the surface of DCs (Fig. 7B). IL-12 secretion from mDCs was also significantly reduced by Dab2 overexpression (Fig. 7C). Dab2 overexpression obliterated the efficacy of the DC vaccine for tumor immunotherapy (Fig. 7D). These results strongly support our conclusion that Dab2 is an intrinsic negative regulator of DC immunogenicity probably to maintain immune homeostasis in vivo.
Figure 7.
Dab2-overexpression impaired the efficacy of the DC vaccine for tumor immunotherapy. (A) Dab2 expression plasmid (pEF-Myc/Dab2) and control vector (pEF-Myc/con) were transfected into DC precursor cells on day 4 during BMDC development. The transfected cells were harvested after 48 h, and matured with LPS (100 ng/mL) for 24 h. Dab2 expression in the transformed mDCs was assessed by Western blot (upper) and FACS analysis (lower) using anti-Myc and anti-Dab2 antibodies. (B) The surface phenotypes of Dab2 transfected mDCs were assessed by flow cytometry, and the gMFI value of each DC surface marker from three independent experiments is shown as mean ± SD of nine samples. *p < 0.05, **p < 0.01 compared with control vector-transfected DCs, Student's t-test. (C) IL-12 levels were assessed by ELISA of the culture supernatant of Dab2-transfected DCs and are shown as mean ± SD of nine samples. *p < 0.05 compared with control vector transfected DCs, Student's t-test. (D) C57BL/6 mice were inoculated s.c. with E.G7 tumor cells (5 × 105) in the right flank, and then immunized on day 3 and day 10 with 1 × 106 Dab2-expressing mDCs (OVA-DC-Dab2) or control vector-transfected mDCs (OVA-DC-con), which were derived from C57BL/6 BM cells and pulsed with OVA peptides (OVA257–264 and OVA323–339). Representative images of E.G7 tumors are shown on day 20 after DC vaccination (left). Tumor growth was monitored every 2–3 d and presented as mean ± SD of four mice from each of two experiments. *p < 0.05, Student's t-test.
Discussion
Adapter proteins play an important role in signal transduction. These proteins contain a variety of protein-binding modules that link binding partners together to create large signaling complexes. The adaptors have no intrinsic enzymatic activity, but they mediate specific protein-protein interactions to drive the formation of a protein complex. The Dab was originally identified in Drosophila as the product of a gene with a key role in a neural development.33 Dab2 (also called p96) is an ortholog of Dab.9 Dab2 was identified as a cytosolic adaptor that regulates endocytosis and growth factor signaling.34 It has also been implicated in several receptor-mediated signaling and cell adhesion functions,35 hematopoietic cell differentiation,36 and angiogenesis.37 Dab2 is broadly expressed in various tissues and organs16-18 and is a Foxp3 target gene required for Treg cells function.14 Dab2 also acts as a tumor suppressor in various tissues like ovary,11 prostate,38 and nasal carcinomas.39 The function of Dab2 in DC differentiation and immunogenicity has not been reported.
In this manuscript, we showed Dab2 expression in DCs and its role in DC immunogenicity. Dab2 expression increased during BMDCs development and was also present in steady-state spDCs and human MoDCs as well (Fig. 1). STAT5 activation, Foxp3 expression, and PI3K/Akt signaling-mediated hnRNPE1 activation, all of which are needed for Dab2 expression in a TGF-β-mediated EMT transition,19,20 were also required for Dab2 expression in DCs during GM-CSF-mediated BMDC development (Fig. 2). Treating BMDCs with PI3K inhibitor for even 20 min before harvest was enough to down-regulate Dab2 expression in DCs (Fig. 2E), suggesting that Dab2 expression is rapidly controlled by intracellular PI3K/Akt signaling in DCs. hnRNP E1 is involved in controlling Dab2 translation by blocking the BAT element of Dab2 mRNA. Once hnRNP E1 is phosphorylated via the PI3K/PKB/Akt pathway under TGF-β signaling, hnRNPE1 is released from the BAT element and Dab2 is expression is significantly induced.21,22 However, TGF-β in culture supernatants of BMDCs was undetectable (Fig. 2F), and the treatment of BMDCs with TGF-β or TGF-β receptor inhibitor did not affect the expression of Dab2 in BMDCs (Fig. 2G), suggesting that Dab2 expression in BMDCs was not associated with TGF-β signaling. Taken together, our data strongly suggest that Dab2 expression during GM-CSF-mediated BMDC development is contingent on the PI3K/Akt-dependent phospho-hnRNP E1-mediated translational control, rather than general transcriptional control, regardless of TGF-β signaling pathway. Silencing Dab2 in DCs enhanced the expression of surface immunogenic molecules (Fig. 3), DC migration and antigen uptake, and expression of IL-12 and other proinflammatory cytokines as compared with WT DCs (Fig. 4). These data indicated that Dab2KD DCs might have higher immunogenic potential than do WT DCs, probably because Dab2 acts as an intrinsic negative regulator. In particular, we found that Dab2KD DCs secreted more IL-12 and IL-6 cytokines than did WT DCs (Fig. 4E). IL-12 inhibits STAT5-mediated activation of the Foxp3 promoter.40 IL-6 is needed with TGF-β for Th17 cell differentiation.41 Dab2 expression requires TGF-β-Smad signaling,19 and IFNγ inhibits Dab2 expression in macrophages.42 These data support our findings that Dab2KD DCs have more immunogenic potential than WT DCs.
