Abstract
Myeloid-derived suppressor cells (MDSCs) contribute to tumor-mediated immune escape and negatively correlate with overall survival of cancer patients. Nowadays, a variety of methods to target MDSCs are being investigated. Based on the intervention stage of MDSCs, namely development, expansion and activation, function and turnover, these methods can be divided into: (I) prevention or differentiation to mature cells, (II) blockade of MDSC expansion and activation, (III) inhibition of MDSC suppressive activity or (IV) depletion of intratumoral MDSCs. This review describes effective mono- or multimodal-therapies that target MDSCs for the benefit of cancer treatment.
Keywords: 5-Fluorouracil, all-trans retinoic acid, bisphosphonates, gemcitabine, immune suppressive mechanisms, myeloid-derived suppressor cells, sunitinib therapeutic vaccination
Abbreviations: 5-FU, 5-fluorouracil; ADAM17, metalloproteinase domain-containing protein 17; APCs, antigen presenting cells; ARG1, arginase-1; ATRA, all-trans retinoic acid; c-kit, Mast/stem cell growth factor receptor; CTLs, cytotoxic T lymphocytes; CD62L, L-selectin; CCL2, chemokine (C-C motif) ligand 2; CDDO-Me, bardoxolone methyl; CXCL12, chemokine (C-X-C motif) ligand 12; CXCL15, chemokine (C-X-C motif) ligand 15; COX2, cyclooxygenase 2; DCs, dendritic cells; ERK1/2, extracellular signal-regulated kinases; Flt3, Fms-like tyrosine kinase 3; FoxP3, forkhead box P3; GITR, anti-glucocorticoid tumor necrosis factor receptor; GM-CSF/CSF2, granulocyte monocyte colony stimulating factor; GSH, glutathione; HIF-1α, hypoxia inducible factor 1α; HLA, human leukocyte antigen; HNSCC, head and neck squamous cell carcinoma; HPV-16, human papillomavirus 16; HSCs, hematopoietic stem cells; ICT, 3, 5, 7-trihydroxy-4′-emthoxy-8-(3-hydroxy-3-methylbutyl)-flavone; IFNγ, interferon γ; IL-1β, interleukin 1 β; IL-4, interleukin 4; IL-6, interleukin 6; IL-10, interleukin 10; IL-13, interleukin 13; IMCs, immature myeloid cells; iNOS2, inducible nitric oxid synthase 2; JAK2, Janus kinase 2; MDSCs, myeloid-derived suppressor cells; MMPs, metalloproteinases (e.g., MMP9); mRCC, metastatic renal cell carcinoma; Myd88, myeloid differentiation primary response protein 88; NAC, N-acetyl cysteine; NADPH, nicotinamide adenine dinucleotide phosphate-oxidase NK cells, natural killer cells; NO, nitric oxide; NOHA, N-hydroxy-L-Arginine; NSAID, nonsteroidal anti-inflammatory drugs; ODN, oligodeoxynucleotides; PDE-5, phosphodiesterase type 5; PGE2, prostaglandin E2; ROS, reactive oxygen species; RNS, reactive nitrogen species; SCF, stem cell factor; STAT3, signal transducer and activator of transcription 3; TAMs, tumor-associated macrophages; TCR, T cell receptor; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α; Tregs, regulatory T cells; VEGFR, vascular endothelial growth factor receptor; WA, withaferin A; WRE, Withaferin somnifera
General Introduction
The clinical efficacies of antitumor therapeutics attempted so far remain limited. One confounding factor imparting this limitation may be observed intratumoral immunosuppression, a typical property of tumor environments that may hamper the desired antitumor effect. Apart from the immunosuppressive mechanisms exerted by cancer cells, suppressive immune cells present within the tumor also play a major role. The main immune cell types contributing to tumor immunosuppression and escape include: tumor-associated macrophages (TAMs), regulatory T cells (Tregs), type 2 natural killer T (NKT) cells and myeloid-derived suppressor cells (MDSCs).1 Therapeutic strategies targeting these cell populations for the benefit of cancer treatment are emerging.
