Abstract
Administration of NGR-TNF, a tumor vessel-targeting and tumor necrosis factor α TNFα) peptide conjugate, with immunotherapy has been shown to inhibit tumor growth in mice. Thus, we planned a Phase I pilot clinical trial to assess safety, immune and clinical response of this combination treatment for advanced melanoma. NA17.A2 and MAGE-3.A1 peptides were used as vaccine. HLA-A*0201 or HLA-A*01 metastatic melanoma patients received human NGR-hTNF i.v. alternating with s.c. weekly injections of either of the peptides emulsified in Montanide. The T-cell response was assessed ex-vivo using peripheral blood mononuclear cells (PBMCs) before, during and after therapy. The serum level of chromogranin A (CgA), soluble TNF receptors (sTNFR1/2), vascular endothelial growth factor (VEGF), and MIP-1β and MCP-1 chemokines, was determined. In 3 subjects, pre- and post-treatment tumor lesions were examined by immunohistochemistry. Clinically, chills were observed in 4 patients during NGR-hTNF infusion and erythema at vaccination site was seen in 7 patients. T-cell response against the vaccine or against other melanoma-associated antigens was detectable after treatment in 6 out of 7 tested patients. Low level or reduction of CgA and sTNFR and increase of MIP-1β and MCP-1 were found in patients sera. In the lesions examined the immune infiltrate was scanty but macrophage number increased in post-therapy lesions. From a clinical standpoint, a long term survival (>4 months) was found in 6 out of 8 evaluable patients (4, 4, 7, 11, 23+, 25+, 25+, 29+ months). The combination of NGR-hTNF and vaccine in metastatic melanoma patients was well tolerated, often associated with an ex-vivo T cell response and long-term overall survival. These findings warrant confirmation in a larger group of patients.
Keywords: anti-vascular target therapy, combination therapy, inflammatory cytokines, melanoma, peptide-based vaccines, T cells
Abbreviations: APC, antigen presenting cell; CgA, chromogranin A; CT, cancer/testis; DFS, disease-free survival; MAA, melanoma-associated antigens; MCP-1, macrophage chemoattractant protein 1; MIP-1β, macrophage inflammatory protein 1β; OS, overall survival; PD, progression of disease; PFS, progression-free survival; PBMC, peripheral blood mononuclear cell; RR, response rate; sTNFR, soluble tumor necrosis factor receptor; TNFα, tumor necrosis factor α
Introduction
The clinical outcome of patients with advanced melanoma has significantly improved during the last 2–3 years. In fact, while in the past Stage IV patients had a median survival time of 6 to 9 months, with 5-year survival rates of only 2%,1 new agents have been developed that increase the progression-free survival (PFS) and/or overall survival (OS) of metastatic melanoma thanks to the targeting of frequently mutated genes (e.g. BRAF) and/or non-specific activation of the immune system by immuno-modulating antibodies (e.g. ipilimumab).2,3
More than 10 years of vaccination trials performed mostly with cancer/testis (CT) and differentiation self melanoma-associated antigens (MAAs) has resulted in a clinical response rate (RR) of 5–27% in Phase II protocols, with even complete and durable regressions in some metastatic melanoma patients. These studies showed that T-cell immune responses against the vaccine were induced in a variable fraction of subjects (10–60%).4,5 Moreover, a recent Phase III trial of vaccination with the peptide gp100 (210A) in combination with high dose of interleukin (IL-2) vs IL-2 alone has resulted in a statistically significant increased RR and disease-free survival (DFS) in melanoma patients.6
In the present study we selected 2 MAA peptides with which to immunize our melanoma patients. The first one (NA17.A2 or N-acetylglucosaminyltransferase; HLA-A*0201-restricted; sequence VLPDVFIRC) (see Table S1) is the result of a splicing alteration encoded by a sequence located in an intron and expressed in 50% of melanomas but not found at significant levels in normal tissues.7 The second one (MAGE-3.A1; sequence EVDPIGHLY) belongs to the group of CT antigens and is expressed approximately by 70% of metastatic melanomas. The reason for such a choice lies also in the fact that both antigens have been previously shown to induce immune and clinical responses in Stage III and IV metastatic melanoma patients8-10 (see also National Cancer Institute Clinical Trials PDQ).
Recent studies in animal models showed that administration of NGR-TNF, a drug consisting of tumor necrosis factor α (TNFα) fused with CNGRCG (a peptide ligand of CD13 expressed by endothelial cells in tumor vessels), can delivery low doses of TNFα to the tumor vasculature and overcome major TNFα counter-regulatory mechanisms, such as shedding of soluble TNFα receptors. NGR-TNFα can alter the endothelial barrier function and increase the penetration of chemotherapeutic drugs in tumors, an effect that could, however, be counteracted by chromogranin A (CgA).11,12 In addition, low-dose NGR-TNFα can upregulate leukocyte adhesion molecules on tumor vessels and induce the release of various chemokines in tumors tissues (such as MCP-1/CCL-2, MCP-3/CCL-7, MIP-2, oncostatin-M and stem cell factor) involved in T-cell activation and migration. These mechanisms associate with increased tumor infiltration of endogenous or adoptively transferred cytotoxic T lymphocytes in transplantable models of melanoma and can enhance the response to adoptive and active immunotherapy even in animal models of spontaneous prostate cancer.13,14
Various Phase I and II studies have been performed with human NGR-TNF (NGR-hTNF) in patients with solid tumors, both as a single agent and in combination with chemotherapy. (reviewed in14) Phase I studies have shown that NGR-hTNF is well tolerated (maximum tolerated dose was 45 μg/m2 administered in 1 h).14,15 Chills and fever were the most frequently observed toxicities. Based on soluble receptors shedding, tolerability, anti-vascular effects and disease control, the dose of 0.8 μg/m2 of NGR-hTNF was chosen for subsequent studies, either alone or with standard chemotherapy. Single-agent Phase II studies conducted with low-dose NGR-hTNF in patients with malignant pleural mesothelioma, hepatocellular carcinoma and colorectal cancer showed radiological anti-vascular effects and significant disease control with low-dose NGR-hTNF.14,15 The results of these studies suggest that such low doses of NGR-hTNF have an optimal safety profile along with anticancer activity, thus rendering this agent suitable for development in combination with immunotherapy.