Next, we demonstrated that Dab2-silencing potentiated DC immunogenicity, thus stimulating both antigen-specific CD4+ T cells and CD8+ T cells significantly (Fig. 5). An analysis of cytokine secretion and intracellular staining of OT-2 T/DC co-cultures revealed that Dab2-silencing in DCs significantly increased the population of Th17 subsets (Figs. 5C and D). Treg cells lacking Dab2 have been reported to be functionally impaired.14 However, in T/DC co-culture experiments, DCs lacking Dab2 did not affect the CD25+Foxp3+ Treg population (Fig. 5E), indicating that the increase in Th17 cells caused by Dab2-silencing in DCs was not due to downregulation of Treg cells. A Dab2KD DC vaccine was more effective at inducing antigens-specific cytotoxic T lymphocytes (CTL) and inhibiting tumor growth than a WT DC vaccine (Fig. 6). Ectopic overexpression of Dab2 in DCs abrogated the DC vaccine efficacy in tumor immunotherapy (Fig. 7). Taken together, our findings indicate that Dab2, which is induced during DC development, is an intrinsic negative regulator of DC differentiation and immunogenicity. Because Dab2 is a phosphoprotein,43 other molecules might interact with Dab2 adaptor protein to activate signaling pathways.
DCs play a pivotal role in activating T cell responses against tumors. The DC-based tumor immunotherapy is an attractive approach to treat cancer because of its safety and feasibility. However, DC-based tumor immunotherapies have not been as effective as expected because of the limited immunogenicity and immunosuppressive conditions in tumor microenvironments. Modifying genes of negative regulators in DCs improves the efficacy of DC vaccines in DC-based tumor immunotherapy. In the present study we found that Dab2, which is expressed during DC development, seems to play an important role in controlling DC immunogenicity, probably to prevent immune exaggeration and maintain homeostasis after immune reactions. Therefore, molecular targeting of Dab2 would be an attractive approach for the development of more effective DC vaccines in tumor immunotherapy. The detailed molecular mechanism underlying Dab2-mediated negative regulation of DC immunogenicity remains to be elucidated by further studies.
