Abstract
The human epidermal growth factor receptor 3 (HER-3/ErbB3) is a unique member of the human epidermal growth factor family of receptors, because it lacks intrinsic kinase activity and ability to heterodimerize with other members. HER-3 is frequently upregulated in cancers with epidermal growth factor receptor (EGFR/HER-1/ErbB1) or human epidermal growth factor receptor 2 (HER-2/ErBB2) overexpression, and targeting HER-3 may provide a route for overcoming resistance to agents that target EGFR or HER-2. We have previously developed vaccines and peptide mimics for HER-1, HER-2 and vascular endothelial growth factor (VEGF). In this study, we extend our studies by identifying and evaluating novel HER-3 peptide epitopes encompassing residues 99–122, 140–162, 237–269 and 461–479 of the HER-3 extracellular domain as putative B-cell epitopes for active immunotherapy against HER-3 positive cancers. We show that the HER-3 vaccine antibodies and HER-3 peptide mimics induced antitumor responses: inhibition of cancer cell proliferation, inhibition of receptor phosphorylation, induction of apoptosis and antibody dependent cellular cytotoxicity (ADCC). Two of the HER-3 epitopes 237–269 (domain II) and 461–479 (domain III) significantly inhibited growth of xenografts originating from both pancreatic (BxPC3) and breast (JIMT-1) cancers. Combined therapy of HER-3 (461–471) epitope with HER-2 (266–296), HER-2 (597–626), HER-1 (418–435) and insulin-like growth factor receptor type I (IGF-1R) (56–81) vaccine antibodies and peptide mimics show enhanced antitumor effects in breast and pancreatic cancer cells. This study establishes the hypothesis that combination immunotherapy targeting different signal transduction pathways can provide effective antitumor immunity and long-term control of HER-1 and HER-2 overexpressing cancers.
Keywords: Antibodies, HER-1, HER-2, HER-3 (erbb3), IGF-1R, immunogenicity, Immunotherapy, peptidomimetics, peptide vaccines, receptor tyrosine kinases
Abbreviations: ADCC, antibody dependent, cellular cytotoxicity; ECD, extracellular domain; ELISA, enzyme-linked immunosorbent assay; FDA, Federal Drug Administration; HER-1 (EGFR or ErbB1), human epidermal growth factor receptor; HER-2 (ErbB2), human epidermal growth factor receptor 2; HER-3 (ErbB3), human epidermal growth factor receptor 3; HER-4 (ErbB4), human epidermal growth factor receptor 4; HPLC, high-pressure liquid chromatography; mAb, monocolonal antibody; MALDI, matrix-assisted laser desorption/ionization; MVF, Measles virus fusion protein; RTK, receptor tyrosine kinase; TKIs, Tyrosine kinase inhibitors.
Introduction
The human epidermal growth factor receptor (HER) family consists of four homologous members: HER-1 (also known as EGFR/ErbB1), EGFR) HER-2, HER-3 and HER-4 (also known as ErbB2, ErbB3 and ErbB4, respectively).1-3 The general structure shared by HER receptors includes an extracellular ligand-binding domain (ECD), a transmembrane region, and an intracellular domain containing a tyrosine kinase and carboxy-terminal region.3 In response to ligand binding, these receptor tyrosine kinases (RTK) can form homodimers or heterodimers and initiate a complex network of cellular signaling that mediates many cellular processes, such as proliferation, differentiation, migration and survival.4-6 HER family receptors are also involved in tumorigenesis when their signaling functions are inefficiently regulated.1-3,7 In particular, EGFR and HER-2 are widely known targets for cancer therapy, with both monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs) directed against these receptors.2,8,9 Despite their structural homology, the HER family members differ with respect to preferred ligands, affinity for ligands, tyrosine kinase activity, and rate of cellular down regulation.10
HER-3 is the only member of the family lacking intrinsic tyrosine kinase activity.11-13 As a result, its role in cancer has long been underestimated and efforts at targeting HER-3 have lagged behind. HER-3 is overexpressed in several human cancers which correlates with tumor progression with worse survival in patients with breast,14 pancreatic 15 and colorectal carcinomas.16 Recent studies have demonstrated that EGFR and HER-2 are the preferred heterodimerization partners for HER-3, and HER-3 is frequently up-modulated in cancers with EGFR or HER-2 over-expression.14,17-19 There is increasing evidence of the importance of HER-2/HER-3 signaling in breast cancer which is also supported by numerous transgenic mouse models.20 HER-3 signaling has also been associated with resistance to: HER-2 inhibitors in HER-2-amplified breast cancers,21-23 EGFR inhibitors in lung cancers,24 pertuzumab resistance in ovarian cancers,25 anti-estrogen therapies in ER-positive breast cancers,25-28 EGFR inhibitors in head and neck cancer, and hormone resistance in prostate cancers.29 Currently, there are no FDA approved therapies that target HER-3, but several mAbs are under investigation, including MM-121 and AMG 888 (U3–1287).30-33 These mAbs have been shown to inhibit tumor cell proliferation in vitro and in vivo, and both antibodies are being evaluated in clinical trials. In addition to HER-3, induction of complex crosstalk with alternate signaling pathways has also been observed in drug resistance to HER family inhibitors. Recent studies have shown that resistance to trastuzumab is mediated by increased signaling and crosstalk through insulin-like growth factor 1 receptor (IGF-IR) and VEGF.28,34-36 Promising and new alternative strategies taken to overcome drug resistance include combination therapy and development of multi-target inhibitors.37 For instance, HER-3 mAbs currently under investigation have been shown to act synergistically with EGFR/HER-2 inhibitors, suggesting that combination treatment may be essential to completely shut-down HER family signaling.33,38 In addition, dual-specific antibodies against HER-2:HER-3 or EGFR:HER-3 heterodimers are also being evaluated.39-41 Thus, strategies to block HER-3 heterodimerization must be at the forefront of any attempt to overcome drug resistance to approved targeted therapies and to develop novel combination treatments.
The main objectives of this study were (1) to identify B-cell epitopes of the HER-3 extracellular domain that could activate the immune system to produce highly specific antibodies that will target tumor cells; and (2) to develop HER-3 peptide mimics that could disrupt HER-3 signaling pathways by preventing ligand binding or heterodimerization. The driving motivation and overarching goal behind these studies rests upon the hypothesis that combination immunotherapy targeting different signal transduction pathways will provide synergistic effective antitumor immunity, tumor regression and long-term control of HER-2 overexpressing cancers. To test this hypothesis we used these novel HER-3 peptides and vaccines in a combination treatment strategy with inhibitors of HER-1, HER-2 or IGF-1R.
HER-3 crystal structures in complex with three mAbs DL11, LMJ716 and RG7116, were used to identify HER-3 amino acid residues involved in binding to the antibodies.40,42,43 We combined the computer predictive algorithms of antigenicity44 together with information gleaned from the crystal structure complexes to identify four HER-3 peptides encompassing residues 99–122 and 140–162 from Domain I, 237–269 from Domain II and 461–479 from Domain III as potential B-cell epitopes/mimics for active immunotherapy (vaccination) against HER-3 positive cancers. We hypothesized that these HER-3 peptide vaccines/mimics could be used to target the receptor in cancer and in a combination approach with our other HER-family established inhibitors. We show that the HER-3 vaccine antibodies and HER-3 peptide mimics induced antitumor responses: inhibition of cancer cell proliferation, inhibition of receptor phosphorylation, induction of apoptosis and ADCC. The peptidomimetics and vaccine antibodies also significantly inhibited growth of xenografts originating from both pancreatic and breast cancers. We also showed synergistic effects of combination treatment with the HER-3 (461–471) epitope with two HER-2 (266–296) and HER-2 (597–626) vaccine antibodies and IGF-1R (56–81) vaccine antibodies in vitro.
Results
Design, synthesis and characterization of (1) HER-3 peptide mimics; and (2) MVF-HER-3 peptide vaccine antibodies
The crystal structure of the unliganded HER-3 extracellular domain was published by Cho et al.8 A ribbon diagram showed that Domains I and III exhibit the expected helical structure and domains II and IV are extended repeats of seven small disulfide-containing modules. Since then, several other crystal structures of the HER-3 extracellular domain have been solved in complex with therapeutic mAbs. The crystal structure of the HER-3 extracellular domain in complex with the Fab portion of a mAb MEDHD7945A (DL11)40 revealed the residues of HER-3 that were important for binding. The residues critical for DL11 and HRG binding are found in Domain III of HER-3 showing that DL11 has the ability to block HRG: HER-3 interaction and inhibit downstream signaling of HER-3. With the information of the critical DL11 binding region playing a significant role in HER-3 signaling, we selected peptide sequence 461–479 which directly overlaps the DL11:HER-3 binding site. The HER-3 461–479 epitope includes part of a β-strand and α-helix in domain III of HER-3 (Fig. S1). Recently, two additional crystal structure complexes were solved for HER-3 binding to mAbs RG7116 and LJM716.42,43 The RG7116: HER-3 complex demonstrates that binding occurs in domain I of the HER-3 ECD. RG7116 inhibits ligand binding and subsequent phosphorylation of HER-3. We selected two HER-3 peptides overlapping this binding region: sequences 99–122 and 140–162. In the native HER-3 protein, residues 99–122 fold into an α-helix followed by a random coil, while the 140–162 peptide sequence contains two anti-parallel β-sheets separated by a random coil (Fig. S1). The complex of HER-3 binding to LJM716 demonstrated that the antibody binds to domains II and IV of the HER-3 extracellular region. LMJ716 was shown to lock HER-3 into an inactive conformation and inhibit downstream signaling of the receptor. The residues encompassing 237–269 sequence overlap with the LJM716:HER-3 binding site, and this epitope folds into two anti-parallel β-sheets separated by a random coil in domain II of the HER-3 ECD (Fig. S1). All HER-3 peptide locations within the native protein are shown in Figure S2. The four chosen epitopes were synthesized by solid-phase peptide chemistry either as the peptide mimic or as the chimeric peptide vaccine incorporating the measles virus fusion protein (MVF residues 288–302) “promiscuous” T-cell epitope.45 Based on our initial screening only two of our selected peptides (HER-3 237–269 and HER-3 461–479) were synthesized as chimeric immunogen with the MVF sequence. Table 1 shows the peptide sequences and their corresponding molecular weights.
Table 1.