These notions, therefore, provide the rationale for combining NGR-hTNF with immunotherapy in melanoma patients.
Results
Detection of antigen- and/or tumor–specific T-cell responses in the peripheral blood of melanoma patients
According to their HLA-A allele, 5 out of 8 HLA-A*0201 melanoma patients (#02, 03, 04, 05 and 08) were immunized with the NA17.A2 epitope while 2 HLA-A*01 patients received MAGE-3.A1 (#01, 06) or, in the case of patient #07, both MAGE-3.A1 and NA17.A2 (#07) peptides.
The presence of circulating T cells recognizing MAAs and/or melanoma cell lines was determined in peripheral blood mononuclear cells (PBMCs) from pre- and post- treatment time points of patients undergoing NGR/Vax/01 treatment. Interferon γ (IFNγ)−based ELISPOT assay was performed by incubation of PBMCs for 24 h with antigen presenting cells (APCs) loaded with the peptide epitope used for vaccination (MAGE-3.A1 and/or NA17.A2) or other CT or differentiation MAA-derived peptides (MAGE-A2; NY-ESO-1; MART-1; gp100; Tyrosinase, Tyr) that are HLA-A*01- or A*0201-restricted. In the other assay, PBMCs were tested on HLA-A-compatible melanoma lines expressing the given MAAs.
Table 1 summarizes the T-cell immune response against melanoma-derived epitopes including those used for immunization, for the 7 assessable patients at week 8 or 9, (i.e., at the end of the first cycle of vaccination) and/or at weeks 17–20 (2nd and 3rd boosts). Three patients (#04, 07 and 08) showed a T-cell response in both assays (target peptides and cell lines) while 4 subjects (#02, 03, 05 and 06) tested positive in only one assay, although a strong but non-HLA-restricted killing of melanoma cells was found in patients 4 and 5. No immunological tests could be performed for patient #01 due to a rapid disease progression.
Table 1.
T cell-mediated immune response of patients enrolled in the trial
| Patient # and peptide vaccine | Recognition of APCs loaded with indicated melanoma peptides | Recognition of melanoma cell lines |
|---|---|---|
| # 02/NA17 | No | Yes; No HLA restriction |
| #03/NA17 | N0 | Yes; No HLA restriction |
| #04/NA17 | Yes (MAGE-A2, gp100 not NA17) | Yes; No HLA restriction |
| #05/NA17 | Yes (NA17, MAGE-A2, MAGE-A3) | Yes; No HLA restriction |
| #06/MAGE-A3 | Yes (MAGE-A2, MAGE-A3, NA-17, NY-ESO-1) | Yes; HLA-restriction |
| #07/NA17, MAGE-A3 | Yes, (NA17, MAGE-A2, MAGE-A3) | Yes; HLA-restriction |
| #08/NA17 | Yes (NA17, MAGE-A2, MAGE-A3, NY-ESO1, Tyr) | Yes; HLA restriction |
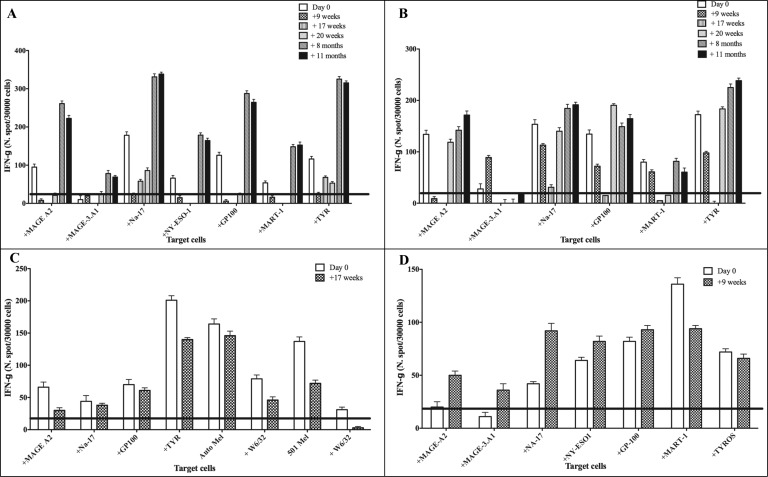
As previously shown,16 circulating T cells specific for the HLA-A*0201- and for HLA-A*01-restricted MAAs, such as gp100, MART-1, Tyr, and MAGE, respectively, were frequently found at the baseline of all the HLA-A*0201 melanoma patients then vaccinated with NA17.A2 (see Fig. 1A, B, C and D; white bars). Patients #07 undergoing vaccination with both MAGE-3.A1 and NA17.A2 peptides showed an increase (p < 0.05) in the frequency of circulating T cells directed to several MAA-derived epitopes recognized in the context of HLA-A*0201 such as MAGE-A2, NA17.A2, NY-ESO-1, GP100, MART-1, Tyr and in the context of of HLA-A*01 such as MAGE-A3 (also designed as MAGE–3A1) (Fig. 1, panel A). Notably, these patterns of reactivity were observed even at long term (+11 and +20 months time points) post-treatments (Fig. 1, panels A and B). A significant increase of T cell reactivity to MAGE-3.A1 (the peptide used for vaccination) was detected in the context of HLA-A*01 molecules in 2 patients, #07 (Fig. 1, panel A) and #06 (not shown). An increased recognition, as compared with the pre-treatment time point, of HLA-A*0201-restricted epitopes (e.g. MAGE-A2, NA17.A2, gp100 and tyrosinase) was found in PBMCs of patient #08 (vaccinated with the NA17.A2 peptide) at long-term post-treatment (Fig. 1, panel B).