Materials and Methods
Mice, cell lines, and reagents
C57BL/6, C57BL/6-background OT-1 (OVA257–264-specific CD8+ T cell receptor transgenic) and OT-2 (OVA323–339-specific CD4+ T cell receptor) mice (6 ∼8 week old) were used for the present study. OT-1/OT-2 mice were Rag-1/Rag-2 normal. All mice were maintained in the animal care facility at Sungkyunkwan University according to the University Animal Care and Use guidelines. EL4 (C57BL/6 mouse-derived thymoma) and E.G7 (OVA-expressing EL4) cells were obtained from the American Type Culture Collection (ATCC). CFSE (5,6-carboxyflouroscein succinimidyl ester) (BioLegendR), Dextran (40 KDa)-FITC (Sigma–Aldrich), STAT5 inhibitor and PI3K inhibitor (Calbiochem), TGF-β receptor inhibitor (TGFβRI) (SB431524, Sigma–Aldrich) and Protein A/G PLUS-Agarose (Santa Cruz Biotech) were used. A PE-conjugated monoclonal antibody to CCR7 was purchased from BioLegend, rabbit anti-mouse Dab2 polyclonal antibody from Protein Tech, Foxp3 monoclonal antibody from eBioscience, and 24-well transwell chambers (8-μM pore size) from Corning Costar (Cambridge, MA). Anti-phospho-tyrosine-STAT5, anti-phospho-Smad2, anti-pan-STAT5, anti-pan-Akt and anti-phospho-serine-Akt antibodies were obtained from Cell Signaling Inc. Anti-β-actin , HRP-conjugated anti-rabbit and anti-mouse IgGs were purchased from Sigma, anti-Smad2 and anti-phospho-serine antibodies from Abcam, murine GM-CSF from Creagene Inc., recombinant human TGF-β1 from R&D system and lipopolysaccharide (LPS, from Escherichia coli O111:B4) from Sigma–Aldrich. Synthetic ovalbumin peptides [OVA257–264 (SIINFEKL] and OVA323–339 (ISQAVHAAHAEINEAGR)] were provided by Peptron (Daejon, Korea). FITC- or PE-conjugated anti-CD4, CD8, CD25, CD11c, CD40, CD80, CD86, MHCII, MHCI, and Foxp3 antibodies were purchased from BD Pharmingen and BioLegend. Cytokine ELISA kits for murine IL-6, IL-12p70, IL-10, IL-17A, IL-1β, TNF-α, TGF-β and γIFN were purchased from BioLegend and the kit for IL-13 was from Abcam.
Bone marrow-derived DCs (BMDC), splenic DCs (spDC) and human monocyte-derived DCs (hMoDC)
BMDCs were generated from mouse BM progenitor cells as described previously.25,26,44 In brief, BM cells collected from the femurs and tibiae of 6-week-old female C57BL/6 mice were washed and cultured in complete RPMI 1640 media [RPMI 1640 (GibcoR) supplemented with 10% FBS and 10 ng/mL mGM-CSF (Creagene Inc.)]. After 2 d, non-adherent cells were washed and re-fed with 2 mL of fresh complete medium containing mGM-CSF. On day 4, 1 mL of fresh medium containing mGM-CSF was added to the culture. On day 6, non-adherent cells were collected as imDCs. imDCs were matured by further culturing in the presence of 100 ng/mL LPS for 24 h. spDCs were isolated from the spleen of 6–8 week old C57BL/6 mice using CD11c microbeads according to manufacturer's instruction (Miltenyi Biotech). Human MoDCs were generated as described45,46 with minor modifications. Peripheral blood mononuclear cells (PBMC) were isolated from the blood of healthy donors by Ficoll gradient centrifugation. Monocytes were isolated by plastic adherence for 1 h and subsequently cultured in the presence of 30 ng/mL rhGM-CSF and 20 ng/mL rhIL-4 (Creagene Inc.) in RPMI 1640 medium supplemented with 1% human AB serum (Lonza). On day 3 of culture, nonadherent cells (used as immature MoDCs) were collected and matured for 18 h with 100 ng/mL lipopolysaccharide (LPS) (Sigma–Aldrich).
Dab2 silencing with small interfering RNA (siRNA)
Dab2-specific siRNAs were designed by BLOCK-IT RNAi Designer (Invitrogen) to achieve full specificity without any off-target effects, such as inducing type-1 interferon. Two siRNAs specific to Dab2 5′-cctgttgtctacagtcctt-3′ (si-Dab2-1) and 5′-ccacctcttgttccctcaa-3′(si-Dab2-2) and a control siRNA 5′-ccttgtatcgacctgtctt-3′(si-con) were synthesized by Invitrogen. DC precursor cells on day 4 or day 5 of mouse BM cell culture were transfected with the 2 Dab2 siRNAs (si-Dab2) or control si-RNA (si-con) using Lipofectamine RNAiMAX (Life Technologies) as reported previously.25,26 After 48 h, cells were washed and used as a Dab2-silenced (Dab2KD) immature BMDCs (imDCs) for subsequent experiments.
