Abstract
Although inflammation has been linked to lung cancer pathogenesis, very little is known about the critical players during lung cancer development. We found that Kras mutation in lung epithelial cells preferentially leads to recruitment of Th17 cells, which produce IL-17, a signature cytokine that promotes inflammation. We demonstrated IL-17 is critical for tumor growth in part by recruiting tumorigenic GR1+ CD11b+ cells.
Keywords: GR1+ CD11b+ cells, Kras, lung adenocarcinoma, Th17
T-helper 17 (Th17) cells, a recently identified lineage of T-helper cells, are very potent drivers of inflammation.1 This is largely accomplished by its product, interleukin (IL)-17, which targets local tissues to produce arrays of effector molecules such as IL-6 and CXCL1.2 IL-17 and Th17 cells have been promising targets for treating autoimmune diseases.3
The intricate relationship between inflammation and cancer offers an opportunity to evaluate the functions of IL-17 and Th17 cells in tumor immunity. Contradictory results have been reported. Adoptive transfer to Th17 cells, if generated in vitro in an antigen-specific manner, can mount effective antitumor immunity. Ectopic transplantation of tumor cells in IL-17-deficient mice, on the other hand, resulted in either enhanced or reduced tumor development.4 These results suggest that the role of IL-17 and Th17 cells in tumor immunity should be understood in a tumor-specific and context-dependent manner. On the other hand, one criticism of the aforementioned studies was whether a transplantable model of cancer can recapitulate the early immune response against cancer in targeted organs. In this regard, the role of IL-17 during the spontaneous development of lung cancer remains undefined. Therefore, we sought to determine whether IL-17 and Th17 cells are active players during endogenous lung cancer development, and if so, to identify the mechanisms that lead to such roles.
When mutated Kras (KrasG12D), most frequently found in lung cancer, was restrictively expressed in lung epithelial cells (CCSPcre), the mononuclear cells that infiltrated in lung tumor were alveolar macrophages, inflammatory macrophages (CD11b+), GR1+ CD11b+ cells, and lymphocytes. Upon tumor development, increased subsets of lymphocytes were IL-17-positive Th17 cells and Foxp3-positive regulatory T cells (Treg). The level of interferon gamma in CD4+ or CD8+ T cells seemed to be constant. When KrasG12D CCSPcre mice were crossed with IL-17-deficient mice, the lung tumor count was reduced by 75%. Although IL-17F was detected in Th17 cells in lung tumors, the tumor burden of IL-17F-deficient mice was similar to that of wild-type mice.5
Lung cancer is often considered to be non-inflammatory. However, increasing evidence suggests that lung inflammation and cancer are linked, especially in individuals who smoke cigarettes. We challenged KrasG12D CCSPcre mice with lysates of non-typable hemophilia influenza as a model of inflammation to accelerate lung tumor development because previous studies showed this type of inflammation can promote Kras-driven lung cancer,6 and this pathogen is closely associated with the exacerbation period of chronic obstructive pulmonary disease, which has a direct correlation with lung cancer occurrence. We found that the number of Th17 cells as well as Th1 cells was increased in this model while the number of Treg cells was unchanged. In IL-17-deficient mice, the tumor burden was reduced by about a 50%. We found that IL-17-deficient mice exhibited about a 50% reduction in the number of tumor.5 Next, we sought to identify pro-tumorigenic factors mediated by IL-17. We evaluated several factors such as tumor-cell proliferation, angiogenesis, inflammatory index, and tumor infiltrated mononuclear cells. Lung histological evaluation showed that tumor-cell proliferation and angiogenesis was lower in IL-17 deficient tumor mice than in WT. Several previous studies have shown that IL-17 is incapable of driving cell proliferation directly. However, IL-6, a key effector molecule driven by IL-17, can activate STAT3, and sustained activation of STAT3 accounted for tumor-cell proliferation mediated by IL-17.7 It is not clear whether neovascularization is directly mediated by IL-17 or indirectly via IL-17-promoted factors.
Interestingly, we observed the number of GR1+ CD11b+ cells was significantly reduced in IL-17-deficient mice (Fig. 1). IL-17 is known to recruit neutrophils during infection. The role of neutrophils in tumor development has been debated as both pro- and anti-tumorigenic. In addition, myeloid-derived suppressive cells, also identified as GR1+ CD11b+ cells, are associated with the repression of effective tumor immunity.8 The functional significance of GR1+ CD11b+ cells in tumor promotion in the current study was demonstrated by GR1+ depletion. When we isolated GR1+ CD11b+ cells from lung tissue, GR1+ CD11b+ effectively suppressed CD8+ T cell proliferation and cytokine production ex vivo, indicating that this subpopulation of myeloid cells can suppress the immune system. Thus, we hypothesized GR1+ CD11b+ cells recruited by IL-17 suppress CD8+ T cells to enhance tumor immunity. Unexpectedly, CD8+ depletion in IL-17-deficient mice did not restore the tumor burden. In fact, CD8+ T cell depletion in this model had no significant effect on tumor burden. This observation suggests GR1+ CD11b+ cells promote tumor development via mechanisms independent from immune suppression, and cytotoxic CD8+ T cells contribute minimally in this model.