Figure 1.
T-cell responses to MAAs in PBMCs of melanoma patients undergoing NGR/VAX treatment. Freshly isolated peripheral blood mononuclear cells (PBMCs) from melanoma patients (#02, 05, 07 and 08) were used to assess their reactivity against melanoma associated antigens (MAA)-derived peptides (MAGE-A2, MAGE-A3, NA-17A, NY-ESO-1, MART-1, gp100, TYR) on HLA-A*01+ 1061 EBV-B (Panel A and D) or HLA-A*0201+ T2 cells (Panels A, B and C) and autologous, when available, or allogeneic HLA-matched tumor cell reactivity (Panel C). Interferon γ (IFNγ)-based ELISPOT assay was used for this analysis. Data are expressed as N. of spots/3 × 104cells and are subtracted of the background of IFNγ release from T cells incubated with EBV-B or T2 control cells alone. Results represent averages of triplicates with SD ≤ 10%; statistical analysis of differences between means of IFNγ released by T cells was performed by 2-tailed Student's t-test; significance defined as p < 0.05.
The increased recognition, albeit in some cases without statistical significance (defined as p < 0.05) as compared with the pre-treatment time point, of HLA-A*02-restricted epitopes (e.g., MAGE-A2, NA-17A2, NY-ESO-1, gp100 and Tyr) was found in PBMCs of patient #08 (vaccinated with the NA17. A2 peptide) at long-term post-treatment (months 8 and 11; Fig. 1, panel B). However, T cells from patient #07 and 08 failed to recognize any allogeneic HLA-matched melanoma cell lines (data not shown). Only for patient #04 a natural killer (NK)-like reactivity against 2 allogeneic HLA-A*0201+ and NA17.A2+ matched melanoma lines was detected following vaccination with NA17.A2 (Table 1).
Baseline T-cell reactivities, including that directed to NA17.A2, decreased at post-treatment time points in 3 patients (#3, 4, 5) showing progressive disease (see below, Table 2). T-cell reactivity against an array of both CT and differentiation MAAs could be found in the peripheral blood of patient #02 at pre-treatment time points and recognition of these antigens decreased at post-vaccination time points (Fig. 1, Panel C).
Table 2.
Clinical outcome of melanoma patients treated with NGR/Vax/01
| Patient # | Stage | Date of treatment onset | Additional therapy | OS§§ | TTP |
|---|---|---|---|---|---|
| 01 | IV M1c | 25/11/2010 | Zelboraf* | 11 | NA |
| 02 | III M1a | 01/03/2011 | Surgery** Chemotherapy** IL-2** Vaccination** (2006) |
29+SD | 26 |
| 03 | IV M1c | 01/07/2011 | Surgery** Chemotherapy** Ipilimumab*,Local RT* |
4 | 1.3 |
| 04 | IV M1b | 22/07/2011 | Chemotherapy** IFNα** |
7 | 1 |
| 05 | IV M1c | 28/7/2011 | Ipilimumab** Chemotherapy** |
4 | 4 |
| 06 | IV M1a | 10/10/2011 | Surgery** |
25+NED | 11 |
| 07 | IV M1a | 14/11/2011 | Surgery*** RT* |
25+NED | 3.3 |
| 08 | IV NED | 19/12/2011 | Surgery*** RT* |
23+NED | 1 |
*After drop out; **Before protocol treatment; ***During protocol treatment; §: Overall Survival (OS) from Protocol Start (months). RT: Radiotherapy; SD: Stable disease; NED: No evidence of disease.
Interestingly, tumor reactivity against the autologous melanoma cell line was found in PBMCs at both pre-and post-treatment time points for patient #02 who was treated with a MAGE-3.A1-based vaccine 4 years ago (Fig. 2, Panel C).17
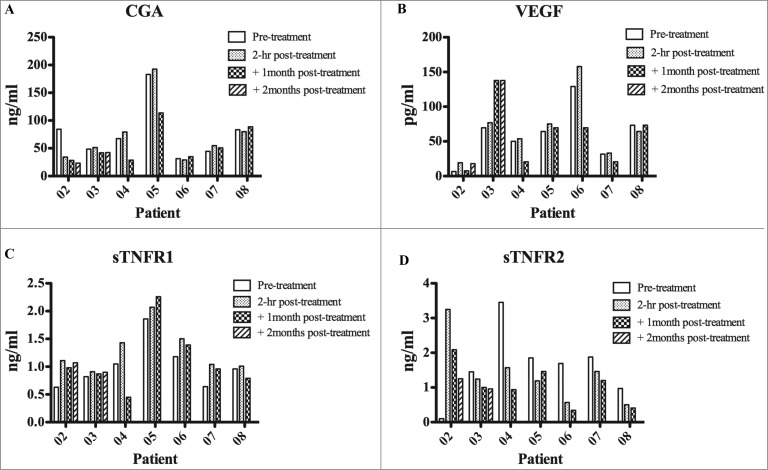
Figure 2.
Detection of TNF inhibitory soluble factors and VEGF in the serum of melanoma patients. Serum from melanoma patients was collected at different time points: baseline, 2 hours, 1 month and 2 months post-treatments. The presence in the serum of chromogranin A (CgA), soluble tumor necrosis factor (sTNFR) was assessed by ELISA. Statistical analysis of differences between means of soluble factors was performed by 2-tailed Student's t-test with significance defined as p < 0.05.
In most melanoma patients undergoing NGR/Vax/01 treatment the phenomenon of antigen spreading was observed since T-cell reactivity induced or augmented following the treatment directed against a number of MAAs (see Fig. 1 and Table 1). Of note, MAAs or tumor recognition in fresh ex-vivo isolated PBMCs at long-term post vaccination time points was observed in 4 patients (# 02, 06, 07 and 08, Table 1) who showed a possible clinical benefit in long term OS (see Table 2).