The amino acid sequences of the synthetic peptide mimics and the chimeric peptides incorporating the MVF promiscuous T-cell epitopes with their molecular weights are depicted. Also shown are the amino acid sequences of the HER-1, HER-2 and IGF-1R peptides
| Peptides | Amino acid sequence of HER-3 peptides | Mol.wt (Da) |
|---|---|---|
| MVF-HER-3(237–269) | KLLSLIKGVIVHRLEGVE-GPSL-VPRCPQPLVYNKLTFQLEPNPHTKYQYGGVCVA-OH | 6099 |
| HER-3 (237–269) | Ac-VPRCPQPLVYNKLTFQLEPNPHTKYQYGGVCVA-OH | 3801 |
| MVF-HER-3 (461–479) | KLLSLIKGVIVHRLEGVE-GPSL-ERLDIKHNRPRRDCVAEGK-NH2 | 4672 |
| HER-3 (461–479) | Ac-ERLDIKHNRPRRDCVAEGK-NH2 | 2333 |
| HER-3 (99–122) | Ac-FVMLNYNTNSSHALRQLRLTQLTE-NH2 | 2891 |
| HER-3 (140–162) | Ac-DTIDWRDIVRDRDAEIVVKDNGR-NH2 | 2798 |
| Peptides | Amino acid sequence of EGFR (HER-1), HER-2 and IGF-1R peptides | Mol.wt(Da) |
| HER-1(418–435) | Ac-SLNITSLGLRSLKEISDG-NH2 | 1944 |
| MVF-HER-1(418–435) | KLLSLIKGVIVHRLEGVE-GPSL-SLNITSLGLRSLKEISDG-NH2 | 4242 |
| MVF-HER-2(597–626) | KLLSLIKGVIVHRLEGVE-GPSL-VARCPSGVKPDLSYMPIWKFPDEEGACQPL-NH2 | 5672 |
| MVF-HER-2(266–296) | KLLSLIKGVIVHRLEGVE-GPSL-LHCPALVTYNTDTFESMPNPEGRYTFGASCV-COOH | 5757 |
| IGF-1R 56–s81 | Ac-LLFRVAGLESLGDLFPNLTVIRGWKL-NH2 | 2969 |
Expression of various receptor tyrosine kinases in cancer cell lines
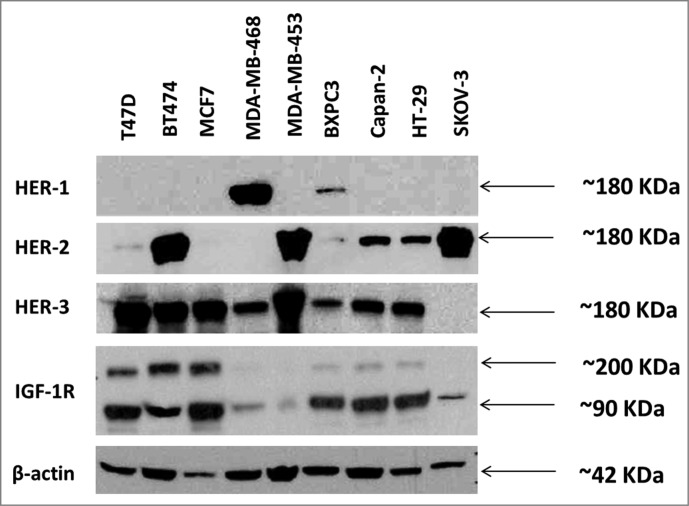
In order to evaluate HER-3 expression in different cancer cells and compare their expression levels with other HER family receptors and IGF-1R, we used protein immunoblotting assays with secondary antibodies that are specific to each of the receptors. Cell lines used included breast (BT-474, MDA-MB-468, MDA-MB 453, T47D, MCF-7), pancreatic (BxPC-3, Capan-2), ovarian (SKOV-3) and colon (HT-29). Western blotting for total HER-3, HER-1, HER-2 and IGF-1R (R & D Systems) was performed to determine total protein expression in cancer cell lines (Fig. 1). HER-3 expression was detected in all cell lines except SKOV-3 cells. HER-1 expression was only detected in MDA-MB-468 cells and BxPC3 cells. High levels of HER-2 were detected in BT474, MDA-MB-453 and SKOV-3 cells. Lower levels of HER-2 were detected in Capan-2 and HT-29 cells. IGF-1R expression was detected in all cell lines, but low amounts were found in MDA-MB-468, MDA-MB-453 and SKOV-3 cells (Fig. 1). Overall, the results indicate that HER-3 expression is predominant in most cancers and is always expressed together with other HER family receptors and/or IGF-1R. This explains why all the cell lines that show HER-3 expression also show a major expression of another HER receptor or IGF-1R.
Figure 1.
Western blot analysis of the expression of human epidermal growth factor receptors (HER) and insulin-like growth factor 1 receptor (IGF-1R) in various cancer cell lines. Cells were grown in 6 wells plates to 70–80% confluency prior to cell lysis. Cell lysates were solved in SDS-PAGE, transferred to PVDF membranes and commercial rabbit antibodies for HER-1 (epidermal growth factor receptor) (cell signaling), HER-2 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2), (cell signaling), HER-3 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 3) (Santa Cruz) and IGF-1R (insulin-like growth factor 1 receptor) (cell signaling) were used to probe for expression of the different receptors. A goat anti-rabbit IgG HRP secondary antibody and ECL reagents (Bio-Rad) were used for detection.
Peptide mimics inhibit cancer cell proliferation
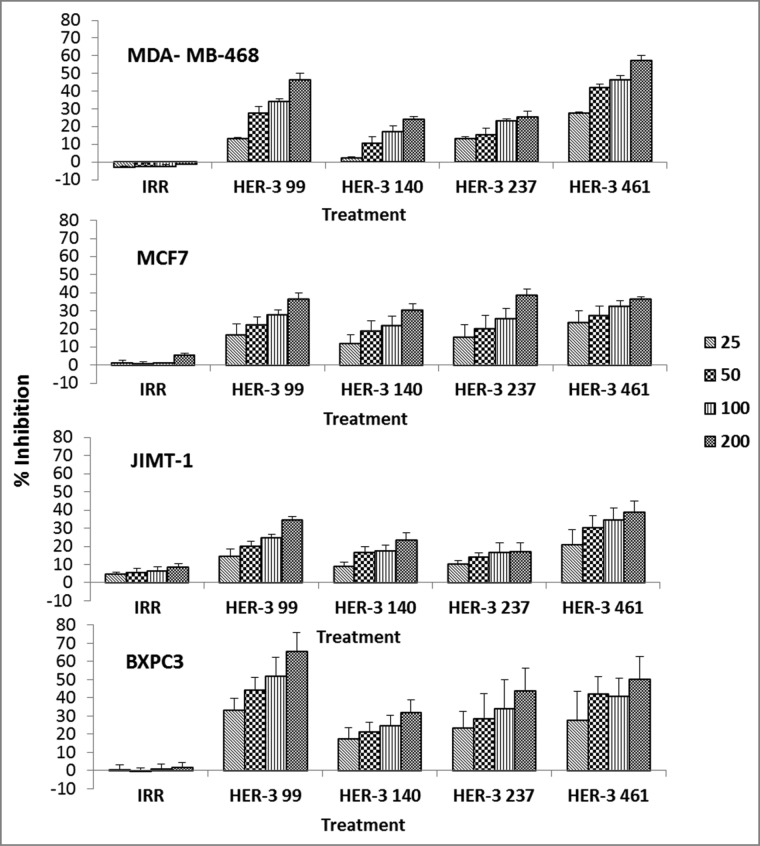
To test the ability of the peptide mimics to elicit antitumor effects, HER-3 positive cells were treated with the peptide mimics and examined in a MTT inhibition assay. The anti-proliferative effects of the peptides were tested against breast (JIMT-1, MCF7, MDA-MB-468) and pancreatic (BXPC3) cancer cells at various concentrations (Fig. 2). Taxol, an inhibitor of mitosis, was used as a positive control (data not shown). Results from the four different cell lines revealed that the HER-3 peptides inhibited proliferation of all cell lines in a dose-dependent manner. The most robust response was observed when all cell lines were treated with the HER-3 99–122 and the HER-3 461–479 peptides, and the cell lines that were most responsive to treatment were the MDA-MB-468 and BXPC3 cells.
Figure 2.
HER-3 peptide mimics inhibit proliferation of HER-3 positive cancer cell lines. Cancer cells (MDA-MB-468, MCF-7, JIMT-1 and BXPC3) were treated with HER-3 peptide mimics at different concentrations (25–200 μg/mL) for 1 h prior to ligand stimulation with HRG (50ng/mL). After 72 h of incubation in the presence of the peptide mimics, MTT was used to measure cell proliferation. Percent inhibition was calculated by taking absorbance (abs) readings at 570 nm and using the following equation: (abs. untreated-abs. treated)/abs. untreated × 100. An irrelevant (IRR) peptide was used as a negative control. Values represent the mean of at least three independent experiments in triplicate (n = 3) with error bars that indicate SEM.
Peptide mimics inhibit phosphorylation of HER-3
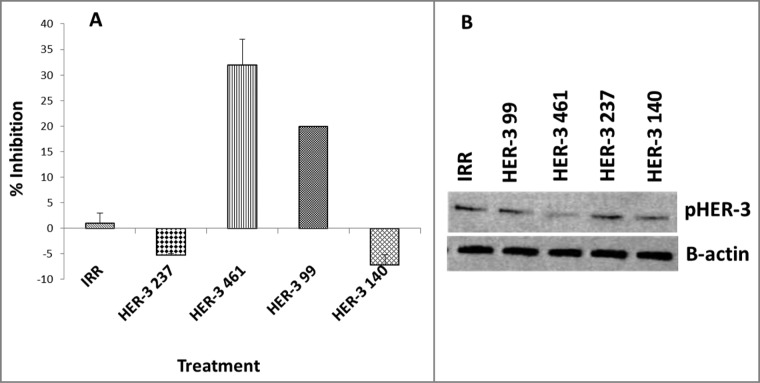
To test the ability of the peptide mimics to block signaling of the HER-3 protein, cells were treated with the inhibitors and phosphorylated HER-3 was measured using the Human phoshoErbb3 ELISA kit (R&D systems) and western blotting (Fig. 3). MDA-MB-468 cells were treated with the peptide mimics for 1 h prior to ligand stimulation and cell lysis. Phosphorylated levels of HER-3 were measured with a human-phospho-HER-3 sandwich ELISA Kit (R + D systems) and by western blotting using a commercial rabbit anti-phospho-HER-3 antibody (cell signaling). No inhibition of phosphorylation was observed when cells were treated with the HER-3 (140–162) and HER-3 (237–269) constructs. The HER-3 (99–122) and HER-3 (461–479) peptides inhibited phosphorylation of HER-3 (∼20% and ∼30%, respectively). Western blotting results from HER-3 phosphorylation also indicated a decrease in phosphorylation levels of HER-3 with the HER-3 99 and HER-3 461, which confirms results already obtained with the ELISA. Both results indicate that these two HER-3 epitopes were able to block receptor phosphorylation either by blocking HER-3 ligand binding, which prevents HER-3 activation, or by blocking receptor heterodimerization with other HER family of receptors.
Figure 3.
HER-3 peptide mimics inhibit phosphorylation of HER-3 positive cancer cell lines (MDA-MB-468). Cancer cells were treated for 1 h prior to ligand stimulation with 10 ng/mL HRG for 15 min. After treatment, cells were lysed 1X in RIPA lysis buffer (Santa Cruz) and phosphorylated HER-3 was measured via a phosphor-HER-3 ELISA kit from R+D Systems (A). Percent inhibition was calculated by taking absorbance (abs) readings at 450 nm and using the following equation: (abs. untreated-abs. treated)/abs. untreated × 100. Results displayed are representative of two independent experiments with duplicate samples. Error bars represent SD of the mean. To confirm ELISA results, cell lysates were also subjected to western blotting. Lysates were solved in SDS-PAGE, transferred to PVDF membrane and probed for expression of pHER-3with a commercial phosphor-tyr-HER-3 antibody from cell signaling (B). Results displayed are representative of two independent experiments (n = 2). In all experiments, an irrelevant peptide was used as a negative control.