Flow cytometry analysis
Direct immunofluorescence staining was performed to analyze the DC surface phenotypes as described previously.26 DCs were stained with appropriate antibodies at 4°C for 20 min. After washing, cells were then analyzed by FACS Caliber (BD) using CellQuest or FlowJo software. For intracellular Dab2 staining, cells prestained with FITC-labeled hamster anti-mouse CD11c antibody were fixed and permeabilized with the BD Cytofix/CytopermTM kit (BD Bioscience Pharmingen). Cells were then stained with PE-labeled rabbit anti-mouse Dab2 antibody (BiossR) or PE-labeled rabbit isotype control antibody in BD perm/wash buffer for 1 h. In the case of intracellular staining for IL-17 or IFNγ in T cells, cells prestained with FITC-labeled anti-mouse CD4 or CD8 antibody were fixed, permeabilized, and then stained with PerCP-Cy5.5-labeled anti-IL-17 or anti-IFNγ antibodies together with PerCP-Cy5.5-labeled mouse isotype control antibody (BioLegend). After washing with BD perm/wash buffer, cells were analyzed by flow cytometry.
T-cell proliferation assay
A T cell proliferation assay was performed with WT and Dab2KD BMDCs as described previously.25 T cells were purified from the spleen or DLN of OT-1 and OT-2 mice using a mouse CD4+ and CD8+ T-cell Isolation Kit II (Miltenyi Biotech) and labeled with CFSE (1 μM for 10 min) as described previously.26 Dab2KD or WT immature BMDCs (imDC) were matured by culturing with LPS (100 ng/mL) for 24 h, and then pulsed with 1 μg/mL OVA peptide (OVA257–264 or OVA323–339) for 1 h. Pulsed mDCs were washed with cold phosphate-buffered saline (PBS), and then co-cultured with CFSE-labeled T cells at different ratios for 3–4 d. Cells grown in co-cultures were harvested and analyzed by flow cytometry. CFSE-positive proliferating (CFSE diluted) T cells were gated and calculated by using the following formula (proliferation index; PI = 1000/geometric sum of gated CFSE). T cell proliferation capacity of antigen-pulsed DCs was represented by fold increase on the basis of the PI of the T cells co-cultured with unpulsed DCs.
Antigen-uptake assay
As described previously,26 Dab2KD or WT immature and mature BMDCs (2 × 105 cells) equilibrated at 37°C or 4°C for 45 min in FACS tubes, were pulsed with 1 mg/mL FITC-conjugated dextran for 1 h. The reaction was stopped with cold PBS buffer. The cells were washed and stained with PE-conjugated anti-CD11c and analyzed with a FACS Calibur flow cytometer.
Chemotaxis of DCs
Dendritic cell chemotaxis was measured by migration in 24-well transwell chambers (Corning Costar, Cambridge, MA, USA). Dab2KD or WT immature and mature BMDC (1 × 105 cells in 0.1 mL) were washed with PBS and resuspended in serum-free RPMI 1640 medium, and then placed in the upper chamber of the transwell plate. The lower chamber of transwell plates contained CCL19 (300 ng/mL) diluted with 0.6 mL of serum free RPMI 1640. Plates were incubated at 37°C for 3 h to allow DC migration. Migrated cells were harvested from the lower chamber and analyzed by flow cytometry.
In vivo migration assay of DCs
As described previously,47 Dab2KD or WT mature BMDCs were labeled with 5 μM CFSE (BioLegend) at 37°C for 10 min. A total of 1 × 106 CFSE-labeled DCs were inoculated subcutaneously (s.c.) in the right flank region of C57BL/6 mice. Twenty-four hour later, inguinal DLNs were obtained from the mice, and the labeled DCs that had migrated from the injection site to the inguinal DLNs were examined by FACS. As a negative control, CFSE-labeled BMDCs that were pre-treated with 50 μg/mL mitomycine C (MMC) for 30 min were used.
Quantitative real time (qRT)-PCR
Total RNA was isolated and purified from BMDCs using the RibospinTM kit (GeneAll). cDNA was synthesized using Maxima Enzyme Mix (Thermo Scientific) and 5X reaction mix (Thermo Scientific). Quantitative PCR was performed using the Fast SYBR® Green Master Mix kit (Life Technologies) with the following primer sets: Dab2 (S)5′-tgctcgtgatgtgacagaca-3′, (AS)5′-agggtcattagggcctcact-3′; GAPDH (S)5′-aatgtgtccgtcgtggatct-3′, (AS)5′-tccaccaccctgttgctgta-3′; and β-actin (S) 5′-gtatgcctcggtcgtacca-3′, (AS) 5′cttctgcatcctgtcagcaa-3′.