Levels of soluble factors, cytokine receptors and chemokines in the serum of melanoma patients undergoing NGR/VAX treatment
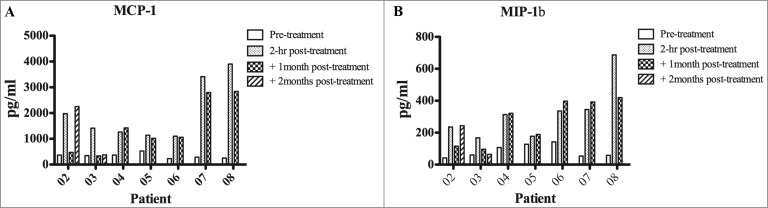
The serum of each patient undergoing NGR/VAX treatment was collected at baseline and 1 or 2 months after treatment, 2 h following the infusion of NGR-TNF. Since we have previously shown that CgA and sTNF-Rs can negatively affect the antitumor activity of NGR-TNF in mice,12,14 the circulating levels of these proteins at baseline were analyzed. Except for patients 05 and 04, who had CgA and soluble tumor necrosis factor receptor 1 (sTNF-R1) or sTNF-R2 levels greater than normal values, all other patients had relatively low levels of these proteins in the circulation. Indeed CgA was found either at relatively low levels or significantly reduced (p < 0.05) as compared with baseline in 2 out of 4 and 1 out of 4 melanoma patients, respectively (Fig. 2, panel A), a finding potentially associated with clinical benefit shown in patients #02, 06, 07, 08. Starting from 2 h after the administration of NGR-TNF, a remarkably increased levels of macrophage chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β) were evident (Fig. 3, panels A and B) with the detection of the highest levels in at least 4 out of 5 patients (#02, 06, 07, 08) showing clinical benefit in terms of OS. IL-1β, IL-6 and IL-8 pro-inflammatory cytokines were found to be under the detection limit without changes during treatment. Of note is the increase of MCP-1 during treatment since this cytokine is known to induce migration from the blood to vascular endothelium where it may promote tissue immunological infiltration of lymphocytes in the microenvironment18 and, along with MIP-1β, an inflammatory reaction that may recruit locally T cells activated by the vaccine.
Figure 3.
Detection of pro-inflammatory factors (MIP1β and MCP1) in the serum of melanoma patients. Serum from melanoma patients was collected at different time points: baseline, 2 hours, 1 month and 2 months post-treatments. The presence in the serum of macrophage chemoattractant protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β) was assessed by ELISA. Data represent pg/mL of each soluble factor. Statistical analysis of differences between means of soluble factors was performed by 2-tailed Student's t-test with significance defined as p < 0.05.
IHC analysis of pre- and post-treatment metastases
Results of this analysis are shown in Fig. S1 for 2 of the 3 representative patients (#06, 07 and 08) who received the whole treatment. In the patients that had tumor nodules excised before and after treatment, although at different times for therapeutic purposes, an analysis of the infiltrate was carried out before and after NGR/Vax/01 therapy. In all cases, the immune infiltrates of lymphoid and dendritic cells were scanty. Such lymphoid infiltrates were observed at the periphery of neoplastic lesions and CD3+CD4+ cells predominated over CD3+CD8+ cells; CD56+cells were only rarely observed and this picture did not change significantly as far as number and distribution is concerned, in pre and post-therapy specimens (not shown). The only significant change observed in post- as compared with pre- therapy lesions was in macrophages (CD16+CD68+): their number increased in post-therapy metastatic lesions with a perivascular distribution in intratumoral vessels (Fig S1, panels A, C vs. panels B and D).
Clinical outcome
Fifteen patients with the appropriate clinical eligibility features were screened for the expression of both class HLA-A*0201 and the NA17.A2 antigen or HLA-A1 and MAGE-3.A1. Eight patients showed the right combination of antigen expression and the HLA-Class I allele and were enrolled in the protocol. The group included 5 males and 3 females with a mean age of 52 and 39 years, respectively.
Toxicity
Four subjects died of progressive disease after having received the first cycle of combined therapy (i.e., NGR-hTNF and vaccine every other week for 4 times each). No serious systemic toxicity related to treatment was observed but Grade I-II constitutional symptoms (chills during infusion of NGR-hTNF) were reported in 4 patients. Of note, patients showing chills fared better than those who did not show such a toxicity, an observation that was already reported on a larger group of NSCLC patients, chills resulting as an independent efficacy factor. (15 Erythema, and/or induration at the site of vaccine injection was seen in 7 out of 8 patients; in 3 of these patients induration persisted after 6 months.
Tumor response and survival
No PR or CR were recorded; 4 patients (#02, 06, 07, 08), showed long-term (>4 months) OS (Table 2). TTP ranged from 1.3 to 26 months (mean 7.0 mos).
OS after the onset of the protocol (1st administration of NGR-hTNF) is shown in Table 2 with patients bearing loco-regional disease (#02, 06, 07, 08) surviving longer (mean 25.5 mos) as compared with the 4 subjects enrolled while in stage IV M1b or c (6.5 mos) (i.e., patients #01, 03, 04, 05). Median OS was not reached at 28 months of observation (data not shown) but we cannot exclude an effect of the selection of clinically favorable patients. Tumor progression and death was the main reasons for termination of the study for 4 patients (#01, 03, 04, 05).