Immunogenicity of the MVF HER-3 peptide constructs and cross-reactivity of vaccine antibodies to human HER-3
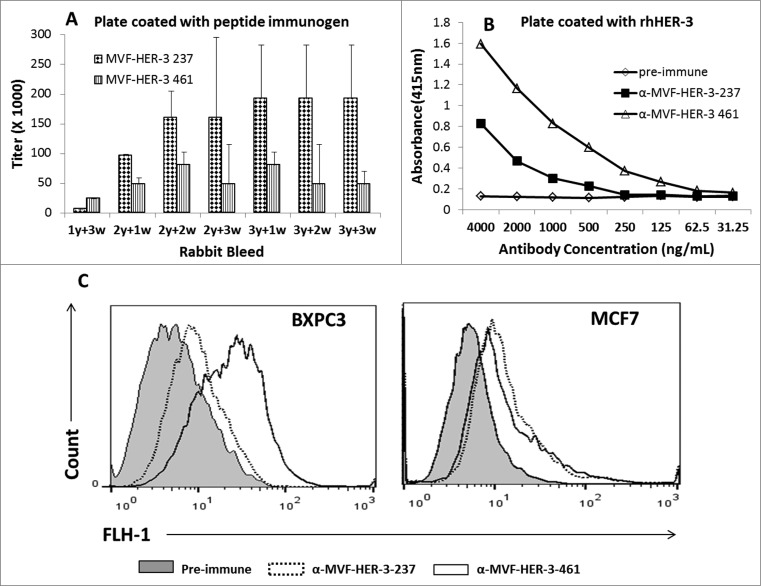
We determined the immunogenicity of the chimeric peptide vaccines MVF-HER-3 (237–269) and MVF-HER-3 (461–479) in outbred New Zealand rabbits. Pairs of rabbits were immunized with the chimeric peptide vaccines emulsified with nor-MDP adjuvant in SEPPIC ISA 720. The MVF-HER-3 237–269 and MVF-HER-3 461–479 peptides were highly immunogenic with antibody titers for MVF-HER-3 (461–479) ≥128,000, and for MVF-HER-3 (237–269) >256,000 (Fig. 4A). High antibody titers were also elicited against the B-cell epitope constructs HER-3 (461–479) and HER-3 (237–269) antibody titers were ≥ 16,000 and ≥ 128,000, respectively (data not shown). The polyclonal antisera to the peptides were purified and the ability of HER-3 anti-peptide antibodies to recognize and bind to recombinant human HER-3 protein was tested in an ELISA (Fig. 4B). The MVF HER-3 anti-peptide antibodies were able to specifically recognize their respective epitopes in a dose-dependent manner. We also tested the ability of the HER-3 vaccine antibodies to bind to the surface of HER-3 positive cancer cells. Flow cytometric analysis was conducted with HER-3 expressing BXPC3 and MCF7 cells (Fig. 4C). Pre-immune antibodies were used as the negative control. The antibodies elicited by the MVF-HER-3-237 and MVF-HER-3-461 constructs bound to the HER-3 protein expressed by the cell lines (Fig. 4) suggesting that the HER-3 anti-peptide antibodies recognize the native protein.
Figure 4.
Immunogenicity of MVF HER-3 peptides and ability of anti-HER-3 vaccine antibodies to recognize recombinant and native HER-3 receptor. Rabbits (n = 2) were immunized with either MVF HER-3 237–269 or MVF HER-3 461–479 every three weeks for a total of three immunizations. Anti-peptide antibody titers were determined by ELISA with plates coated with the appropriate MVF HER-3 peptide immunogen (A). Titers are defined as the reciprocal of the highest dilution of sera that gave an absorbance reading above 0.2 after subtracting the pre-immune sera. Results represent the average titer for two rabbits with error bars representing the SD of the mean. Anti-HER-3 peptide antibodies were purified from high titered rabbit anti-serum on a protein A/G column. The ability of the peptide specific antibodies to recognize recombinant HER-3 was determined by ELISA (B). Results are representative of three independent experiments with duplicate samples (n = 2). Direct binding of the anti-peptide antibodies to the native HER-3 receptor was determined by flow cytometric analysis with BXPC3 and MCF7 cells (C). Cells were treated with 50 ug/mL anti-HER-3 antibodies for 2 h. HER-3 binding was detected with goat anti-rabbit IgG-Alexa fluor 488 secondary antibody. Cells were analyzed on a BD FACS Calibur system. Pre-immune serum was used as a negative control.
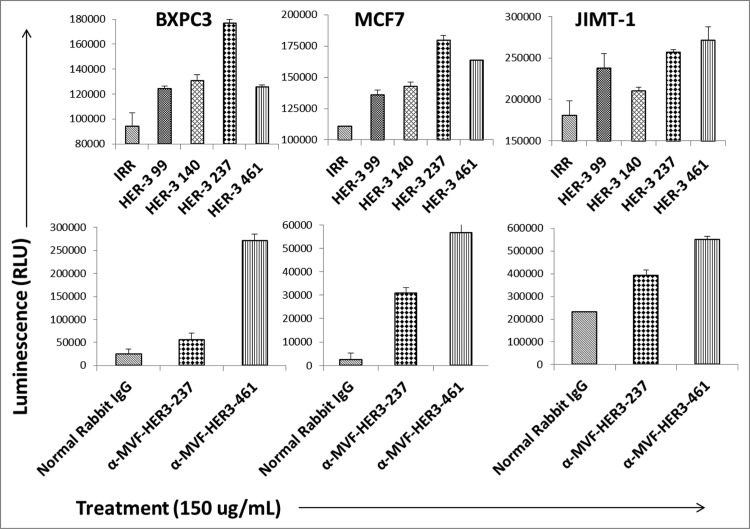
HER-3 peptide mimics and HER-3 vaccine antibodies induce apoptosis
Targeting apoptotic regulatory pathways in cancer is a promising strategy for therapeutic agents. The ability of our peptide mimics and vaccine antibodies to induce apoptosis was tested using the Caspase 3/7 Glo kit (Promega). MCF7, BXPC3 and JIMT-1 cells were treated with the inhibitors for 24 h prior to measuring activity of caspases 3 and 7. The results obtained showed a significant increase in the amount of caspase 3 and 7 activity when compared to the negative control (normal rabbit IgG or IRR peptide), indicating that the HER-3 peptide mimics and vaccine antibodies can induce apoptosis (Fig. 5). The highest amount of apoptosis was observed when cells were treated with the HER-3 461 and 237 peptide mimics (Fig. 5, top panel). Antibodies raised to these two peptides also caused substantial apoptosis (Fig. 5, bottom panel). Treatment caused approximately a two-fold increase in the amount of caspases 3/7 released, indicating increased apoptosis or cell death.
Figure 5.
HER-3 peptide mimics induce apoptosis. HER-3 positive breast (JIMT-1 and MCF7) and pancreatic (BXPC3) cancer cells were plated in 96-well plates and treated with the peptide mimics (top panel) or vaccine antibodies (bottom panel) for 24 h prior to cell lysis. Apoptosis (directly proportional to amount of luminescence produced) was measured using the Caspase Glo 3/7 kit (Promega). After 24 h treatment, caspase glo reagent was added, and plates were incubated for 3 h before being read on a luminometer. Pre-immune antibodies and an irrelevant (IRR) peptide were both used as negative controls. Results are representative of three independent experiments with duplicate samples (n = 2). Error bars represent SD of the mean.
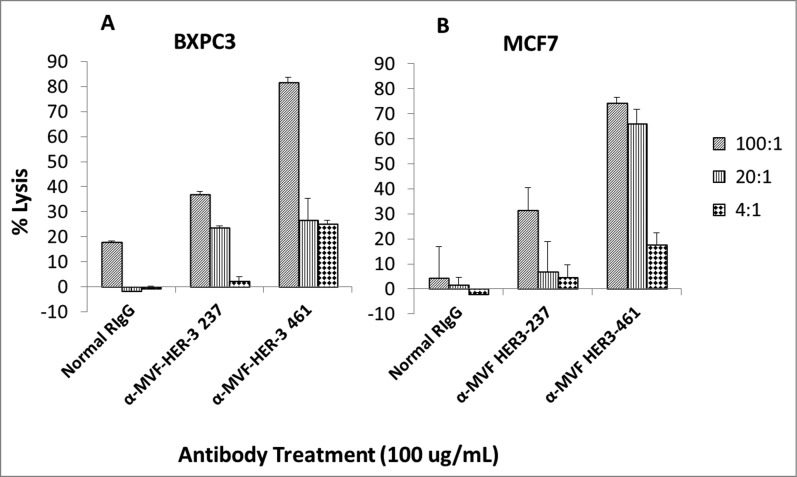
HER-3 vaccine antibodies mediate ADCC
Antibodies can exert their antitumor effects via ADCC, because the Fc region can interact with peripheral blood mononuclear cells (PBMCs) and attract them to specific targets. As a result, we examined the ability of the HER-3 vaccine antibodies to induce ADCC against various cancer cell lines (Fig. 6). Target cells (MCF7 and BXPC3) were treated with the vaccine antibodies and human PBMCs at different concentrations (effector cells). Cell lysis was measured using a bioluminescence cytotoxicity assay kit (cell technology). A significant increase in cell lysis was observed following treatment with the HER-3 461 vaccine antibodies in both the pancreatic and breast cancer cell lines, and the effects were greater when an effector to target ratio of 100:1 was used. These results suggest that the vaccine antibodies are capable of stimulating PBMCs to cause cancer cell death (ADCC).
Figure 6.
MVF HER-3 antibodies have the ability to elicit ADCC. HER-3 positive cancer cells BxPC3 (A) and MCF7 cells (B) were used as target cells. Target cells were seeded and incubated in the presence of human PBMCs at different effector: target cell ratios (100:1, 20:1, 4:1). Cells were then treated for one hour with MVF HER-3 antibodies (100 μg/mL) prior to cell lysis. The Acella-tox kit (Cell Technology) was used to measure the relative amount of ADCC and cell lysis was measured according to the manufacturer's instructions. Results represent average of three different experiments (n = 3)) and display the % lysis of treatment groups when compared to 100% target cell lysis. . Normal rabbit IgG (Pierce) was used as a negative control.
Antitumor effects of HER-3 peptide mimics/vaccine antibodies in two transplantable cancer mouse models (BALB/c SCID mice and BXPC3 cells or JIMT-1 cells)
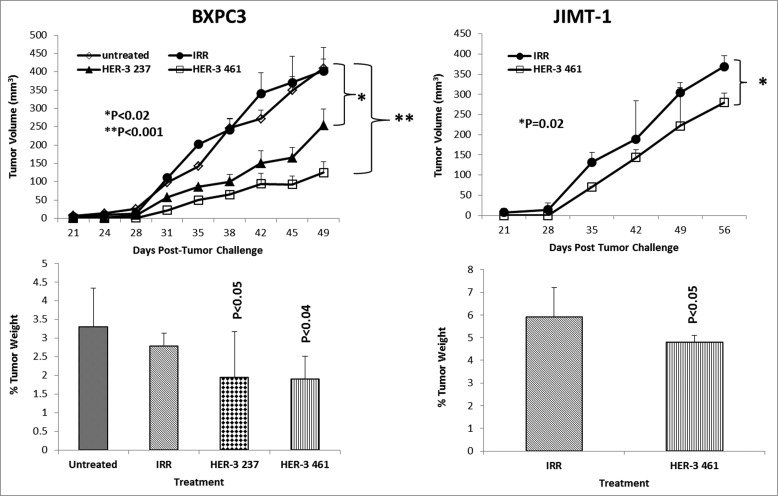
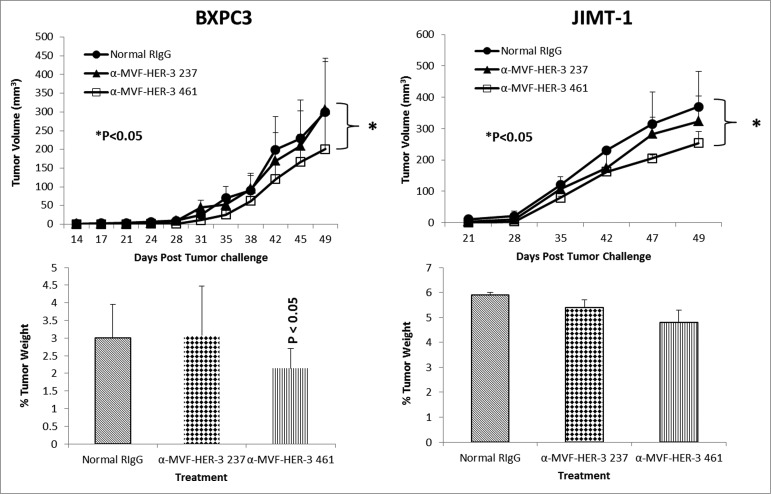
To test the in vivo effects of the peptide mimics and peptide vaccine antibodies, two epitopes (HER-2 237–269 and HER-3 461–479) were used in two transplantable mouse models. Mice were challenged with either BXPC3 or JIMT-1 cells and treated with the peptide mimics or vaccine antibodies starting at day zero (day of tumor challenge) and weekly for a total of 8–9 treatments. Tumor growth was measured biweekly, and all mice were euthanized at the end of treatment; tumors were extracted and weighed, and the percentage of tumor weight was calculated. The results in Fig. 7 demonstrate that both peptide constructs HER-3 (237–269) and HER-3 (461–479) had the ability to significantly delay tumor growth in mice challenged with BXPC3 cells (P < 0.2 and P < 0.001, respectively). The HER-3 (461–479) peptide construct showed greater antitumor effects and significantly decreased the % tumor weight (P < 0 .04). As a result, only the HER-3 461–479 peptide was used in JIMT-1 tumor model (Fig. 7). The HER-3 (461–479) peptide also had the ability to significantly inhibit tumor growth in our breast cancer mouse model (P = 0.02). We also used the BALB/c SCID mice BXPC3 and JIMT-1 cell tumor models to explore the ability of the vaccine antibodies to delay tumor growth (Fig. 8). After tumor challenge, mice were passively treated weekly with the vaccine antibodies, and tumor volume was measured biweekly. Fig. 8 shows the effect of theHER-3 anti-peptide antibodies in both mouse models. In both the BXPC3 and JIMT-1 transplantable mouse models, only the MVF-HER-3 461 vaccine antibodies resulted in a significant reduction in tumor growth and development (P < 0.05) (Fig. 10, top). Treatment with the MVF-HER-3 461 vaccine antibodies also demonstrated a significant reduction of tumor weight in BXPC3 xenografts (Fig. 10, bottom) thereby confirming the best HER-3 epitope being the HER-3 (461–479) that could be used in the combination studies.