In healthy individuals MDSCs, present in low numbers in the circulation, are involved in regulation of immune responses and tissue repair.1 During immunological responses to infections, inflammation and cancer this population rapidly expands. MDSCs are a heterogeneous population of myeloid lineage defined by an immature state and the capacity to suppress T-cell responses. Because of their incomplete differentiation, MDSCs differ from mature myeloid cells.1 In mouse models, MDSCs are identified by co-expression of CD11b (αM-integrin) and Gr-1 (Ly6 C-Ly6G)2 on their cell surface. Recently, the immature marker CD31 was reported as necessary for proper identification of MDSCs.3 Human MDSCs are more difficult to characterize due to the absence of lineage specific antigens, such as CD3 or CD19. Most often, human MDSCs are identified as CD14-CD11b+CD33+, without co-expression of the MHC Class-II molecule HLA-DR typical of mature myeloid and lymphoid cells.4,5 A more detailed account of their histological and functional characteristics can be found in this review.6
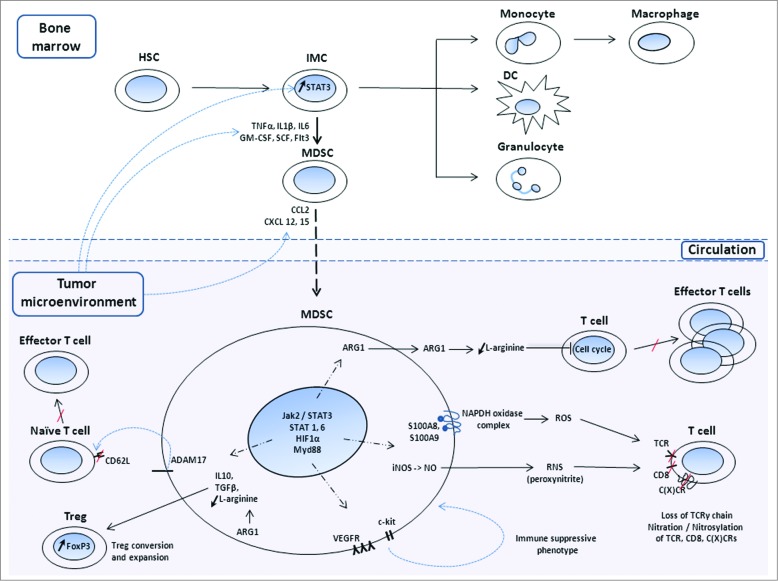
Recent studies have shown that circulating MDSCs significantly increase in early and late stage cancer patients and correlate with clinical cancer stage and metastatic disease.7 In addition, MDSCs accelerate angiogenesis, tumor progression and metastasis via multiple mechanisms (Fig. 1). Ample evidence demonstrates that cancer tissues with high MDSC infiltration are associated with poor patient prognosis and resistance to various therapies.6,8 Therefore, several mono-, bi- or multi-therapeutic strategies are now being developed to target MDSCs coincident with the stimulatation of potent antitumor immune responses. This review provides an overview of therapeutic strategies that are grouped based on the processes they target: MDSC development, expansion and activation and strategies that specifically target the suppressive mechanisms employed by MDSCs (Table 1).
Figure 1.
Mechanisms used by MDSCs to decrease antitumor immunity and contribute to tumor immune escape. Under normal circumstances, hematopoietic stem cells (HSCs) located in the bone marrow give rise to immature myeloid cells (IMCs), which differentiate into mature macrophages, dendritic cells (DCs) or granulocytes. In the context of cancer, the tumor microenvironment releases mediators that signal development of IMCs to myeloid-derived suppressor cells (MDSCs) and chemokines that signal MDSC migration to tumors. Immunosuppressive intratumoral MDSCs can: (i) block migration of naïve CD62L+ (L-selectin) T cells to lymphoid organs and subsequent formation of effector T cells; (ii) release factors that stimulate regulatory T cell (Treg) conversion and expansion; (iii) induce intracellular pathways that promote self-expansion; (iv) produce high levels of arginase 1 (ARG-1) that depletes T cells of L-arginine, inducing cell cycle arrest; (v) stimulate production of reactive oxygen and nitrogen species (ROS, RNS) that decrease T cell receptor (TCR) functionality; (vi) nitrate / nitrosylate CD8 and chemokine C-C or C-X-C motif ligands and receptors that contribute to MDSC and, respectively, T cell migration. TNFα, tumor necrosis factor α; TGFβ, transforming growth factor β; IL1β, interleukin 1 β; IL6/10, interleukin 6/10; GM-CSF, granulocyte macrophage colony stimulating factor; SCF, stem cell factor; Flt3, Fms-like tyrosine kinase 3; ARG1, arginase 1; iNOS, inducible nitric oxide synthase; NO, nitric oxide; S100A8 and S100A9, S100 calcium binding proteins; ADAM17,ADAM disintegrin and metallopeptidase domain 17; STAT, signal transducer and activation of transcription; HIF-1α, hypoxia inducible factor 1α; Myd88, myeloid differentiation primary response protein 88; proto-oncogene c-kit, SCF receptor; VEGFR, vascular endothelial growth factor receptor.