Western blot analysis
Western blot analysis was performed as described previously.26,48 In brief, cells were washed in cold PBS, and lysed with lysis buffer containing 50 mM Tris-HCl (pH-7.4), 150 mM NaCl, 1 mM DTT, 30 mM NaF, 10 mM Na3VO4, 0.5% NP40, and a protease inhibitor cocktail (Roche). Cell lysates were subjected to 8–12% SDS-PAGE and transferred to a PVDF membrane (Millipore). Membranes were incubated overnight at 4°C with primary antibodies in 4% nonfat dry milk, and then further incubated for 1 h with HRP-conjugated secondary antibodies. Bound antibodies were detected by using chemiluminescent HRP substrate (Millipore, USA) and a Chemiluminescent Imaging System (Davinch ChemiTM).
Immunoprecipitation
Immunoprecipitation (IP) was performed as described previously.49 In brief, BMDCs were lysed with IP lysis buffer containing 25 mM HEPES (pH-7.4), 150 mM NaCl, 1 mM EDTA and 0.5% TritonX-100, incubated for 5 min on ice, and sonicated for 3–4 five-second pulses on ice using an ultrasonicator (Sonic Vibra-Cell VC 750). After centrifugation, the supernatants were incubated with primary antibody at 4°C for overnight, followed by incubation with Protein A/G PLUS-Agarose (Santa Cruz Biotech). Agarose bead pellets were washed, resuspended in a loading buffer, and subjected to SDS-PAGE. The resolved proteins were transferred to nitrocellulose membranes and stained with specific antibodies. Blotted protein was assessed by ECL.
ELISA
BMDC precursor cells on day 4 of DC culture were transfected with si-Dab2 or si-con. After 48 h, cells were stimulated with LPS (100 ng/mL) for 24 h. The BMDC (si-con/si-Dab2) culture supernatants were assessed using ELISA kits for IL-6, IL-12p70, TNF-α, IL-1β, TGF-β and IL-10. The amounts of IFNγ, IL-17A, and IL-4 cytokines were also assessed in the supernatants of DC/T cell co-cultures at day 3 with an ELISA reader (Molecular Device).
Tetramer assay
Tetramer assay was performed as described previously.50 OVA-pulsed Dab2KD or WT BMDCs (1 × 106) and OT-1 T cells (5 × 106) were transferred intravenously into naive C57BL/6 mice through a tail vein. After 7 d, the mice were boosted by intravenous (i.v) injection with 0.1 mM OVA I peptide (100 μL). Three day later, splenocytes from these mice were strained with PE-labeled H-2Kb/OVAI tetramer (kindly provided by Dr. Chang, Ewha Womans University, Seoul, Korea) and FITC-anti-CD8 antibodies, followed by flow cytometry analysis.
CTL assay and ELISA for IFNγ
OT-1 mice were injected twice at a 1-week interval with WT (si-con) or Dab2KD (si-Dab2) DCs pulsed with OVA257–264 peptide. Fourteen day after injection, splenocytes and draining lymph nose (DLN) cells from vaccinated mice were cultured for 7 d in the presence of OT-1 peptide (10 μg/mL) in 6-well plates (2 × 106 cells/well) and co-cultured with CFSE-labeled target cells (EL4 and E.G7 tumor cells) at different ratios for 4 h. After PI staining, CTL activity of splenocytes and DLN cells were analyzed by flow cytometry as described.26,51 IFNγ levels in the culture supernatants were assessed by ELISA.
DC-based tumor immunotherapy
Tumor immunotherapy was performed on tumor-bearing C57BL/6 mice as described previously.26 In brief, mice were injected with EL4 and E.G7 cells (5 × 105 cells /mouse) s.c. in the right flank. On day 3 and day 10 after tumor inoculation, the mice were vaccinated with WT (si-con), Dab2-silenced, or Dab2 overexpressing DCs that had been pulsed with OVA257–264 peptide. Tumor growth was monitored every 2–3 d using a slide caliper. Tumor masses were calculated with the formula V = (A2 × B)/2, where A is the short axis (width) and B is the long axis.