Figure 7.
HER-3 peptide mimics delay tumor growth in 2 transplantable mouse models. SCID Mice (n = 5) at the age of 5–6 weeks old were challenged subcutaneously with either BXPC3 cells or JIMT-1 cancer cells and treated weekly with the indicated peptide mimics (200 μg) intravenously. Tumor growth was monitored over time. After euthanasia, tumors were extracted and weighed. Results displayed include average tumor volume over time (top panel) and % weight tumors when compared to total mouse weight (bottom panel). Error bars represent standard deviations from the mean. An irrelevant peptide (IRR) was used as a negative control. In BXPC3 mouse model, peptide treatment caused a significant delay in tumor growth with both peptide constructs HER-3 237–269 (p < 0.02) and HER-3 461–479 (p < 0.001), and the effects were also observed in percentage tumor weight per body mass; HER-3 237–269 (p < 0.05) and HER-3 461–479 (p < 0.04). Only the HER-3 461–479 construct was used in JIMT-1 mouse model, and it caused both a significant delay in tumor growth and % tumor weight (p = 0.02 and p < 0.05, respectively).
Figure 8.
HER-3 vaccine antibodies delay tumor growth in a transplantable mouse model. SCID Mice (n = 5) were challenged subcutaneously with either pancreatic (BXPC3) or breast (JIMT-1) cancer cells and treated weekly with the indicated peptide vaccine antibodies (500 μg) via IP injection. JIMT-1 cells and treated weekly with peptide vaccine antibodies. Tumor growth was monitored over time. After euthanasia, tumors were extracted and weighed. Results displayed include average tumor volume over time (top panel) and % weight tumors (bottom panel) when compared to total mouse weight (bottom panel). Error bars indicate standard deviation of the mean. Normal rabbit IgG (Pierce) was used as a negative control. In both mouse models, only treatment with the MVF-HER-3 461 vaccine antibodies caused a significant delay in tumor growth (p < 0.05 for both mouse models), and the effects were also observed in percentage tumor weight per body mass (p < 0.05).
Figure 10.
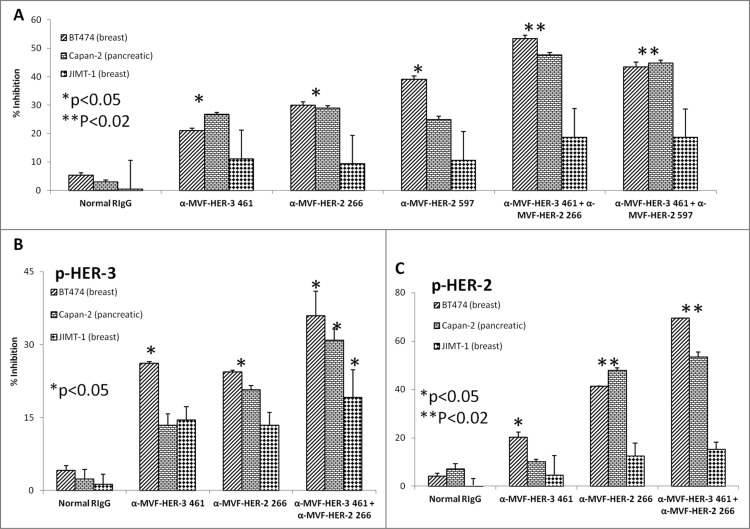
HER-3 vaccine antibodies produce synergistic antitumor effects when used in combination with HER-2 vaccine antibodies. Cancer cells (BT474, Capan-2 and JIMT-1) were seeded and treated for 1 h with either HER-3 461 vaccine antibodies, HER-2 266 vaccine antibodies, HER-2 597 vaccine antibodies or a combination of antibodies prior to ligand stimulation with HRG (50 ng/mL). After 72 h of incubation in the presence of the inhibitors, cells were analyzed for proliferation via MTT inhibition assay (A). Percent inhibition was calculated by taking absorbance (abs) readings at 570 nm and using the following equation: (abs. untreated-abs. treated)/abs. untreated × 100. Cells were also seeded and treated with the various combinations of vaccine antibodies for 1 h prior to ligand stimulation (HRG) and cell lysis. Cell lysates were used to measure inhibition of phosphorylation of both HER-3 (B) and HER-2 (C) via ELISA kits from R+D Systems. Percent inhibition was calculated by taking the absorbance at 450 nm and using the same equation mentioned above (in A). In all experiments, normal rabbit IgG (Pierce) was used as a negative control. Results display averages of two independent experiments with triplicate samples (n = 3). Error bars represent SD of the average. Combination treatment showed a better significant effect in both the proliferation and phosphorylation assays (**p < 0.02 in both proliferation and HER-2 phosphorylation assays with the BT-474 breast and Capan-2 pancreatic cancer cells) while single treatment with the HER-3 or HER-2 inhibitors showed a lesser significant value (*p < 0.05) or was not significant.
Combination treatment of HER-3 (461–479) and IGF-1R (56–81) peptide mimics exhibit enhanced cancer cell proliferation and phosphorylation
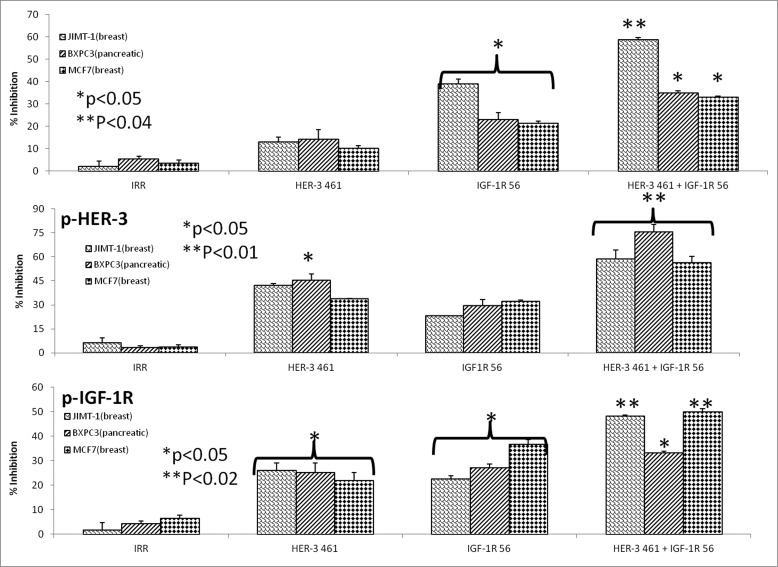
There are several lines of evidence that suggest extensive crosstalk exists between HER family receptors and IGF-1R.46 As shown in Fig. 1, HER-3 is expressed with other HER family receptors or IGF-1R in several cancer cell lines. Thus it is possible to target dual receptors in order to overcome any potential resistance mechanism to single treatment. To test the effects of combination treatment with HER-3 and IGF-1R inhibitors, we used three different cancer cell lines: JIMT-1 and MCF7 (breast) and BXPC3 (pancreatic) cancer cells. To measure proliferation, we used the MTT assay where the cells were treated with the single HER-3 (461–479) and IGF-1R (56–81) and combination [HER-3 (461–479) + IGF-1R (56–81)] inhibitors and incubated for three days before adding MTT. The peptide mimics single and combination treatment were able to prevent cancer cell proliferation in all three cell lines as shown in Fig. 9A. Combination treatment caused significant inhibition of proliferation of about 60% or greater (**p < 0.04) while single treatment showed an inhibitory rate of about 40% and 30% for HER-3-(461–479) and IGF-1R-56 respectively in all three cell lines (*p < 0.05). We also evaluated the effects of combination treatment on HER-3 and IGF-1R phosphorylation in the presence of IGF-1 and HRG ligand signaling in MCF-7, JIMT-1 and BXPC-3 cells. After treatment was used to measure phosphorylated levels of the receptors in a sandwich ELISA method using Human-phospho-HER-3/IGF-IR ELISA kit from R&D Systems (Saint Paul, MN). Results in Figs. 9B and C shows the effects of combination treatment on receptor phosphorylation were markedly increased (**p < 0.02) compared to individual treatments (*p < 0.05). Overall, the results point to the potential benefits of targeting HER-3 and IGF-IR as novel combinations in cancers that express these two receptors. Combining treatment with the HER-3 and IGF-1R peptide mimics enhances their ability to inhibit cellular proliferation and decrease receptor phosphorylation. These results indicate that targeting these two alternative signaling pathways in cancer may be beneficial for cancer therapy in the future.
Figure 9.
HER-3 peptide mimics produce synergistic antitumor effects when used in combination with IGF-1R peptide mimics. Cancer cells (JIMT-1, MCF7 and BXPC3) were plated in 96-well plates and treated for 1 h with either the HER-3 461 peptide mimic, IGF-1R 56 peptide mimic or HER-3 461 + IGF-1R 56 combination prior to ligand stimulation with both IGF-1 and HRG (50ng/mL). After 72 h of incubation in the presence of the peptide mimics, MTT was used to measure cell proliferation (A). Percent inhibition was calculated by taking absorbance (abs) readings at 570 nm and using the following equation: (abs. untreated-abs. treated)/abs. untreated × 100. Cells were also analyzed for inhibition of phosphorylation of both HER-3 (B) and IGF-1R (C). For phosphorylation assays, cells were seeded and treated for 1 h with peptide mimics prior to ligand stimulation for 15 min. with both IGF-1 and HRG. After cell lysis, phosphorylated HER-3 and IGF-1R were measured using ELISA kits from R+D Systems. Percent inhibition was measured by taking the absorbance readings at 450 nm and using the same equation as described above (in A). In all experiments an irrelevant (IRR) peptide was used as a negative control. Results display averages of two different experiments plated in triplicate (n = 3). Error bars represent SD of the mean. In both assays, combination treatment showed a better significant effect (**p < 0.04, **p < 0.02 and **p < 0.01 for proliferation, IGF-1R phosphorylation and HER-3 phosphorylation respectively) while single treatment with the HER-3 or IGF-IR inhibitors showed a lesser significant value (*p < 0.05) or was not significant when compared to the negative control rabbit IgG.