Discussion
The results of this combination Phase I, pilot trial provide useful information concerning safety, T-cell responses and a potentially clinical response in metastatic, drug-resistant melanoma patients. To see whether combination therapy with MAA-based vaccine and the anti-vascular targeting agent NGR-hTNF could be safe and potentially effective in patients resistant or not eligible for the new drugs, we performed a Phase I, pilot study in metastatic melanoma patients whose tumor cells expressed either NA17.A2 or MAGE-3.A1 MAAs that are known to be recognized by T cells in HLA-A*0201 or HLA-A*01-restricted fashion.7-10
As previously reported, the administration of low dose NGR-TNF (0.8 μg/sqm) failed to show any major systemic toxicity. However, serum level of some pro-inflammatory cytokines known to favor immune cell recruitments like MIP-1β and MCP-1 did increase. Mild local reactions, as expected, were common at the site of vaccine injections. Thus, this biological drug combination was shown to be safe and well tolerated in all the treated subjects. A different combination approach (i.e., peptide-based vaccine and anti-VEGF) was used in a recent study of 9 advanced pancreatic cancer patients all showing a T-cell response against the vaccine with a trend for clinical benefit in 4 of them.19 A trial of tumor lysate loaded dendritic cell (DC)-based vaccination and the anti-angiogenic drug Bevacizumab was also recently conducted in ovarian cancer patients who additionally received adoptive immunotherapy of ex-vivo vaccine-primed T cells.20 Four of 6 patients developed an immune response and showed clinical benefit similar to our melanoma subjects, suggesting that an anti-vascular targeting and immunotherapy combination may be effective in treating advanced cancer patients.
All our 4 patients bearing loco-regional disease did show an increased T-cell recognition of both MAAs used for vaccination regimen and other MAAs possibly depending on antigen spreading phenomenon.21 It is of interest that some patients spontaneously mounted an immune response against MAAs, particularly against NA17.A2 that faded away with tumor progression during the NGR/Vax treatment. This observation may be explained by the strong immunogenicity of NA17.A2, an epitope that derives by altered splicing of an intron of the gene ɣ-acetylglucosaminyl-transferase V and is, therefore, foreign to the immune system. 7 In addition, NA17.A2 immunogenicity appears to be consistent since T-cell responses against NA17. A2 could be induced in melanoma patients immunized with DCs loaded with this peptide or with NA17.A2 tumor-derived apoptotic bodies22 and by different routes.23 Also the finding of an eclipse of T-cell reactivity against MAAs included in the vaccine, associated with the intermediate immunizations (weeks 11, 17) but reappearing after the late boosting (8 and 11 months) can be attributed to a transient sequestering of antitumor T cells in neoplastic tissues or at the site of peptide-Montanide injection24 and to different T-cell clone dynamics that may reduce their concentration in the blood.25 However, we could not test this hypothesis due to a lack of sufficient number and quality of melanoma lesions. When the serum concentration of pro-inflammatory cytokines was analyzed, we observed MCP-1/CCL2 and MIP-1 increased level during therapy as compared with baseline in all patients (Fig. 3). This may have caused a more intense infiltration of M1 antitumor macrophages rather then T cells in tumor lesions that were found in the 2 cases examined (#06, 08: see Fig. S1) resulting in a better tumor control as occurred in patients #02, 06, 07, 08 who showed only loco-regional tumor nodules and/or invaded lymph nodes.
The possible clinical benefit of this combined treatment was evident in at least 6 of 8 evaluable patients who experienced a long-term OS (>4 months from onset of treatment i.e. 7, 11, 23+ 25+, 25+, 29+ months) (see Table 2). Thus, it is possible that the combination of NGR-TNF and vaccines may induce synergistic effects due to a better tumor penetration by lymphocytes or other immune cells thanks to vessels disruption caused by NGR-hTNF in the microenvironment.
When the serum concentration of pro-inflammatory cytokines was analyzed, we observed MCP-1/CCL2 and MIP-1 increased levels during therapy as compared with baseline in all patients (Fig. 3), thus recapitulating what we have previously found in mouse models (13). These findings suggest that human melanoma lesions were also rapidly infiltrated by T cells. Our clinical trial was not designed to measure T-cell infiltrate in melanoma lesions 2 h after NGR-hTNF treatment. However, in mice we also investigated persistence and effector function of migrating tumor-specific T cells in NGR-TNF treated mice. While the absolute numbers of tumor-specific and IFNγ+ T cells quantified at 2 and 24 h in melanoma lesions were similar (13), their number rapidly declined thereafter (Calcinotto A. unpublished observations). Thus, we concluded that the effect of NGR-TNF is a precocious and rapid event (13). Because human melanoma lesions were obtained weeks after NGR-hTNF treatment, it came as no surprise that lymphocyte infiltration did not increase after treatment, aswe found in the mouse system. Nevertheless, IHC analyses were very informative, and showed a significant increase in infiltrating CD16+CD68+ macrophages post-therapy. While we do not believe that this could be attributed to a direct effect of NGR-hTNF, we speculate that such enrichment in infiltrating macrophages is the spillover of the intense inflammation induced by NGR-hTNF and subsequent T-cell infiltration. ”
In conclusion, the combination of NGR-hTNF and vaccine in metastatic melanoma patients was well tolerated, associated with an ex-vivo T-cell response and long terms OS. In our opinion then, these findings warrant confirmation in a larger group of patients.
Patients and Materials and Methods
Toxicity was evaluated by NCI Common Toxicity Criteria for Adverse Events.V.4 and the clinical outcome by RECIST.
Lymphocytes and tumor cell lines
PBMCs were obtained before, during and after treatment (see flow chart in Table S3) from patients with diagnosis of metastatic melanoma admitted for treatment to the San Raffaele Hospital, Milan, Italy. The MHC Class I typing of the patients was performed on their PBMCs by single-stranded oligonucleotide probe–PCR typing.26 The melanoma cell lines used in this study were established in vitro by our group from human melanoma tissues. Other cell lines used were the 1061 EBV-B (HLA-A*01), the 501 mel (a gift of Dr. Paul F. Robbins, Surgery Branch, NCI, Bethesda MD), and the T2 and K562 cells (commercially obtained from ATCC, Manassa, VA, USA). All the lines were cultured with RPMI 1640 plus 10% FBS and periodically checked for bacterial and Mycoplasma contaminations.