Statistical analysis
All experiments were performed at least three times with consistent results. Statistical data are presented as mean ± SD. Group comparisons were analyzed with student's t-test. A p-value of less than 0.05 (p < 0.05) was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Ministry of Education Science and Technology (2012M3A9B402826), and in part by the Bio New Drug Grants (A102130 and A110054) from the Korean Ministry of Health and Welfare.
References
- 1. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 2012; 30:1-22; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-100311-102839 [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011; 35:323-35; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563-604; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39:38-48; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prunier C, Hocevar BA, Howe PH. Wnt signaling: physiology and pathology. Growth Factors 2004; 22:141-50; PMID:; http://dx.doi.org/ 10.1080/08977190410001720860 [DOI] [PubMed] [Google Scholar]
- 6. Maurer ME, Cooper JA. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci 2005; 118:5345-55; PMID:; http://dx.doi.org/ 10.1242/jcs.02650 [DOI] [PubMed] [Google Scholar]
- 7. Yang DH, Cai KQ, Roland IH, Smith ER, Xu XX. Disabled-2 is an epithelial surface positioning gene. J Biol Chem 2007; 282:13114-22; PMID:; http://dx.doi.org/ 10.1074/jbc.M611356200 [DOI] [PubMed] [Google Scholar]
- 8. Kim JA, Bae SH, Choi YJ, Kim KH, Park SS. Feed-back regulation of disabled-2 (Dab2) p96 isoform for GATA-4 during differentiation of F9 cells. Biochem Biophys Res Commun 2012; 421:591-8; PMID:; http://dx.doi.org/ 10.1016/j.bbrc.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 9. Fulop V, Colitti CV, Genest D, Berkowitz RS, Yiu GK, Ng SW, Szepesi J, Mok SC. DOC-2/hDab2, a candidate tumor suppressor gene involved in the development of gestational trophoblastic diseases. Oncogene 1998; 17:419-24; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1201955 [DOI] [PubMed] [Google Scholar]
- 10. Xu XX, Yi T, Tang B, Lambeth JD. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene 1998; 16:1561-9; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1201678 [DOI] [PubMed] [Google Scholar]
- 11. Mok SC, Wong KK, Chan RK, Lau CC, Tsao SW, Knapp RC, Berkowitz RS. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol Oncol 1994; 52:247-52; PMID:; http://dx.doi.org/ 10.1006/gyno.1994.1040 [DOI] [PubMed] [Google Scholar]
- 12. Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene 1999; 18:3104-13; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1202649 [DOI] [PubMed] [Google Scholar]
- 13. Xu S, Zhu J, Wu Z. Loss of Dab2 expression in breast cancer cells impairs their ability to deplete TGF-beta and induce Tregs development via TGF-beta. PloS One 2014; 9:e91709; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0091709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain N, Nguyen H, Friedline RH, Malhotra N, Brehm M, Koyanagi M, Bix M, Cooper JA, Chambers CA, Kang J. Cutting edge: Dab2 is a FOXP3 target gene required for regulatory T cell function. J Immunol 2009; 183:4192-6; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0902041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shevach EM, Davidson TS, Huter EN, Dipaolo RA, Andersson J. Role of TGF-Beta in the induction of Foxp3 expression and T regulatory cell function. J Clin Immunol 2008; 28:640-6; PMID:; http://dx.doi.org/ 10.1007/s10875-008-9240-1 [DOI] [PubMed] [Google Scholar]
- 16. Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J 2002; 21:1555-64; PMID:; http://dx.doi.org/ 10.1093/emboj/21.7.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu XX, Yang W, Jackowski S, Rock CO. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem 1995; 270:14184-91; PMID:; http://dx.doi.org/ 10.1074/jbc.270.23.14184 [DOI] [PubMed] [Google Scholar]
