Abstract
Indoleamine 2,3-dioxygenase (IDO) is an immunoregulatory enzyme. Remarkably, we discovered IDO-specific T cells that can influence adaptive immune reactions in patients with cancer. Further, a recent phase I clinical trial demonstrated long-lasting disease stabilization without toxicity in patients with non-small-cell lung cancer (NSCLC) who were vaccinated with an IDO-derived HLA-A2-restricted epitope.
Keywords: cancer vaccine, CD8, IDO, NSCLC, PD-L1
Abbreviations: DC, dendritic cells; IDO, indoleamine 2,3-dioxygenase; NSCLC, non-small-cell lung cancer; OS, overall survival; PBMC, peripheral blood mononuclear cells; PD, progressive disease; Tregs, regulatory T cells; SD, stable disease
The immune system maintains a delicate balance between immunity and tolerance, protecting the host from pathogens while minimizing local tissue damage. IDO is a critical endogenous cellular factor that contributes to immune suppression. IDO activity constitutes a counter-regulatory mechanism induced by pro-inflammatory signals. This is also a crucial mechanism in cancer,1 and cancer patients exhibit IDO elevation in a subset of plasmacytoid dendritic cells (DC) in tumor-draining lymph nodes. IDO may also be expressed by tumor cells and tumor stromal cells, where it inhibits the effector phase of immune responses.2
We recently reported spontaneous CD8+ and CD4+ T-cell reactivity against IDO in the tumor microenvironment and in the peripheral blood of various cancer patients. We demonstrated that these IDO-reactive CD8+ T cells were peptide-specific cytotoxic effector cells that can recognize and kill IDO-expressing cells, including tumor cells and DC. We further found in vitro, that the presence of these IDO-specific T cells enhanced other T-cell responses, both directly and indirectly.3 Thus, IDO may serve as a widely applicable target for immunotherapeutic strategies with a completely different function and expression pattern compared to previously described antigens, and such IDO-based immunotherapy could potentially act synergistically with other types of immunotherapy.
To evaluate the efficiency and safety of IDO-based vaccinations, we initiated a phase I first-in-man clinical phase I vaccination trial. The study comprised 15 HLA-A2+ patients with advanced NSCLC who were vaccinated with an IDO-derived A2 peptide in Montanide adjuvant (www.clinicaltrials.gov; NCT01219348).4 The vaccine was well tolerated with manageable side effects and no CTCAE grade 3/4 toxicities. Local reactions at the vaccine site occurred in 90% of patients, most likely due to Montanide5 and Imiquimod.6 The median overall survival (OS) was >2 y, which was markedly higher than expected for this patient group. Of the seven patients with stable disease (SD), six are still alive. Based on this cohort's expected median progression-free survival of ∼6–7 mo,7 we defined clinical benefit as objective response or SD for ≥8.5 mo, which was observed in 47% of the treated patients. One patient obtained an objective partial response after demonstrating continuous tumor regression on vaccine treatment for 1 y. He and one other patient remain enrolled in the trial.
The vaccine comprised an IDO-derived HLA-A2-restricted epitope. We compared the vaccinated HLA-A2+ patients with the HLA-A2− patients in the intent-to-treat population who were excluded due to HLA type. Respectively, the two groups had median OS of 25.9 mo (778 d) and 7.7 mo (237 d), demonstrating significant longer OS in the vaccinated HLA-A2+ group (P = 0.03). The clinical significance of HLA phenotype in cancer patients has been widely investigated. A large study recently reported HLA-A2 expression to be an unfavorable prognostic factor in stage I NSCLC patients,8 highlighting the potential importance of the significantly longer OS observed in vaccinated HLA-A2+ patients compared to unvaccinated HLA-A2− NSCLC patients. However, these observations must be confirmed in a larger clinical trial.
All examined patients showed IDO expression in the tumor microenvironment but there was no apparent correlation between IDO expression intensity and the clinical response to vaccine treatment. However, two patients with progressive disease (PD) and very short-term survival exhibited grade 3+ IDO expression, which could potentially be associated with an unfavorable prognosis.
Immune monitoring using ELISPOT confirmed the presence of IDO-specific T cells in all patients, not only in the patients that seemed to benefit clinically. Two patients with SD showed an IDO-specific ELISPOT response during 1 y of treatment, suggesting sustained long-term IDO reactivity. Notably, compared to those who showed no clinical response, patients who achieved a clinical response to the vaccine showed significantly higher pre-treatment levels of IDO-specific T cells. Furthermore, all treated patients exhibited a significantly reduced Treg population after the sixth vaccine. Kynurenine produced by IDO may effectively hamper the immune response by binding the aryl hydrocarbon receptor, which favors local Treg formation. We previously showed that IDO-specific T-cell activation decreased Treg frequency in vitro.3 Based on these promising clinical results, we recently initiated an additional phase I trial combining IDO vaccination with standard ipilimumab treatment for stage IV melanoma patients (www.clinicaltrial.gov NCT02077114).