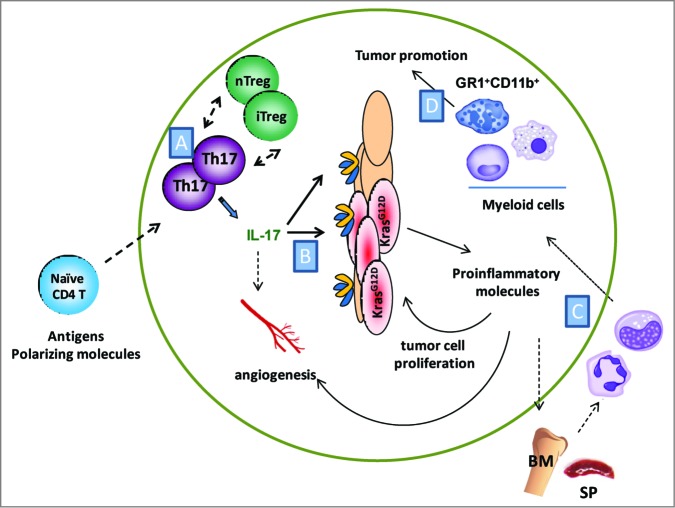
Figure 1.
Promotion of tumor growth mediated by IL-17 in a spontaneous model of lung cancer. (A) Oncogenic Kras mutation in lung epithelial cells leads to generation of Th17 and Treg cells. How these cells are generated and maintained in tumor lung is not clear. (B) IL-17 stimulates tumor epithelium and stromal cells to produce proinflammatory molecules, which promote tumor-cell proliferation, angiogenesis and recruitment of myeloid cells. (C) Myeloid cells are likely generated, expanded and migrated to tumor lung upon the activation of mutated Kras. Less is known about the mechanisms and how IL-17 modulates these pathways. (D) Accumulated GR1+ CD11b+ cells in tumor lung promote lung adenocarcinoma (BM; Bone marrow, SP; Spleen).
Our study prompts other critical questions. First, what are the pathways for the generation and regulation of Th17 cells within the tumor microenvironment? Kras mutation in lung epithelial cells is reported to increase inflammatory response.9 Either lung stromal cells or hematopoietic cells that infiltrate the tumor or tumor itself may secrete cytokines or metabolites that promote the polarization of Th17 or Treg cells polarizing. Alternatively, memory or innate Th17 cells may expand in the lung upon K-ras mutation, or Th17 could be generated via the conversion of another CD4+ T cell subset that resides in the lung such as Treg cells. The identification of antigens of Th17 or Treg cells would increase our understanding of the nature of these cells. Second, while we have shown the role of IL-17 in primary lung tumors, whether IL-17 plays a similar role during metastasis is unknown. The function of IL-17 or Th17 cells depends on the tumor microenvironment. This aspect is particularly significant if the purpose is to target Th17 pathways for the treatment of end-stage or fully grown tumors. Third, it is unknown whether blockage of IL-17 or Th17 cells can be combined with existing therapies so that non-redundant pathways of tumorigenesis can be targeted efficiently. Since CCSPcre/K-rasG12D mice accurately recapitulated both the genetic and histopathologic progression from the earliest lesions of human lung cancer, future studies will provide a unique platform to test the suppression of IL-17 and Th17 cells for lung cancer treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann New York Acad Sci 2008; 1143:188-211; PMID:; http://dx.doi.org/ 10.1196/annals.1443.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal 2011; 23:1069-75; PMID:; http://dx.doi.org/ 10.1016/j.cellsig.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Dis 2012; 11:763-76; PMID:; http://dx.doi.org/ 10.1038/nrd3794 [DOI] [PubMed] [Google Scholar]
- 4. Zou WP, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy (vol 10, pg 248, 2010). Nat Rev Immunol 2011; 11:565; PMID:; http://dx.doi.org/ 10.1038/nri3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Nat Acad Sci U S A 2014; 111:5664-9; PMID:; http://dx.doi.org/ 10.1073/pnas.1319051111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba II, Ji L, Kurie JM, Dickey BF, Demayo FJ. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol 2009; 40:443-53; PMID:; http://dx.doi.org/ 10.1165/rcmb.2008-0198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009; 206:1457-64; PMID:; http://dx.doi.org/ 10.1084/jem.20090207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, et al. . K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006; 25:2105-12; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1209237 [DOI] [PubMed] [Google Scholar]