Isolation of MAA reactive T lymphocytes
An IFNγ-based ELISPOT assay (Mabtech, Nacka Strand Sweden) was used to measure the T cell reactivity to MAA-derived peptides and/or HLA-A matched melanoma cell lines. Briefly, 3 × 104 cells/well PBMCs () were incubated in flat bottom 96-well plates in the presence of 1.7 × 104cells/well of T2 (HLA-A*0201) or 1061 EBV-B (HLA-A*01) antigen presenting cells (APC) loaded or not with a single peptide or pools of peptides. The peptides and their sequence used in this study have been reported above and are listed in Table S1. PBMCs were also co-incubated with autologous, when available, (patients #02 and 07), or HLA-A-matched allogeneic melanoma cell lines. The specificity of T-lymphocyte recognition was assessed by inhibition of cytokine release after pre-incubation of the melanoma cells with 10 μg/mL each of the anti-HLA Class I mAb W6/32, and the anti-HLA Class II (DR) mAb L243. T lymphocytes incubated with mitogens, such as Con-A or PHA were the positive controls. Unstimulated T lymphocytes represented the negative control (background). K562 cells were used as target cells to detect NK-type reactivity in patients PBMCs. Results represent averages of triplicates, subtracted of the background, with SD ≤ 10%; statistical analysis of differences between means for cytokine release assays was performed using 2-tailed Student's t-test, with significance defined as p < 0.05.
Analysis of soluble factors in the serum of melanoma patients
Serum was collected from the peripheral blood of melanoma patients undergoing NGR/VAX treatment at baseline, and 2 h following the first (week 1) and the fourth (week 7) NGR-hTNF infusion. The following soluble factors were measured by ELISA: chromogranin A (CgA), VEGF, sTNF-R1 and -2, IL-1β, IL-6, IL-8, macrophage chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-1β. CgA was analyzed by ELISA as previously described.27 Human sTNF-R1, sTNF-R2, VEGF, IL-6, CCL8/IL-8, were detected using DuoSet® Kits from R&D Systems (Minneapolis,USA). CCL4/MIP-1β, CCL2/MCP-1 and IL-1β/IL-1F2 were analyzed by using Quantikine® ELISA Kits (R&D Systems). Statistical analysis of differences between means of soluble factors was performed by 2-tailed t-Test (P < 0.05).
Immunohistochemistry (IHC) analysis of melanoma lesions before and after therapy
Three patients, namely #06, 07 and 08 developed subcutaneous and/or lymph node metastases that were excised before and after the protocol treatment, providing the opportunity to analyze these lesions for immune cells infiltration by IHC. Thus the excised lymph nodes and cutaneous metastasis were evaluated with a panel of antibodies listed in Table S2S, with a polymer based diaminobenzidine (DAB) detection kit (Powervision, Novocastra, UK) in a BOND III automated immunostainer (Leica Biosystems), developed with DAB and counterstained with Heamatoxylin.
Trial design
This is a Phase I proof-of-principle pilot study aimed at determining safety, immune response and, as secondary objective, clinical response in high risk metastatic melanoma patients with measurable lesions or made disease-free by surgery, and treated with a vaccine including the HLA Class I-restricted melanoma peptides NA17.A2 and/or MAGE-3.A1 emulsified in Montanide in combination with the anti-vascular targeting agent NGR-hTNF (Molmed, Milano). Fourteen (14) evaluable patients were planned to be included with a protocol duration of 18 months. The first patient was enrolled on November 3, 2010. The trial closed earlier (last patient enrolled on November 2, 2011) for slow accrual due to new therapies that became available for the metastatic melanoma.
Eligibility criteria
After approval of the final NGR/Vax/01 protocol in June 2010, several new promising Phase II and III protocols became available in 2011 and 2012 for metastatic melanoma patients whose tumor cells expressed mutated genes (e.g., BRAF, MEK, c-Kit) along with immunomodulating agents like ipilimumab (Yervoy®).2,3 In case patients were not eligible for the above studies they were screened for the present protocol. Eligibility criteria included: 1. Histologically confirmed AJCC (modified) stage IV (M1a, M1b, M1c) or Stage IV disease-free metastatic melanoma; 2. Adult subjects of 18–70 years of age; 3. ECOG score 0–1; 4. Life expectancy of at least 6 months;. 5. Adequate organ function as assessed by a) hematologic test: Hb ≥ 10 mg/ dL; leukocytes ≥ 3000/ μL; lymphocytes ≥ 1000/ μL; absolute neutrophil count ≥ 1500/ μL; platelet count ≥ 100’000/ μL; b) Hepatic function: AST and ALT ≤ 4 times upper limit of normal (ULN); bilirubin ≤ 2 ULN; c) Renal function: creatinine ≤ 1.5 mg/ dL or creatinine clearance ≥ 60mL/ min; 6. Fertile females had to practice adequate contraception. 7. Patients had to sign written informed consent according to the institutional and national guidelines; HLA-A*0201- and/or HLA-A*01-positive typing on PBMCs; melanoma tissue expressing NA17.A2 and/or MAGE-3.A1.
Exclusion criteria. (a) Current other malignancy at other sites or previous other cancer within the last 5 years, (b) Presence of active brain metastases; (c) Concurrent chemotherapy or immunotherapy; (d) Prior chemotherapy, radiotherapy or immunotherapy, unless ended at least 4, 6, and 8 weeks, respectively before admission to the present study; (e) Significant cardiovascular diseases requiring medical intervention; (f) Primary or secondary immunodeficiency; (g) Active or chronic infection (including HIV, HBV, HCV); (h) Women pregnant or breast-feeding; (i) Simultaneous participation in another clinical trial.