Table 1.
Summary of pre-clinical and clinical compounds that target MDSC development, expansion and activation
| Compound | Class of compounds | Targeted processes | Mechanisms of action | Refs. |
|---|---|---|---|---|
| Zolendronic acid | Bisphosphonates | MDSC migration | c-kit cleavage | 18-21, 39 |
| Curcumin derivatives | Natural compounds | MDSC formation MDSC maturation |
Selective inhibition of the JAK2/STAT3 pathway | 16,17,32 |
| Sunitinib | Tyrosine kinase inhibitors | MDSC formation MDSC depletion |
- Inhibition of STAT3 phosphorylation and activation - Inhibition of c-kit and VEGFR functions |
12-15, 20 |
| All-trans retinoic acid (ATRA) | Vitamin A metabolites | MDSC maturation | Activation of ERK pathway | 5, 25-27 |
| Vitamin D3 | Vitamins D | Not known | 3,29 | |
| 3, 5, 7-trihydroxy-4′-emthoxy-8-(3-hydroxy-3-methylbutyl)-flavone (ICT) | Icariin derivatives | - Inhibition of S100A8/9 expression - Inhibition of STAT3 and AKT pathways |
30 | |
| MPSSS poly-saccharide | Lentinus edodes derived compounds | Stimulation of the Myd88-dependent NF-κB signaling pathway | 31 | |
| Bevacizumab (Avastin) | Humanized monoclonal antibodies | MDSC expansion | Blockade of VEGF signaling | 38 |
| Celecoxib | Nonsteroidal anti-inflammatory drugs (NSAID) | T cell nutrient depletion | Inhibition of COX2 | 48 |
| Sildenafil and tadalafil (Viagra and Cialis) | PDE-5 inhibitors | Inhibition of PDE-5 in MDSCs | 49 | |
| N-hydroxy-L-Arginine (NOHA) | Intermediate of NO biosynthesis | Inhibition of ARG1 function | 6 | |
| Nitroaspirin | NSAID | Induction of oxidative stress | Inhibition of ARG1 and iNOS functions | 52 |
| N-acetyl cysteine (NAC) | Cysteine derivatives | Inhibition of ROS production | 53 | |
| CpG oligodeoxy-nucleotides (ODN) | Short single-stranded synthetic DNA molecules | Inhibition of ARG1 and iNOS production | 54-56 | |
| Bardoxolone methyl (CDDO-Me) | Synthetic triterpenoids | STAT3 inhibition and upregulation of antioxidant genes | 57,58 | |
| Withaferin A | Withaferin somnifera derived compounds | Stimulates glutathione, superoxide dismutase and catalase production | 59,60 | |
| Monoclonal anti-Gr1 antibody | Monoclonal antibodies | Local intra-tumoral MDSCs | Specific targeting of all Gr1+ cells | 64 |
| IL4Rα aptamer | RNA aptamers | - Inhibition of STAT6 signaling - Induction of MDSC apoptosis |
65-67 | |
| Gemcitabine | Nucleoside analogs | Induction of MDSC apoptosis and necrosis | 31 | |
| 5-fluorouracil (5-FU) | Pyrimidine analogs | Induction of MDSC apoptosis | 70,71 | |
| Peptibodies | Genetically fused peptide sequences | Local intra-tumoral and intra-splenic MDSCs, circulating MDSCs | Targeting of MDSC-membrane proteins (S100 family) | 72 |
*Abbreviations: JAK2 / STAT3, Janus kinase 2 / signal transducer and activator 3; proto-oncogene c-kit, Mast/stem cell growth factor receptor; VEGFR, vascular endothelial growth factor receptor; ATRA, all-trans retinoic acid; ERK, extracellular signal-regulated kinases; ICT, 3, 5, 7-trihydroxy-4′-emthoxy-8-(3-hydroxy-3-methylbutyl)-flavone; Myd88, myeloid differentiation primary response protein 88; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NSAID, nonsteroidal anti-inflammatory drugs; COX2, cyclooxygenase 2; PDE-5, phosphodiesterase type 5; NOHA, N-hydroxy-L-arginine; NO, nitric oxide; NAC, n-acetyl cysteine; ODN, oligodeoxynucleotides; ARG1, arginase 1; iNOS, inducible nitric oxid synthase; CDDO-Me, bardoxolone methyl; IL4Rα, interleukin 4 receptor α; 5-FU, 5-fluorouracil.