Combination treatment with HER-3:HER-2 and HER-3:HER-1 vaccine antibodies on cancer cell proliferation and phosphorylation
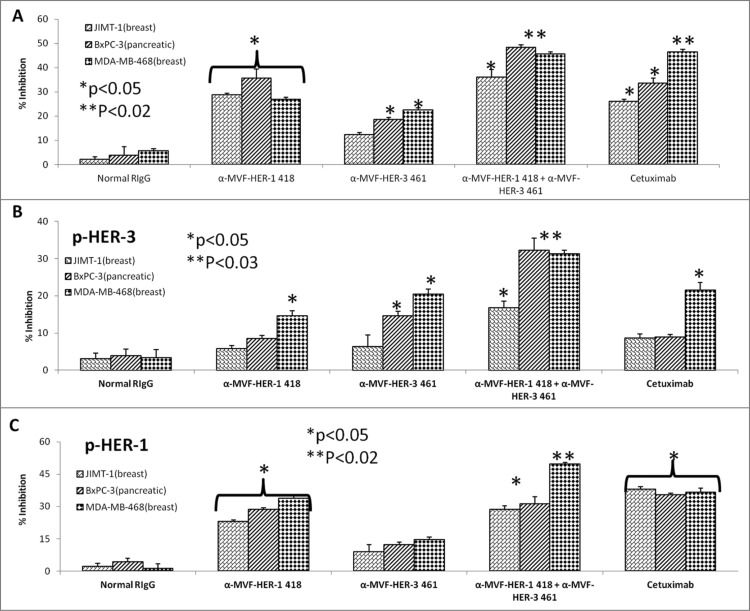
Considerable horizontal crosstalk also exists between HER family receptors, and HER-3 signaling has been implicated in drug resistance to EGFR (HER-1) and HER-2 inhibitors. 21,47,48 In this experiment, we evaluated two novel combination approaches which involves (1) the targeting HER-1 and HER-3; and (2) targeting of HER-2 and HER-3. Cell lines used in these two approaches are shown to express moderate to high levels of the receptors. To test the effects of combination treatment against HER family receptors, breast (JIMT-1 and MDA-468) and pancreatic (BXPC3) cancer cells were treated with either the (1) HER-3 (461–479) vaccine antibodies, (2) HER-2 (266–296) vaccine antibodies (pertuzumab-like), (3) HER-2 (597–626) vaccine antibodies (trastuzumab-like), (4) HER-1 (418–435) vaccine antibodies (cetuximab-like), (5) combination of HER-2 (266–296) and HER-3 (461–479) vaccine antibodies, (6) combination of HER-2 (597–626) and HER-3 (461–479) antibodies or (7) combination of HER-1 (418–435) and HER-3 (461–479) vaccine antibodies in MTT inhibition and phosphorylation assays (Figs. 10 and 11). Figs. 10A–C demonstrates the effects of combination treatment with HER-2 and HER-3 vaccine antibodies on cellular proliferation and receptor phosphorylation. In all cases, combination treatment with HER-2 and HER-3 vaccine antibodies resulted in an increase in both inhibition of proliferation and receptor phosphorylation (**p < 0.02) when compared to single treatment alone (*p < 0.05). Following treatment and ligand stimulation, cell lysates were collected to measure phosphorylated levels of both HER-3 (Fig. 10B) and HER-2 (Fig. 10C) using sandwich ELISA phospho-HER-2/HER-3 kits (R+D Systems). Combination treatment resulted in a superior reduction of both phosphorylated HER-2 and HER-3, indicating that co-targeting the receptors with these novel peptide mimics is a promising alternative approach for inhibiting breast and pancreatic cancers.
Figure 11.
HER-3 vaccine antibodies produce synergistic antitumor effects when used in combination with HER-1 vaccine antibodies. Cancer cells (JIMT-1, BXPC3 and MDA-MB-468) were treated for 1 h with either HER-3 461 vaccine antibodies, HER-1 418 vaccine antibodies or combination of antibodies prior to ligand stimulation with both EGF and HRG (50 ng/mL). Cells were analyzed for proliferation via MTT inhibition assay as described above (A) and inhibition of phosphorylation of both HER-3 (B) and HER-1 (C) via ELISA kits from R+D Systems as described above. In all experiments, normal rabbit IgG (Pierce) was used as a negative control, and Cetuximab was used as a positive control. Results display averages of two independent experiments with triplicate samples (n = 3). Error bars represent SD of the average. In both assays, combination treatment HER-1 and HER-3 vaccine antibodies showed a better significant effect (**p < 0.02 for proliferation and HER-1 phosphorylation and **p < 0.03 for HER-3 phosphorylation respectively) while single treatment with the HER-1 and HER-3 inhibitors showed a lesser significant value (*p < 0.05) or was not significant in some of the cell lines.
Figs. 11A–C displays similar results for combination treatment with HER-1 and HER-3 vaccine antibodies (**p < 0.02) when compared to single treatment alone (*p < 0.05). Breast (JIMT-1 and MDA-MB-468) and pancreatic (BXPC3) cancer cell proliferation was modestly effected by single treatment with the HER-1 and HER-3 vaccine antibodies and combination treatment resulted in a significant increase of inhibition of proliferation (Fig. 11A). Phosphorylated levels of HER-1 and HER-3 were measured following treatment with sandwich ELISA phospho-HER-1/HER-3 ELISA kits (R+D Systems) (Fig. 11B and, C). When compared to single agents alone, combination treatment resulted in reduced phosphorylation of both receptors, suggesting that there are many potential benefits of targeting multiple HER family members in cancers.
Combination treatment with HER-3:HER-2, HER-3:HER-1 or HER-3:IGF-1R vaccine antibodies causes increased apoptosis and ADCC
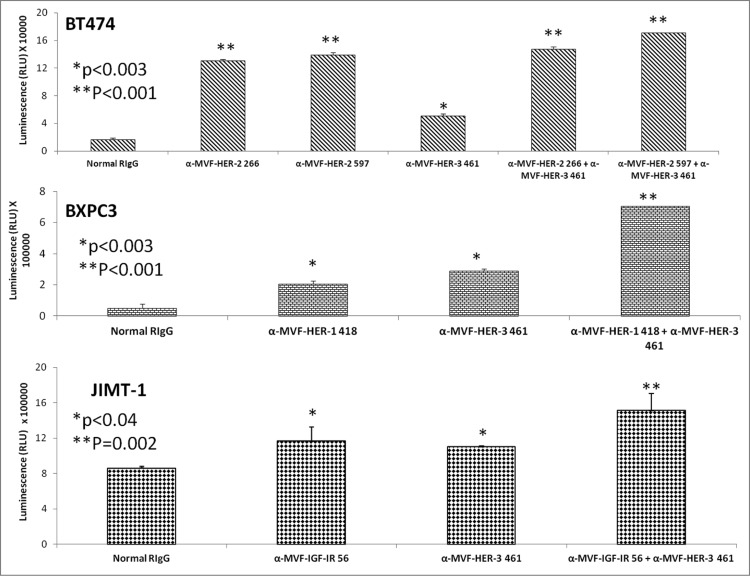
To test the ability of peptide vaccines to synergistically induce apoptosis and ADCC of cancer cells, we compared single vs. combination treatments using the Caspase glo 3/7 kit (Promega) and a bioluminescence cytotoxicity kit (cell technology). Human breast cancer cells (BT474) were treated with a combination of the HER-2 and HER-3 vaccine antibodies as described above, and combination treatment resulted in an increase in the amount of apotosis (**p < 0.001) (Fig. 12, top) when compared to single treatment alone. Significant apoptosis was observed when human pancreatic cancer cells (BXPC3) were treated with a combination of the HER-1-418 and HER-3-461 antibodies (**p < 0.001) (Fig. 12, middle) as compared to individual treatment (*p < 0.003). Finally, when JIMT-1 breast cancer cells were used to monitor apoptotic activity, a combination of antibodies to HER-416 and IGF-1R 56 showed increase in the amount of caspase 3 and 7 activity was observed (**p = 0.002) vs. single treatment (*p < 0.04).
Figure 12.
HER-3 vaccine antibodies used in combination with HER-1, HER-2 or IGF-1R vaccine antibodies induces a greater level of apoptosis. Various cancer cell lines (BT474, BXPC3 and JIMT-2) were treated for 1 h with antibodies/combinations of antibodies prior to ligand binding. After 24 h of incubation in the presence of the inhibitors, apoptosis was measured using the Caspase glo 3/7 kit from Promega. Plates were read on a luminometer and normal rabbit IgG (Pierce) was used as a negative control. Results are representative of two independent experiments with triplicate samples (n = 3). Error bars represent SD of the average. Combination treatment HER-3 caused greater induction of apoptosis as indicated by significant release in caspase enzymes (**p < 0.002) while single treatment with the HER-1, HER-2, HER-3 or IGF-IR inhibitors showed a lesser significant value (*p < 0.003 for BT-474 breast and BxPC-3 pancreatic cancer cells and *p < 0.04 for JIMT-1 breast cancer cells).
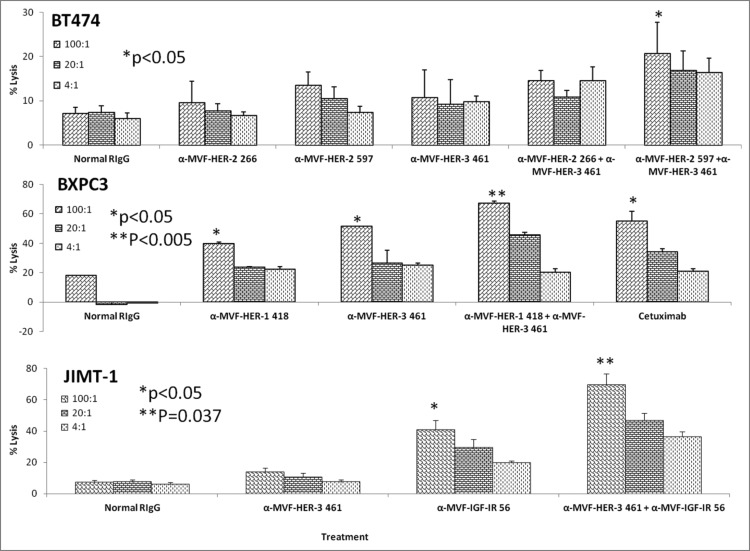
Results from the ADCC bioluminescence assay demonstrated an increase in the amount of cell lysis (Fig. 13), indicating that combination treatments induced significantly higher levels of ADCC than single treatment alone. Combination treatment effects on ADCC with HER-2/HER-3, HER-1/HER-3 and HER-3/IGF-1R were also evaluated using BT-474, BxPC-3 and JIMT-1 cells respectively. Single treatments with the vaccine antibodies showed inhibitory effects that were dependent on the effector to target concentrations with ratio of 100:1 showing the greatest induction of ADCC indicating that the increase in numbers of PBMCs caused more stimulation by the vaccine antibodies thereby inducing ADCC. Combination treatment showed greater induction of ADCC in all three cases but these effects were highly evident in the case of HER-3-461 and IGF-1R-56 vaccine combination using the trastuzumab resistant JIMT-1 cells (**p = 0.037). Combination treatment caused upto 70% lysis using the 100:1 target ratio while single treatments with HER-3-461 and IGF-1R-56 caused about 10% and 38% (*p < 0.05) lysis respectively (Fig. 13, bottom). These results clearly establish the potential clinical benefits of the combination approach in causing additive/synergistic antitumor effects in various cancer types that express these receptors.
Figure 13.