Vaccine formulation and treatment schedule
The vaccine included either the NA17.A2 or the MAGE-3.A1 clinical grade peptide (manufactured by Bachem, Weil am Rhein, Germany; their use was approved by the National Regulatory Agency for Drugs of Human Use, AIFA) or both (in case of patient positive for both HLA-A alleles) emulsified in Montanide ISA (incomplete Seppic adjuvant) 51. Peptides were administered at the dose of 330 μg by subcutaneous route. Vaccination was given 4 times (1st cycle) at alternate weeks at different sites (deltoid or inguinal regions) in 2 mL volume in 2 different, closed sites (2–3 cm apart). Vaccination was then repeated at weeks 12, 16, 20 and boosted at 8 and 11 months afterwards (see Table S3). In case of no progression patients could receive additional boosts every 3–6 months at discretion of the principle investigator (see flow chart in Table S3). Only stage IV M1a, M1b, M1c (AJCC classification) and stage IV disease-free, i.e., high-risk melanoma patients, were considered for this trial.1
Once the clinical-pathological stage of patients was assessed, the standard work-up included total body CT and/or PET scan, and NA17.A2 and MAGE-3.A1 typing of tumor tissues by RT-PCR. Only HLA-A*0201 and/or HLA-A*01 subjects whose tumor express either NA17.A2 and/or MAGE-3.A1 were selected for the trial. Blood and plasma samples were withdrawn before, during and after treatment in order to assess antitumor immune response (see above) and the plasma level of angiogenesis/inflammation-related factors (see Table S3).
Primary efficacy parameters
The major objective of the study was safety and assessment of treatment-induced specific T-cell immunity against MAA-derived epitopes, including that contained in the vaccine, and/or expressed by tumor cells.
An immune response was considered “positive” based on the ex-vivo recognition of at least one MAA-derived epitope or of HLA-A*0201- or –A*01- positive melanoma cell lines and on a statistically significant increase in the number of spots comparing pre-vaccination values with those obtained 2 weeks or longer (+20 weeks and/or +8, +11 months) after the 1st and 2nd cycles of vaccinations. 50 mL of heparinized blood was withdrawn per sample, to be used for PBMCs isolation.
Secondary efficacy parameters
Tumor evaluation was performed on the following occasions (see flow chart in Table S3): at the pre-treatment visit (baseline evaluation); 1 month after the end of the 1st and 2nd cycle of vaccination (week 12 and 20, respectively); then tumor assessment was scheduled according to the guidelines of our department. Once progression of disease (PD) was documented by at least 2 assessments performed by the same technique at 4-week intervals, evaluations were performed as clinically indicated and the subject considered “off-study.” However, since immune response may take several weeks and even months to reach measurable levels in the blood, at the discretion of the principle investigator, vaccination boosts could be continued even in the presence of PD until deemed potentially useful for the patient.
Time to progression (TTP) and tumor response (according to RECIST) was measured from the time of first treatment (first NGR-hTNF administration) until the time at which there was evidence of PD.
Laboratory parameters were evaluated at the baseline (week -1), and at weeks 5, 8 (just before the last vaccination of the 1st cycle), 12 (before starting the 2nd cycle), 16 and 20 (the day of the last vaccination of the 2nd cycle), and, thereafter, at the day of every additional boosting vaccination (see Table S3). Then, laboratory parameters were scheduled according to the guidelines of the department (see page 16). Analysis of potential auto-immune reactions was performed by ELISA (Quanta Lite, Innova Diagnostics, Milan); anti-DNA, anti-thyroglobulin, anti-microsomal antibodies were measured by assessing antibody at week -1 and at week 9 and 20 after the last 2nd cycle vaccine dose, then at each boosting vaccination or as indicated by the principle investigator.
Post treatment assessment. Post-treatment clinical study evaluations (TTP, RECIST) were performed 4 weeks after the last vaccine dose of the 1st cycle (week 12) and after the last dose of the 2nd cycle of vaccination (week 20). The laboratory tests were those described above that included auto-antibodies and immunological assays.
Follow-up assessments
After the completion of treatment, in case of objective response or SD, subjects were offered continuation of the treatment at regularly scheduled intervals of every 3 months for the 2nd year and 6 months for the 3rd year, or until PD. After PD was observed, subjects were followed as clinically indicated. Survival duration was measured from initiation of study treatment and data cutoff for survival analysis was January 2014.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A Corti is the inventor of NGR-TNF (patent).
Acknowledgment
The procedures of this study were approved by the Internal Ethics Committee of San Raffaele Hospital on February 4, 2010 (code N. 49/932) and by the Italian Agency for Pharmacological Agents on April 19 and May 18, 2010 respectively and are in accordance with the Helsinki Declaration of 1975. The final authorization to start the protocol was released by the Ethics Committee of the San Raffaele Hospital on June 7, 2010. EUDRACT N. 2009-017818-67 Received.