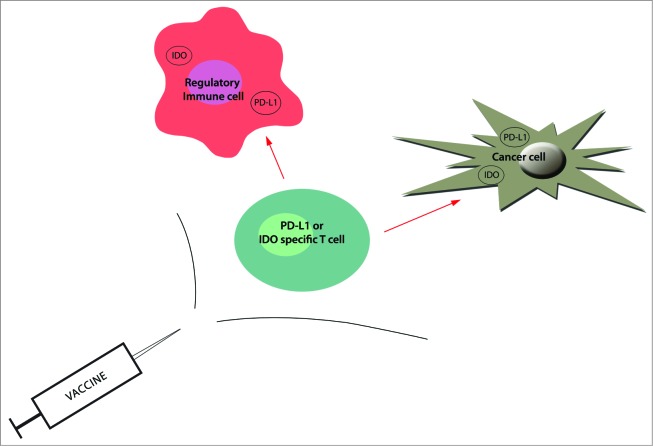
We have also characterized self-reactive T cells that specifically recognize additional proteins expressed in regulatory immune cells, e.g., programmed death ligand 1 (PD-L1) and Foxp3.9,10 In particular, PD-L1-specific T cells are an interesting example of the immune system's ability to influence adaptive immune reactions by directly reacting against immune-suppressive mechanisms that have been adopted by cancerous cells. Targeting of antigens with an immune regulatory function offers a form of immunotherapy in which specific cell depletion is not limited to targeting products expressed on the cell surface. By definition, almost any successful immune therapy strategy aims to induce immunological activation and inflammation. Since immune-suppressive cells might antagonize the desired effects of immunotherapeutic approaches, the targeting of such cells – for example, by vaccination – is obvious, easily implementable and probably highly synergistic (Fig. 1). Thus, boosting specific T cells that recognize immune regulatory proteins, such as IDO or PD-L1, may directly modulate immune regulation, potentially altering tolerance to tumor antigens.
Figure 1.
The targeting of immunosuppressive cells as well as cancer cells by vaccination. Following protein processing, antigen-derived peptides are presented on the cell surface by HLA class I molecules, where they are recognized by CD8+ T cells. Hence, antigen-specific T cells can recognize HLA-restricted epitopes on the surface of regulatory immune cells (e.g., myeloid-derived suppressor cells and tumor-associated dendritic cells), resulting in suppression and/or delay of local immune suppression. They can also eliminate antigen-expressing cancer cells (red arrows). The boosting of IDO or PD-L1-specific T cells by vaccination may consequently directly modulate immune regulation and potentially altering tolerance to tumor antigens. Because immune-suppressive cells might antagonize the desired effects, the addition of antigens like PD-L1 and IDO should be an easily implementable and vastly synergistic method to improve the efficiency of cancer vaccines.
Authorship and Conflict of Interest Statements
The author declares no competing financial interests. However, please note that Mads Hald Andersen has filed a patent application based on the use of IDO for vaccination. The rights of the patent application have been transferred to the University Hospital at Herlev according to the Danish Law of Public Inventions at Public Research Institutions.
Funding
The study was supported by Herlev Hospital, the capital region of Denmark, the Danish Cancer Society, and the Danish Medical Research Council. The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
References
- 1. Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets 2007; 7:31-40; PMID:; http://dx.doi.org/ 10.2174/156800907780006896 [DOI] [PubMed] [Google Scholar]
- 2. Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9: 1269-74; PMID:; http://dx.doi.org/ 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 3. Sorensen RB, Hadrup SR, Svane IM, Hjortso MC, thor Straten P, Andersen MH. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 2011; 117:2200-10; PMID:; http://dx.doi.org/ 10.1182/blood-2010-06-288498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iversen TZ, Engell-Noerregaard L, Ellebaek E, Andersen R, Larsen SK, Bjoern J, Zeyher C, Gouttefangeas C, Thomsen BM, Holm B, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res 2014; 20:221-32; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1560 [DOI] [PubMed] [Google Scholar]
- 5. Harris RC, Chianese-Bullock KA, Petroni GR, Schaefer JT, Brill LB, Molhoek KR, Deacon DH, Patterson JW, Slingluff CL, Jr. The vaccine-site microenvironment induced by injection of incomplete Freund's adjuvant, with or without melanoma peptides. J Immunother 2012; 35:78-88; PMID:; http://dx.doi.org/ 10.1097/CJI.0b013e31823731a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishelevich R, Zhao Y, Tuchinda P, Liu H, Nakazono A, Tammaro A, et al. Imiquimod-induced TLR7 signaling enhances repair of DNA damage induced by ultraviolet light in bone marrow-derived cells. J Immunol 2011; 187:1664-1673; PMID: 21765012; http://dx.doi.org/ 10.4049/jimmunol.1100755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:2191-7; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.25.4052 [DOI] [PubMed] [Google Scholar]
- 8. Nagata Y, Hanagiri T, Mizukami M, Kuroda K, Shigematsu Y, Baba T, Ichiki Y, Yasuda M, So T, Takenoyama M, Sugio K, et al. Clinical significance of HLA class I alleles on postoperative prognosis of lung cancer patients in Japan. Lung Cancer 2009; 65:91-97; PMID:; http://dx.doi.org/ 10.1016/j.lungcan.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 9. Andersen MH. FOXP3-specific immunity. Oncoimmunology 2013; 2:e26247; PMID:; http://dx.doi.org/ 10.4161/onci.26247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad SM, Larsen SK, Svane IM, Andersen MH. Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia 2014; 28:236-38; PMID:; http://dx.doi.org/ 10.1038/leu.2013.261 [DOI] [PubMed] [Google Scholar]