HER-3 vaccine antibodies used in combination with HER-1, HER-2 or IGF-1R vaccine antibodies results in increased levels of ADCC. Various cancer cells (target cells) were seeded and incubated in the presence of human PBMCs at different effector: target cell ratios (100:1, 20:1, 4:1). Cells were then treated for one hour with antibodies/combinations of antibodies prior to cell lysis. The Acella-tox kit (Cell Technology) was used to measure the relative amount of ADCC. Results display the % lysis of treatment groups when compared to 100% target cell lysis. Normal rabbit IgG (Pierce) was used as a negative control. Results display averages for two independent experiments with triplicate samples (n = 3). Error bars represent SD of the average. Combination treatment showed significant induction of ADCC in all three cell lines used with a significant value of *p < 0.05 for BT-474 cells, **p < 0.005 for BxPC-3 cells and **p = 0.037 for JIMT-1 cells. Single treatment with the HER-1 and HER-3 in BxPC-3 cells or IGF-IR inhibitors in JIMT-1 cells showed a lesser significant value (*p < 0.05).
Discussion
Due to its lack of intrinsic kinase activity and inability to homodimerize, HER-3 was not considered a target for cancer immunotherapy until recently. This view has changed because of mounting evidence suggesting that HER-3 heterodimers (with HER-1 or HER-2) are the most active signaling dimers, and that HER-3 offers a major mechanism by which HER-driven tumors escape from targeted therapy.21,24–29,49–55 Currently, there are no FDA approved HER-3 targeted therapies available in the clinic today. As a result, development of novel HER-3 inhibitory therapies that help overcome resistance to other HER family therapies is of great priority. In this study, we have evaluated the in vitro and in vivo efficacy of a type of targeted therapy that involves using peptide therapeutics to inhibit HER-3. Peptides therapeutics offer benefits such as: low cost, high specificity and potency, ability to penetrate the cell membrane, low immunogenicity and safety.56-61 This research project stems from work conducted in our laboratory over the last several years. Our lab has developed effective vaccines against HER-2 and novel therapies based on blockade of receptor: ligand interactions, such as B7:CD28.62-64 Two of our HER-2 B-cell epitope vaccine candidates established antitumor effects in preclinical studies, and we were able to translate our novel findings into a phase I clinical trial in which patients were vaccinated with a combination of the two vaccine epitopes.65-69 We have also made efforts in validating our combination therapy approach by targeting HER-2 and VEGF in transgenic and transplantable breast cancer mouse models. Studies from our laboratory have shown that a combined approach aimed toward simultaneously shutting down multiple cancer signaling pathways produces superior antitumor effects when compared to single treatments alone.58
Several published crystal structures of HER-3 in complex with mAbs (DL11, LMJ716 and RG7116) have provided our laboratory with significant insights into the key HER-3 amino acids involved in binding to the antibodies.40,42,43 In pre-clinical studies, these mAbs have been shown to 1) inhibit signaling of HER-3 and 2) elicit antitumor effects both in vitro and in vivo. As a result, we developed four HER-3 vaccine epitopes that mimic the binding sites of these mAbs (Figs. S1 and S2;Table 1). We evaluated the antitumor effects of our peptide mimics on pancreatic and breast cancer cells that overexpress HER-3 (Fig. 1). The peptide mimics were able to inhibit proliferation of HER-3 positive cells in a dose dependent manner, suggesting that the peptides have the ability to block ligand-induced activation of HER-3 (Fig. 2). The HER-3 99–122 and HER-3 461–479 peptides elicited the strongest anti-proliferative effect, and these were the only two peptides that were able to slightly inhibit HER-3 phosphorylation (Fig. 2). The peptide mimics did not fully inhibit HER-3 phosphorylation, suggesting that the peptides do not completely inhibit activation and subsequent signaling of the HER-3 protein.
Two of our peptide vaccine constructs were highly immunogenic in rabbits (no data for MVF HER-3 99–122 and MVF HER-3 140–162 constructs), and the antibodies raised against each of the vaccines were able to bind rhHER-3 protein in an ELISA assay (Fig. 4A and B). In order for the vaccine antibodies to inhibit growth of HER-3 expressing cancer cells, they must be able to recognize the native receptors on the surface of the cancer cells. As a result, we also tested the ability of the vaccine antibodies to bind HER-3 expressing cells via flow cytometry (Fig. 4C). The binding effect was highest with the HER-3 461 vaccine antibodies (Fig. 4). These results suggest that we were able to engineer a vaccine that is immunogenic with high binding affinity to the HER-3 receptor. The inhibitory effects of the vaccine antibodies greatly rely on their ability to inhibit proliferation of cancer cells and induce apoptosis. One major mechanism of cancer cell growth is to block apoptosis and anticancer agents should be able to induce apoptosis, resulting in programmed cancer cell death. We then used the caspase assay to show that these peptide inhibitors or vaccine Abs were able to cause release of caspase enzymes (Fig. 5). To further study the mechanism of action of these vaccines, we performed an assay to measure ADCC. ADCC is a key mechanism of action of most Abs, and we showed that two of the peptide vaccine polyclonal Abs (237 and 461 constructs) were able to stimulate PBMCs to cause killing of breast and pancreatic cancer cells (Fig. 6). These studies indicate that either vaccination with the chimeric epitopes or therapeutic treatment with the peptide mimics would be effective candidates for targeting HER-3. After demonstrating the in vitro antitumor effects of these peptide inhibitors, we evaluated the in vivo effects using two transplantable mouse models, one of which is driven by high expression of the HER family of receptors (JIMT-1) and the other is highly dependent on EGFR: HER-3 expression (BXPC3). Two of our peptide mimics (HER-3 237–269 and HER-3 461–479 constructs) were tested in these models, in which both elicited a significant delay in onset of tumor growth (Fig. 7). In addition, two of our vaccine antibodies were also tested in these mouse models. Only the MVF-HER-3 461–479 peptide vaccine antibodies caused a significant delay in onset of tumor growth (Fig. 8). Unfortunately, these mouse models were not ideal for studying active immunization with our peptide mimics. We were limited to using passive immunotherapy with xenografted nude mice. We anticipate that a better response would have been observed if mice were immunized with our peptide constructs. Our lab is also currently working on evaluating the in vivo effects of our two remaining peptide mimics (HER-3 99–122 and HER-3 140–162) and vaccine constructs.
Although efforts at targeting HER-3 have lagged behind, we, and others, have shown that HER-3 may be an attractive target against many types of cancer. An increasing amount of evidence indicates that crosstalk between the HER family members represents a major factor affecting clinical efficacy. Blocking the function of one HER receptor can be compensated by another HER family member (or alternative signaling pathway) via several molecular mechanisms that are not yet fully understood. For instance, HER-3, IGF-1R and EGFR have been identified as key contributors to acquired resistance against HER-2 targeting agents, while HER-3and IGF-1R have also been shown to be involved in regulating acquired resistance to EGFR inhibitors.50,70,71 Overall, the future success of targeted therapeutics will be dependent on overcoming these molecular mechanisms of drug resistance. A better clinically promising strategy could be to combine anti-HER-3 therapies with HER-1 and HER-2 targeting agents.37 Several studies have shown that combination therapy against multiple HER family members is superior to single treatment alone. Some investigators have combined HER-3 mAbs with HER-1 or HER-2 inhibitors and shown synergistic antitumor effects, while others have taken the approach of developing dual specific mAbs that specifically bind to two of the HER family receptors.38,40,41,72-74 Recent studies have also suggested that blocking both the IGF-1R and HER family signaling pathways simultaneously produces synergestic/superior antitumor effects.75-79 We hypothesized that targeting more than one receptor at the same time with our peptide therapeutics would prevent cross-talk and decrease the possibility of drug resistance due to activation of alternative signaling pathways. To test our hypothesis, we combined our HER-3 461 inhibitors with an IGF-1R peptide mimic (and vaccine antibodies), HER-1 vaccine antibodies or HER-2 vaccine antibodies (previously established in our lab) and tested the antitumor effects in vitro by measuring proliferation, inhibition of phosphorylation, apoptosis and ADCC. In all cases, combination treatment of the HER-3 peptide with IGF-1R, HER-1 or HER-2 additively inhibited proliferation and phosphorylation (Figs. 9–11), indicating that targeting two relevant signaling pathways may be beneficial in future therapies. Combination treatments also resulted in an increase in apoptosis and ADCC (Figs. 12 and 13), suggesting synergism between HER-3 peptides/vaccine antibodies and other inhibitors previously established in our lab. In conclusion, we plan on further evaluating the potential synergistic antitumor effects of combination therapy with our HER-3 peptide inhibitors in vivo. We hypothesize that dual targeting either HER-3 and IGF-1R or HER-3 and HER-2 will demonstrate superior antitumor activity and help prevent emergence of drug resistance.
Materials and Methods
Synthesis and characterization of the HER-3 peptide mimics
Peptide synthesis was carried out on a Milligen/Biosearch 9600 peptide solid phase synthesizer (Bedford, MA) using Fmoc/t-butyl chemistry. CLEAR acid or amide resins were used for synthesis of the constructs. After synthesis, a fraction of each peptide was cleaved from the resin using cleavage reagent B (trifluoroacetic acid/phenol/water/TIS 90:4:4:2), and crude peptides were purified by semi-preparative reversed-phase HPLC. The remaining peptide resin was linked to a promiscuous T-helper cell epitope derived from the measles virus fusion protein (MVF residues 288–302). Each peptide was synthesized collinearly with the MVF epitope and a flexible residue linker (GPSL) to allow independent folding of each epitope. After synthesis, cleavage and purification, the MVF HER-3 peptides were characterized by analytical HPLC (high pressure liquid chromatography) and MALDI (matrix-assisted laser desorption ionization mass spectroscopy) at Chemical Instrumentation Center (The Ohio State University, Columbus, OH). After confirming the correct molecular weight, the peptides were then lyophilized and dissolved prior to use in subsequent assays.
Protein expression in cancer cell lines
Western blotting for total HER-3, HER-1, HER-2 and IGF-1R was performed to determine total protein expression in cancer cell lines. One million cells/well were plated in 6 well plates and incubated at 37⁰C until cells were 70–80% confluent. Culture media was then removed from the wells, and cells were washed with PBS. Cells were lysed with 1X RIPA Lysis Buffer (Santa Cruz) for 2.5 h at 4°C. Cell lysates were spun at 13000× g and debris-free supernatants were transferred into clean tubes. Protein concentration of each sample was measured by Coomassie plus protein assay reagent kit (Pierce). Lysates were frozen at −80°C. Protein expression was measured using western blotting with rabbit polyclonal antibodies for HER-1 (Cell Signaling #4405), HER-2 (Cell Signaling #4290), HER-3 (Santa Cruz sc-285) and IGF-1R (Cell Signaling #9750). A β-actin antibody (Abcam Ab8227) was used to control for loading. Detection was accomplished using goat anti-rabbit HRP secondary antibody (Bio-rad 170–5046) and Immun-StarTM HRP Chemiluminescent Kit (Bio-Rad). All procedures were performed according to the manufacturer's instructions.
Rabbits
Two female New Zealand white outbred rabbits (Harlan) were immunized intramuscularly with 1mg of MVF-HER-3 237 or MVF-HER-3 461 peptide dissolved in 500 μL PBS and emulsified in 500 uL of Montanide ISA720 vehicle with 100 ug muramyl dipeptide adjuvant, nor-MDP (N-acetylglucosamine-3yl-acetyl-L-alanyl-D-isoglutamine). Subsequent booster injections were given every three weeks after primary immunization. Sera of rabbits immunized with MVF-HER-3 237 or MVF-HER-3 461were collected weekly, and complement was inactivated by heating to 56°C for 30 min. The titer of anti-HER-3 peptide antibody was quantified by ELISA. High-titered sera was purified on a protein A/G column (Pierce, Rockford, IL) and eluted antibodies were concentrated and exchanged in PBS using 100 kDa cutoff centrifuge filter units (Millipore). Antibody concentrations were determined by Coomassie plus protein assay reagent kit (Pierce).