- 18. Jokubaitis VG, Gresle MM, Kemper DA, Doherty W, Perreau VM, Cipriani TL, Jonas A, Shaw G, Kuhlmann T, Kilpatrick TJ, et al. Endogenously regulated Dab2 worsens inflammatory injury in experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 2013; 1:32; PMID:; http://dx.doi.org/ 10.1186/2051-5960-1-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J 2001; 20:2789-801; PMID:; http://dx.doi.org/ 10.1093/emboj/20.11.2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prunier C, Howe PH. Disabled-2 (Dab2) is required for transforming growth factor beta-induced epithelial to mesenchymal transition (EMT). J Biol Chem 2005; 280:17540-8; PMID:; http://dx.doi.org/ 10.1074/jbc.M500974200 [DOI] [PubMed] [Google Scholar]
- 21. Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 2010; 12:286-93; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussey GS, Link LA, Brown AS, Howley BV, Chaudhury A, Howe PH. Establishment of a TGFbeta-induced post-transcriptional EMT gene signature. PloS One 2012; 7:e52624; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0052624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shapira KE, Hirschhorn T, Barzilay L, Smorodinsky NI, Henis YI, Ehrlich M. Dab2 inhibits the cholesterol-dependent activation of JNK by TGF-beta. Mol Biol Cell 2014; 25:1620-8; PMID:; http://dx.doi.org/ 10.1091/mbc.E13-09-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou J, Hsieh JT. The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. DOC-2/DAB2-mediated inhibition of ERK phosphorylation via binding to Grb2. J Biol Chem 2001; 276:27793-8; PMID:; http://dx.doi.org/ 10.1074/jbc.M102803200 [DOI] [PubMed] [Google Scholar]
- 25. Miah MA, Byeon SE, Ahmed MS, Yoon CH, Ha SJ, Bae YS. Egr2 induced during DC development acts as an intrinsic negative regulator of DC immunogenicity. Eur J Immunol 2013; 43:2484-96; PMID:; http://dx.doi.org/ 10.1002/eji.201243046 [DOI] [PubMed] [Google Scholar]
- 26. Miah MA, Yoon CH, Kim J, Jang J, Seong YR, Bae YS. CISH is induced during DC development and regulates DC-mediated CTL activation. Eur J Immunol 2012; 42:58-68; PMID:; http://dx.doi.org/ 10.1002/eji.201141846 [DOI] [PubMed] [Google Scholar]
- 27. Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25- effector T cells. Int Immunol 2008; 20:421-31; PMID:; http://dx.doi.org/ 10.1093/intimm/dxn002 [DOI] [PubMed] [Google Scholar]
- 28. Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007; 178:280-90; PMID:; http://dx.doi.org/ 10.4049/jimmunol.178.1.280 [DOI] [PubMed] [Google Scholar]
- 29. Nyga R, Pecquet C, Harir N, Gu H, Dhennin-Duthille I, Regnier A, Gouilleux-Gruart V, Lassoued K, Gouilleux F. Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J 2005; 390:359-66; PMID:; http://dx.doi.org/ 10.1042/BJ20041523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koral K, Erkan E. PKB/Akt partners with Dab2 in albumin endocytosis. Am J Physiol Renal Physiol 2012; 302:F1013-24; PMID:; http://dx.doi.org/ 10.1152/ajprenal.00289.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767-811; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- 32. Cao W, Tan P, Lee CH, Zhang H, Lu J. A transforming growth factor-beta-induced protein stimulates endocytosis and is up-regulated in immature dendritic cells. Blood 2006; 107:2777-85; PMID:; http://dx.doi.org/ 10.1182/blood-2005-05-1803 [DOI] [PubMed] [Google Scholar]
- 33. Gertler FB, Bennett RL, Clark MJ, Hoffmann FM. Drosophila abl tyrosine kinase in embryonic CNS axons: a role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell 1989; 58:103-13; PMID:; http://dx.doi.org/ 10.1016/0092-8674(89)90407-8 [DOI] [PubMed] [Google Scholar]
- 34. Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 2002; 21:4915-26; PMID:; http://dx.doi.org/ 10.1093/emboj/cdf487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang CL, Cheng JC, Liao CH, Stern A, Hsieh JT, Wang CH, Hsu HL, Tseng CP. Disabled-2 is a negative regulator of integrin alpha(IIb)beta(3)-mediated fibrinogen adhesion and cell signaling. J Biol Chem 2004; 279:42279-89; PMID:; http://dx.doi.org/ 10.1074/jbc.M402540200 [DOI] [PubMed] [Google Scholar]