Funding
This spontaneous protocol was financially supported by a Grant of the Alliance against Cancer (Italian Ministry of Health, Rome) to G.P.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001; 19:3622-34; PMID: [DOI] [PubMed] [Google Scholar]
- 2. Eggermont AMM, Robert C. New drugs in melanoma: it's a whole new word. Eur J Cancer 2011; 47:2150-57; PMID:; http://dx.doi.org/ 10.1016/j.ejca.2011.06.052 [DOI] [PubMed] [Google Scholar]
- 3. Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol 2013; 24:2174-80; PMID:; http://dx.doi.org/ 10.1093/annonc/mdt161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, Anichini A. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst 2002; 94:805-18; PMID:; http://dx.doi.org/ 10.1093/jnci/94.11.805 [DOI] [PubMed] [Google Scholar]
- 5. Rosenberg SA, Yang J, Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Med 2004; 10:909-15; PMID:; http://dx.doi.org/ 10.1038/nm1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartzentruber D, Lawson DH, Richards JM, Conry PM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. NEJM 2011; 364:2119-27; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1012863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guilloux Y, Lucas S, Brichard VG, Van Pel A, Viret C, De Plaen E, Brasseur F, Lethé B, Jotereau F, Boon T, et al. A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanoma is encoded by an intron sequence of the N-acetylglucosyltransferase V gene. J Exp Med 1996; 183:1173-83; PMID:; http://dx.doi.org/ 10.1084/jem.183.3.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brichard VG, Machiels J-P, Piperno S, Dorvai T. Peptide-based immunization against tumor-specific antigen NA17.A2 in HLA-A2 patients with metastatic cutaneous melanoma. Proc ASCO 2001; 20. [Google Scholar]
- 9. Gajewski TF, Fallarino F, Ashikari A, Sherman M. Immunization of HLA-A2+ melanoma patients with MAGE-3 or MelanA peptide-pulsed autologous peripheral blood mononuclear cells plus recombinant human interleukin 12. Clin Cancer Res 2001; 7:895s-901s; PMID: [PubMed] [Google Scholar]
- 10. Marchand M, Van Baren N, Weynants P, Brichard V, Drèno B, Tessier M-H, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer 1999; 80:219-30; PMID:; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 11. Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration.J Clin Invest 2002; 110:475-82; PMID:; http://dx.doi.org/ 10.1172/JCI0215223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dondossola E, Gasparri A, Colombo B, Sacchi A, Curnis F, Corti A. Chromogranin A restricts drug penetration and limits the ability of NGR-TNF to enhance chemotherapy efficacy. Cancer Res 2011; 71:5881-90; PMID: [DOI] [PubMed] [Google Scholar]
- 13. Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, Corti A, Bellone M. Targeting tumor necrosis factor–α to neo-angiogenesis vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol 2012; 188; 2687-94: www.jimmunol.org et al. 2012); PMID:; http://dx.doi.org/ 10.4049/jimmunol.1101877 [DOI] [PubMed] [Google Scholar]
- 14. Corti A, Curnis F, Rossoni G, Marcucci F, Gregorc V. Peptide-mediated targeting of cytokines to tumor vasculature: the NGR-hTNF example. Biodrugs, 2013; 27:591-60; http://dx.doi.org/ 10.1007/s40259-013-0048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gregorc V, De Braud FG, De Pas TM, Scalamogna R, Citterio G, Milani A, Boselli S, Catania C, Donadoni G, Rossoni G, et al. Phase I study of NGR-hTNF, a selective vascular targeting agent, in combination with cisplatin in refractory solid tumors. Clin Cancer Res 2011; 17:1964-72; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1376 [DOI] [PubMed] [Google Scholar]
- 16. Germeau C, Ma W, Schiavetti F, Lurquin C, Henry E, Vigneron N, Coulie PG, Boon T. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens, J Exp Med 2005; 201:249-57; PMID:; http://dx.doi.org/ 10.1084/jem.20041379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russo V, Pilla L, Lunghi F, Crocchiolo R, Greco R, Ciceri F, Maggioni D, Fontana R, Mukenge S, Rivoltini L, et al. Clinical and immunological responses in melanoma patients vaccinated with MAGE-3-genetically modified lymphocytes. Int J Cancer 2013; 132 (11): 2557-66; PMID:; http://dx.doi.org/ 10.1002/ijc.27939 [DOI] [PubMed] [Google Scholar]
- 18. Deshmane SL, Kremlev S, Amini S, and Sawaia BE. Monocyte chemoattractant protein 1(MCP-1): an overview. J Interf Cytokine Res 2009; 29; 313-26; http://dx.doi.org/ 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okuyama R, Aruga A, Hatori T, Takeda K, Yamamoto M. Immunological responses to a multi-peptide vaccine targeting cancer-testis antigens and VEGFRs in advanced pancreatic cancer patients. OncoImmunology 2013; 2:e27010; PMID:; http://dx.doi.org/ 10.4161/onci.27010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandalaft LE, Powell DJ, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stmulated T cells in recurrent ovarian cancer. Oncoimmunology 2013; 2:2e222664; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corbiére V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethé B, van Baren N, Van den Eynde BJ, Boon T, Coulie PG. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res 2011; 71:1253-62; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2693 [DOI] [PubMed] [Google Scholar]
- 22. Labarrière N, Bretaudeau L, Gervois N, Bodiner M, Bougras G, Diez E, Lang F, Gregoire M, Jotereau F. Apoptotic body-loaded dendritic cells efficiently cross-prime cytotoxic T lymphocytes specific for NA17-A antigen but not for Melan-A/MART1 antigen. Int J Cancer 2002; 101:280-6; http://dx.doi.org/ 10.1002/ijc.10605 [DOI] [PubMed] [Google Scholar]
- 23. Lesimple T, Neidhard E-M, Vignard V, Lefeuvre C, Adamski H, Labarrière, Carsin A, Monnier D, Collet B, Clapisson G, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res 2006; 12:7380-8; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1879 [DOI] [PubMed] [Google Scholar]
- 24. Hailemichael H, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang X-F, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induced tumor-specific CD8+T cell sequestration, dysfunction and deletion. Nature Med 2013; 19:465-71; PMID:; http://dx.doi.org/ 10.1038/nm.3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lurquin C, Lethé B, De Plaen E, Corbiere V, Théate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med 2005; 201:249-57; PMID:; http://dx.doi.org/ 10.1084/jem.20041378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tiercy JM, Djavad N, Rufer N, Speiser DE, Jeannet M, Roosnek E. Oligotyping of HLA-A2, -A3 and B44 subtypes. Detection of subtypes incompatibilities between patients and their serologically matched unrelated bone marrow donors. Human Immunol 1994; 41:207-15; http://dx.doi.org/ 10.1016/0198-8859(94)90038-8 [DOI] [PubMed] [Google Scholar]
- 27. Crippa L, Bianco M, Colombo B, Gasparri AM, Ferrero E, Loh YP, Curnis F, Corti A. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 2013; 121:392-402; PMID:; http://dx.doi.org/ 10.1182/blood-2012-05-430314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.