Cell lines
BT474, MCF7, MDA-MB-453, MDA-MB-468, BxPC3, HT-29, SKOV-3, T47D cancer cells were purchased from the ATCC. JIMT-1 cells were generously provided by Rita Nahta's lab in Atlanta, Georgia. BxPC3 and T47D cells were cultured in RPMI1640 supplemented with 10% FBS, 1% pen-strep. MCF7, MDA-MB-453, MDA-MB-468, JIMT-1 and BT474 cells were cultured in DMEM supplemented with 10% FBS, 1% pen-strep. All cells were grown at 37⁰C in 95% air, 5% CO2.
Flow cytometry
Five x 105cells were washed twice in 1 mL of staining buffer (PBS, 1%BSA,0.02% sodium azide). After washing, cells were treated with 100 ug of anti-HER-3 peptide antibody in 100uL staining buffer for 30 min. Following incubation, cells were washed twice with 1mL staining buffer and treated for 30 min with 1:100 dilution of goat anti-rabbit IgG–Alexa Fluor 488 secondary antibody (Invitrogen) in 100 uL staining buffer. After washing, cells were fixed in 3.7% paraformaldehyde in PBS and analyzed on a BD FACSCalibur System (DHRLI Flow Cytometry Core Lab, OSU). Normal rabbit IgG was used as the negative control, and a commercial anti-HER-3 antibody was used as the positive control (Abgent).
rhHER-3 ELISA
Ninety-six well plates were coated with 100 uL of rh HER-3 at 2μg/mL in PBS overnight at 4°C. Nonspecific binding sites were blocked for 1 h with 200 uL PBS-1% BSA, and plates were washed with PBST. Vaccine antibodies in PBT were added to antigen-coated plates in duplicate wells, serially diluted 1:2 in PBT, and incubated for 2 h at room temperature. After washing the plates, 100 uL of 1:500 goat anti-rabbit IgG conjugated to horseradish peroxidase (Pierce) were added to each well and incubated for 1 h. After washing, the antibody was detected using 50 μL of 0.15% H2O2 in 24 mM citric acid and 5mM sodium phosphate buffer (pH 5.2) with 0.5mg/mL 2,2′-aminobis(3ethylbenzthiazole-6-sulfonic acid) as the chromophore. Color development proceeded for 10 min, and the reaction was stopped with 25 uL of 1% SDS. Absorbance was read at 415 nm using a BioRad Benchmark ELISA plate reader (Hercules, CA).
HER-3 phosphorylation assay
A Human phosphor-ErbB3 ELISA kit (R & D Systems) was used to measure the amount of phosphorylated HER-3. One million cells/well were plated in six well plates and incubated at 37°C overnight. Culture media was removed from the wells, and cells were washed with PBS. Cells were treated with 150 μg HER-3 peptide or anti-peptide antibodies in binding buffer (0.2& BSA, RPMI 1640, 10 mM HEPES (pH 7.2) for 1 h at 37°C. 5nM Heregulin was added, and plates were incubated at RT for 10 min. After stimulation, cells were lysed with 1X RIPA Lysis Buffer (R + D systems) for 2.5 h at 4°C. Cell lysates were spun at 13000×g and debris-free supernatants were transferred into clean tubes. Protein concentration of each sample was measured by Coomassie plus protein assay reagent kit (Pierce). Lysates were frozen at −80°C. Phosphorylated HER-3 was measured using the DuoSet IC for human phospho-ErbB3 (R + D Systems, Minneapolis, MN).
MTT inhibition assay
Cells were seeded in 96 well flat bottom plates at 1 × 104 cells/well in 100 uL growth media and allowed to adhere overnight at 37°C. Growth media was then replaced with inhibitors dissolved in low-sera (1% FBS) media. Plates were incubated for 1 h at 37°C, and 50 ng/mL HRG was added in 1% growth medium. Plates were incubated an additional 72 h at 37°C before 25 uL of 5mg/mL MTT was added to each well. Plates were incubated for 2 h at 37°C, then 100 uL extraction buffer (20% SDA,50% DMF, pH 4.7) was added. Plates were incubated overnight at 37°C and read on an ELISA reader at 570 nm.
Apoptosis assay
The capsase glo 3/7 kit (Promega) was used to measure the ability of the peptides and vaccine antibodies to induce apoptosis. 1 × 106 cells were seeded and incubated overnight at 37ºC. Cells were then treated with the peptides or vaccine antibodies for 1 h prior to ligand stimulation. Cells were incubated for 8, 24 or 48 h before addition of the caspase glo detection reagent. Results only show data for 24 h treatment. Apoptosis is directly related to the amount of luminescence (RLU).
ADCC assay
A biolouminescence cytotoxicity assay was used to detect the ability of anti-peptide antibodies to elicit ADCC (aCella-TOXTM). All procedures were done according to the manufacturer's instructions. Non-radioactive reagents were used to measure the amount of GAPDH enzyme released by dead or dying cells. The effector cells were normal human PBMC's from healthy donors (American Red Cross). Target cells used were HER-3 positive cancer cells. Effector: target cell ratios used were 100:1, 20:1 and 4:1. Percent lysis was calculated by the following equation: (sample – E:T spontaneous lysis)/maximum lysis of target cells × 100).
Xenograft studies
Female BALB/c SCID mice 5–6 weeks old (Jackson Laboratories) were challenged subcutaneously with 5 × 106 human cancer cells (BXPC3 or JIMT-1). Starting at day zero (day of tumor challenge), mice were treated intravenously with 200 μg of each peptide mimic or 500 ug/mL vaccine antibodies weekly for 7–8 weeks. Tumor growth was monitored twice a week using Vernier calipers. Tumor volume was calculated by the formula (long measurement × short measurement2)/2.
Combination treatment with HER family peptide therapeutics and IGF-1R inhibitors
The HER-1 418, HER-2 266 and HER-2 597 vaccine antibodies were previously described and established by our lab.2,80,81 Cells were treated with 100 μg/mL single agent/various combinations and analyzed by MTT inhibition assay, apoptosis assay and ADCC (as previously described above).
Statistical analysis
Tumor sizes and weights were analyzed using Stata's XTGEE cross-sectional generalized estimating equation, which fits general linear models that allow you to specify within animal correlation structure in data involving repeated measurements. For other experiments, t-test was carried out to observe the statistical relevance in between different sets of experiments as well as the significant difference between treated and untreated cells.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Rita Nahta is a Glenn Breast Cancer Research Scholar at the Winship Cancer Institute of Emory University.
Funding
This work was supported by funding from NIH R01 CA 84356 (PTPK), OSU Peptide Research Fund (PTPK) and R01CA157754 (RN).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Ueno Y, Sakurai H, Tsunoda S, Choo MK, Matsuo M, Koizumi K, Saiki I. Heregulin-induced activation of ErbB3 by EGFR tyrosine kinase activity promotes tumor growth and metastasis in melanoma cells. Int J Cancer 2008; 123:340-7; PMID:; http://dx.doi.org/ 10.1002/ijc.23465 [DOI] [PubMed] [Google Scholar]
- 2. Allen SD, Garrett JT, Rawale SV, Jones AL, Phillips G, Forni G, Morris JC, Oshima RG, Kaumaya PT. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol 2007; 179:472-82; PMID:; http://dx.doi.org/ 10.4049/jimmunol.179.1.472 [DOI] [PubMed] [Google Scholar]
- 3. Koutras AK, Fountzilas G, Kalogeras KT, Starakis I, Iconomou G, Kalofonos HP. The upgraded role of HER3 and HER4 receptors in breast cancer. Crit Rev Oncol Hematol 2010; 74:73-8; PMID:; http://dx.doi.org/ 10.1016/j.critrevonc.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 4. Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12:553-63; PMID:; http://dx.doi.org/ 10.1038/nrc3309 [DOI] [PubMed] [Google Scholar]
- 5. Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 2014; 79:34-74; PMID:; http://dx.doi.org/ 10.1016/j.phrs.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 6. Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 2012; 16:15-31; PMID:; http://dx.doi.org/ 10.1517/14728222.2011.648617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iorio MV, Casalini P, Piovan C, Di Leva G, Merlo A, Triulzi T, Menard S, Croce CM, Tagliabue E. microRNA-205 regulates HER3 in human breast cancer. Cancer Res 2009; 69:2195-200; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2920 [DOI] [PubMed] [Google Scholar]
- 8. Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 2002; 297:1330-3; PMID:; http://dx.doi.org/ 10.1126/science.1074611 [DOI] [PubMed] [Google Scholar]
- 9. Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med 2004; 55:433-57; PMID:; http://dx.doi.org/ 10.1146/annurev.med.55.091902.104433 [DOI] [PubMed] [Google Scholar]
- 10. Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 2003; 284:2-13; PMID:; http://dx.doi.org/ 10.1016/S0014-4827(02)00105-2 [DOI] [PubMed] [Google Scholar]
- 11. Sierke SL, Cheng K, Kim HH, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochem J 1997; 322 (Pt 3):757-63; PMID:; http://www.biochemj.org/bj/322/0757/3220757.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A 1994; 91:8132-6; PMID:; http://dx.doi.org/ 10.1073/pnas.91.17.8132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci U S A 2009; 106:21608-13; PMID:; http://dx.doi.org/ 10.1073/pnas.0912101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naidu R, Yadav M, Nair S, Kutty MK. Expression of c-erbB3 protein in primary breast carcinomas. Br J Cancer 1998; 78:1385-90; PMID:; http://dx.doi.org/ 10.1038/bjc.1998.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friess H, Yamanaka Y, Kobrin MS, Do DA, Buchler MW, Korc M. Enhanced erbB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1995; 1:1413-20; PMID:; http://clincancerres.aacrjournals.org/content/1/11/1413.full.pdf [PubMed] [Google Scholar]
- 16. Maurer CA, Friess H, Kretschmann B, Zimmermann A, Stauffer A, Baer HU, Korc M, Buchler MW. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol 1998; 29:771-7; PMID:; http://dx.doi.org/ 10.1016/S0046-8177(98)90444-0 [DOI] [PubMed] [Google Scholar]
- 17. Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008; 68:5878-87; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0380 [DOI] [PubMed] [Google Scholar]
- 18. Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, Di Fiore PP, Kraus MH. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995; 10:1813-21; PMID:; http://www.ncbi.nlm.nih.gov/pubmed/7538656 [PubMed] [Google Scholar]
- 19. Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res 2001; 7:1957-62; PMID: [PubMed] [Google Scholar]
- 20. Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer 2007; 7:389-97; PMID:; http://dx.doi.org/ 10.1038/nrc2127 [DOI] [PubMed] [Google Scholar]
- 21. Sergina NV, Moasser MM. The HER family and cancer: emerging molecular mechanisms and therapeutic targets. Trends in molecular medicine 2007; 13:527-34; PMID:; http://dx.doi.org/ 10.1016/j.molmed.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nahta R, Shabaya S, Ozbay T, Rowe DL. Personalizing HER2-targeted therapy in metastatic breast cancer beyond HER2 status: what we have learned from clinical specimens. Curr Pharmacogenomics Person Med 2009; 7:263-74; PMID:; http://dx.doi.org/ 10.2174/187569209790112337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2012; 2:62; PMID:; http://dx.doi.org/ 10.3389/fonc.2012.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007; 316:1039-43; PMID:; http://dx.doi.org/ 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 25. Amler LC. HER3 mRNA as a predictive biomarker in anticancer therapy. Expert Opin Biol Ther 2010; 10:1343-55; PMID:; http://dx.doi.org/ 10.1517/14712598.2010.512003 [DOI] [PubMed] [Google Scholar]
- 26. Osipo C, Meeke K, Cheng D, Weichel A, Bertucci A, Liu H, Jordan VC. Role for HER2/neu and HER3 in fulvestrant-resistant breast cancer. Int J Oncol 2007; 30:509-20; PMID:; http://dx.doi.org/ 10.3892/ijo.30.2.509 [DOI] [PubMed] [Google Scholar]