- 36. Tseng CP, Chang P, Huang CL, Cheng JC, Chang SS. Autocrine signaling of platelet-derived growth factor regulates disabled-2 expression during megakaryocytic differentiation of K562 cells. FEBS Lett 2005; 579:4395-401; PMID:; http://dx.doi.org/ 10.1016/j.febslet.2005.06.080 [DOI] [PubMed] [Google Scholar]
- 37. Cheong SM, Choi SC, Han JK. Xenopus Dab2 is required for embryonic angiogenesis. BMC Dev Biol 2006; 6:63; PMID:; http://dx.doi.org/ 10.1186/1471-213X-6-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou J, Scholes J, Hsieh JT. Characterization of a novel negative regulator (DOC-2/DAB2) of c-Src in normal prostatic epithelium and cancer. J Biol Chem 2003; 278:6936-41; PMID:; http://dx.doi.org/ 10.1074/jbc.M210628200 [DOI] [PubMed] [Google Scholar]
- 39. Tong JH, Ng DC, Chau SL, So KK, Leung PP, Lee TL, Lung RW, Chan MW, Chan AW, Lo KW, et al. Putative tumour-suppressor gene DAB2 is frequently down regulated by promoter hypermethylation in nasopharyngeal carcinoma. BMC Cancer 2010; 10:253; PMID:; http://dx.doi.org/ 10.1186/1471-2407-10-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, Nguyen ET, Mathur AN, Levy DE, Kaplan MH. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology 2009; 127:587-95; PMID:; http://dx.doi.org/ 10.1111/j.1365-2567.2008.03037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 2007; 8:1390-7; PMID:; http://dx.doi.org/ 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- 42. Rosenbauer F, Kallies A, Scheller M, Knobeloch KP, Rock CO, Schwieger M, Stocking C, Horak I. Disabled-2 is transcriptionally regulated by ICSBP and augments macrophage spreading and adhesion. EMBO J 2002; 21:211-20; PMID:; http://dx.doi.org/ 10.1093/emboj/21.3.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chetrit D, Ziv N, Ehrlich M. Dab2 regulates clathrin assembly and cell spreading. Biochem J 2009; 418:701-15; PMID:; http://dx.doi.org/ 10.1042/BJ20081288 [DOI] [PubMed] [Google Scholar]
- 44. Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother 2007; 56:1817-29; PMID:; http://dx.doi.org/ 10.1007/s00262-007-0325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frankenberger B, Schendel DJ. Third generation dendritic cell vaccines for tumor immunotherapy. Eur J Cell Biol 2012; 91:53-8; PMID:; http://dx.doi.org/ 10.1016/j.ejcb.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 46. Wolfl M, Greenberg PD. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nat Protocols 2014; 9:950-66; PMID:; http://dx.doi.org/ 10.1038/nprot.2014.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao C, Wood MW, Galyov EE, Hopken UE, Lipp M, Bodmer HC, Tough DF, Carter RW. Salmonella typhimurium infection triggers dendritic cells and macrophages to adopt distinct migration patterns in vivo. Eur J Immunol 2006; 36:2939-50; PMID:; http://dx.doi.org/ 10.1002/eji.200636179 [DOI] [PubMed] [Google Scholar]
- 48. Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Nat Acad Sci U S A 2009; 106:7852-7; PMID:; http://dx.doi.org/ 10.1073/pnas.0812148106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoon CH, Miah MA, Kim KP, Bae YS. New Cdc2 Tyr 4 phosphorylation by dsRNA-activated protein kinase triggers Cdc2 polyubiquitination and G2 arrest under genotoxic stresses. EMBO Rep 2010; 11:393-9; PMID:; http://dx.doi.org/ 10.1038/embor.2010.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yokouchi H, Chamoto K, Wakita D, Noguchi D, Yamazaki K, Dosaka-Akita H, Nishimura M, Ikeda H, Nishimura T. Tetramer-blocking assay for defining antigen-specific cytotoxic T lymphocytes using peptide-MHC tetramer. Cancer Sci 2006; 97:148-54; PMID:; http://dx.doi.org/ 10.1111/j.1349-7006.2006.00149.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010; 120:1111-24; PMID:; http://dx.doi.org/ 10.1172/JCI40269 [DOI] [PMC free article] [PubMed] [Google Scholar]