- 27. Frogne T, Benjaminsen RV, Sonne-Hansen K, Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ, de Cremoux P, Stenvang J, et al. Activation of ErbB3, EGFR and Erk is essential for growth of human breast cancer cell lines with acquired resistance to fulvestrant. Breast Cancer Res Treat 2009; 114:263-75; PMID:; http://dx.doi.org/ 10.1007/s10549-008-0011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G, Gonzalez-Angulo AM, Hennessy BT, Mills GB, Kennedy JP, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res 2009; 69:4192-201; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamburger AW. The role of ErbB3 and its binding partners in breast cancer progression and resistance to hormone and tyrosine kinase directed therapies. J Mammary Gland Biol Neoplasia 2008; 13:225-33; PMID:; http://dx.doi.org/ 10.1007/s10911-008-9077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal 2009; 2:ra31; PMID:; http://dx.doi.org/ 10.1126/scisignal.2000352 [DOI] [PubMed] [Google Scholar]
- 31. Li C, Brand TM, Iida M, Huang S, Armstrong EA, van der Kogel A, Wheeler DL. Human epidermal growth factor receptor 3 (HER3) blockade with U3-1287/AMG888 enhances the efficacy of radiation therapy in lung and head and neck carcinoma. Discov Med 2013; 16:79-92; PMID:; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3901945/pdf/nihms544643.pdf [PMC free article] [PubMed] [Google Scholar]
- 32. LoRusso P, Janne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, Yee L, Copigneaux C, Hettmann T, Wu CY, et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2013; 19:3078-87; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3051 [DOI] [PubMed] [Google Scholar]
- 33. Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res 2010; 70:2485-94; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307:58-62; PMID:; http://dx.doi.org/ 10.1126/science.1104819 [DOI] [PubMed] [Google Scholar]
- 35. Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 2005; 65:11118-28; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3841 [DOI] [PubMed] [Google Scholar]
- 36. Huang X, Gao L, Wang S, McManaman JL, Thor AD, Yang X, Esteva FJ, Liu B. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-i receptor in breast cancer cells resistant to herceptin. Cancer Res 2010; 70:1204-14; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3321 [DOI] [PubMed] [Google Scholar]
- 37. Gossage L, Eisen T. Targeting multiple kinase pathways: a change in paradigm. Clin Cancer Res 2010; 16:1973-8; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A 2011; 108:5021-6; PMID:; http://dx.doi.org/ 10.1073/pnas.1016140108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamath AV, Lu D, Gupta P, Jin D, Xiang H, Wong A, Leddy C, Crocker L, Schaefer G, Sliwkowski MX, et al. Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother Pharmacol 2011; 69(4):1063-9; PMID:; http://dx.doi.org/ 10.1007/s00280-011-1806-6 [DOI] [PubMed] [Google Scholar]
- 40. Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472-86; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 41. McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther 2012; 11:582-93; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0820 [DOI] [PubMed] [Google Scholar]
- 42. Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, Sineshchekova O, Saxena P, Sutton CR, Chen D, et al. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res 2013; 73:6024-35; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mirschberger C, Schiller CB, Schraml M, Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G, Kolm I, et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013; 73:5183-94; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0099 [DOI] [PubMed] [Google Scholar]
- 44. Lairmore MD, Lal RB, Kaumaya PT. Evaluation of immunodominant epitopes of human T-lymphotropic virus type 1 (HTLV-I) using synthetic peptides. Biomed Peptides Proteins Nucleic Acids: Struct, Syn Biol Act 1995; 1:117-22. [PubMed] [Google Scholar]
- 45. Kaumaya PT, Kobs-Conrad S, Seo YH, Lee H, VanBuskirk AM, Feng N, Sheridan JF, Stevens V. Peptide vaccines incorporating a 'promiscuous' T-cell epitope bypass certain haplotype restricted immune responses and provide broad spectrum immunogenicity. J Mol Recognit 1993; 6:81-94; PMID:; http://dx.doi.org/ 10.1002/jmr.300060206 [DOI] [PubMed] [Google Scholar]
- 46. Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol 2008; 13:485-98; http://dx.doi.org/ 10.1007/s10911-008-9107-3 [DOI] [PubMed] [Google Scholar]
- 47. Amin DN, Sergina N, Lim L, Goga A, Moasser MM. HER3 signalling is regulated through a multitude of redundant mechanisms in HER2-driven tumour cells. Biochem J 2012; 447:417-25; PMID:; http://dx.doi.org/ 10.1042/BJ20120724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang Y, Opresko L, Shankaran H, Chrisler WB, Wiley HS, Resat H. HER/ErbB receptor interactions and signaling patterns in human mammary epithelial cells. BMC Cell Biol 2009; 10:78; PMID:; http://dx.doi.org/ 10.1186/1471-2121-10-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeon CH, Pegram MD. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest New Drugs 2005; 23:391-409; PMID:; http://dx.doi.org/ 10.1007/s10637-005-2899-8 [DOI] [PubMed] [Google Scholar]
- 50. Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene 2008; 27:3944-56; PMID:; http://dx.doi.org/ 10.1038/onc.2008.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vlacich G, Coffey RJ. Resistance to EGFR-targeted therapy: a family affair. Cancer Cell 2011; 20:423-5; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007; 445:437-41; PMID:; http://dx.doi.org/ 10.1038/nature05474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jiang N, Saba NF, Chen ZG. Advances in targeting HER3 as an anticancer therapy. Chemother Res Pract 2012; 2012:817304; PMID:; http://dx.doi.org/ 10.1155/2012/817304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res 2006; 8:215; PMID:; http://dx.doi.org/ 10.1186/bcr1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 2006; 3:269-80; PMID:; http://dx.doi.org/ 10.1038/ncponc0509 [DOI] [PubMed] [Google Scholar]
- 56. Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs 2009; 12:779-84; PMID:; http://www.ncbi.nlm.nih.gov/pubmed/19943221 [PubMed] [Google Scholar]
- 57. Reichert J. Development trends for peptide therapeutics. Tufts Center for the Study of Drug Development Tufts University, 2008. Peptide Therapeutics Foundation, San Diego CA 92121; http://www.peptidetherapeutics.org/PTF_report_summary_2010.pdf [Google Scholar]
- 58. Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J Biol Chem 2011; 286:13626-37; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.216820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Foy KC, Miller MJ, Moldovan N, Carson Iii WE, Kaumaya PT. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. Oncoimmunology 2012; 1:1048-60; PMID:; http://dx.doi.org/ 10.4161/onci.20708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv 2013; 4:1443-67; PMID:; http://dx.doi.org/ 10.4155/tde.13.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller MJ, Foy KC, Kaumaya PT. Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov Med 2013; 15:166-76; PMID:; http://www.discoverymedicine.com/Megan-Jo-Miller/2013/03/28/cancer-immunotherapy-present-status-future-perspective-and-a-new-paradigm-of-peptide-immunotherapeutics/ [PubMed] [Google Scholar]
- 62. Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PT, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J Immunol 2002; 169:2180-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.169.4.2180 [DOI] [PubMed] [Google Scholar]
- 63. Srinivasan M, Wardrop RM, Gienapp IE, Stuckman SS, Whitacre CC, Kaumaya PT. A retro-inverso peptide mimic of CD28 encompassing the MYPPPY motif adopts a polyproline type II helix and inhibits encephalitogenic T cells in vitro. J Immunol 2001; 167:578-85; PMID:; http://dx.doi.org/ 10.4049/jimmunol.167.1.578 [DOI] [PubMed] [Google Scholar]
- 64. Allen SD, Rawale SV, Whitacre CC, Kaumaya PT. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J Pept Res 2005; 65:591-604; PMID:; http://dx.doi.org/ 10.1111/j.1399-3011.2005.00256.x [DOI] [PubMed] [Google Scholar]
- 65. Dakappagari NK, Douglas DB, Triozzi PL, Stevens VC, Kaumaya PT. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer Res 2000; 60:3782-9; PMID:; http://cancerres.aacrjournals.org/content/60/14/3782.full.pdf+html [PubMed] [Google Scholar]
- 66. Dakappagari NK, Pyles J, Parihar R, Carson WE, Young DC, Kaumaya PT. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. J Immunol 2003; 170:4242-53; PMID:; http://dx.doi.org/ 10.4049/jimmunol.170.8.4242 [DOI] [PubMed] [Google Scholar]
- 67. Dakappagari NK, Sundaram R, Rawale S, Liner A, Galloway DR, Kaumaya PT. Intracellular delivery of a novel multiepitope peptide vaccine by an amphipathic peptide carrier enhances cytotoxic T-cell responses in HLA-A*201 mice. J Pept Res 2005; 65:189-99; PMID:; http://dx.doi.org/ 10.1111/j.1399-3011.2005.00212.x [DOI] [PubMed] [Google Scholar]
- 68. Dakappagari NK, Lute KD, Rawale S, Steele JT, Allen SD, Phillips G, Reilly RT, Kaumaya PT. Conformational HER-2/neu B-cell epitope peptide vaccine designed to incorporate two native disulfide bonds enhances tumor cell binding and antitumor activities. J Biol Chem 2005; 280:54-63; PMID:; http://dx.doi.org/ 10.1074/jbc.M411020200 [DOI] [PubMed] [Google Scholar]
- 69. Kaumaya PT, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, Lamb T, Mani A, Kane Y, Balint CR, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol 2009; 27:5270-7; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.22.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer 2004; 108:334-41; PMID:; http://dx.doi.org/ 10.1002/ijc.11445 [DOI] [PubMed] [Google Scholar]
- 71. Haluska P, Carboni JM, TenEyck C, Attar RM, Hou X, Yu C, Sagar M, Wong TW, Gottardis MM, Erlichman C. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther 2008; 7:2589-98; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, von Mehren M, Shchaveleva I, Simmons HH, Marks JD, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer 2008; 99:1415-25; PMID:; http://dx.doi.org/ 10.1038/sj.bjc.6604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Larbouret C, Gaborit N, Chardes T, Coelho M, Campigna E, Bascoul-Mollevi C, Mach JP, Azria D, Robert B, Pelegrin A. In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors' downregulation and dimers' disruption. Neoplasia 2012; 14:121-30; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, Sliwkowski MX, Harari PM. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res 2013; 73:824-33; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF-IR inhibitor, NVP-AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer 2013; 13:41; PMID:; http://dx.doi.org/ 10.1186/1471-2407-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dong J, Sereno A, Aivazian D, Langley E, Miller BR, Snyder WB, Chan E, Cantele M, Morena R, Joseph IB, et al. A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 2011; 3:273-88; PMID:; http://dx.doi.org/ 10.4161/mabs.3.3.15188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dong J, Sereno A, Snyder WB, Miller BR, Tamraz S, Doern A, Favis M, Wu X, Tran H, Langley E, et al. Stable IgG-like bispecific antibodies directed toward the type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J Biol Chem 2011; 286:4703-17; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.184317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, Rimkunas V, Xu L, Kohli N, Rennard R, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther 2014; 13:410-25; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0255 [DOI] [PubMed] [Google Scholar]
- 79. Chen C, Zhang Y, Li J, Tsao SW, Zhang MY. Superior antitumor activity of a novel bispecific antibody cotargeting human epidermal growth factor receptor 2 and type I insulin-like growth factor receptor. Mol Cancer Ther 2014; 13:90-100; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0558 [DOI] [PubMed] [Google Scholar]
- 80. Garrett JT, Rawale S, Allen SD, Phillips G, Forni G, Morris JC, Kaumaya PT. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. J Immunol 2007; 178:7120-31; PMID:; http://dx.doi.org/ 10.4049/jimmunol.178.11.7120 [DOI] [PubMed] [Google Scholar]
- 81. Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PT. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J Immunol; 191:217-27; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1300231 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.