MDSC Development—Prevention or Differentiation to Mature Cells
MDSCs increase in abundance under the duress of pathological conditions, such as in response to inflammation and cancer. In the case of malignant disease, a tumor-driven ‘microenvironment’ arises6 characterized by alterations in cytokine homeostasis.3 Immature myeloid cells (IMCs) are blocked en route during differentiation from hematopoietic stem cells (HSCs) to mature granulocytes, macrophages or dendritic cells. This cell-fate specification blockage results in accumulation of IMCs with immunosuppressive activities, which are collectively called MDSCs.1 Local cell-cell signaling mediators, such as the interleukins IL-1β and IL-6, as well as tumor necrosis factor α (TNFα), granulocyte monocyte colony stimulating factor (GM-CSF), and vascular endothelial growth factor (VEGF) stimulate development of MDSCs in the bone marrow. In contrast, tumor-associated cytokines, such as the C-C and C-X-C motif chemokines CCL2, CXCL12 and CXCL15, induce recruitment of the newly formed MDSCs to the tumor site.9
Strategies that prevent MDSC formation and migration
MDSC differentiation depends on signaling through cellular tyrosine kinases. For example, intracellular signal transducer and activator of transcription 3 (STAT3) activation stimulates expression of proliferative genes in IMCs and promotes their subsequent development to MDSCs.10 Thus, compounds able to block STAT3 activation are currently being investigated as potential anticancer agents.11
Sunitinib is a broad-spectrum tyrosine kinase inhibitor that selectively targets MDSCs. Treatment with sunitinib strongly decreases functionality of c-Kit and vascular endothelial growth factor receptor (VEGFR), 2 receptors localized on both cancer cells and MDSCs, and involved in skewing of MDSCs toward an immunosuppressive phenotype. Sunitinib was shown to have significant, though not curative, clinical therapeutic effects in metastatic renal cell carcinoma (mRCC), as circulating MDSCs in mRCC patients were reduced by 50% after treatment. In addition, sunitinib treatment has been shown to diminish Treg levels and improv cytotoxic T lymphocyte (CTL) response.12,13 Sunitinib has been reported to have inhibitory effects on various tyrosine kinases and to selectively decrease MDSC levels, in spleens alone13 or in both tumors and spleens of various preclinical models,14 while concurrently increasing numbers and functionality of CD8+ and CD4+ T cells. Importantly, one cycle of sunitinib treatment \ significantly reduced IL-4 production and increased ]interferon γ (IFNγ) producing T cells in mRCC patients.15
Curcumin is a diarylheptanoid (diferuloylmethane) derived from the rhizome of the East Indian plant Curcuma longa, a member of the ginger family (Zingiberaceae). A component of the spice turmeric, curcumin downregulates the activity of several pro-inflammatory enzymes, such as cyclooxygenase-2 (COX2) and lipoxygenase. Curcumin derivatives are naturally occurring phenols usually used for their anti-oxidant and anti-inflammatory activities. Recently, curcumin derivatives were described to modulate cell signaling, by inhibiting the Janus kinase 2 (JAK2) and STAT3 signaling pathways in MDSCs,16 sensitizing tumor cells to antigen-specific CTLs and inducing differentiation of immature dendritic cells (DCs)17 Furthermore, treatment with curcumin derivatives has no negative effects on the viability and activity of human peripheral blood mononuclear cells.16 More studies in several murine tumor models are warranted to further elucidate the therapeutic potential of curcumin derivatives.
An alternative approach is to block mobilization of the already formed MDSCs from the bone marrow into the circulation. Bisphosphonates have been used primarily to prevent loss of bone mass in patients with bone metastases. However, recent studies have shown that bisphosphonates inhibit enzymes responsible for the generation of prenyl groups, which are added to metalloproteinases (MMPs) during post-translational modifications.18 Decreased prenylation of MMPs, such as MMP9, affect c-Kit cleavage and diminish MDSC mobilization from the bone marrow.19 MMP9-induced mobilization of VEGF and subsequent binding to it's cognate receptor VEGFR on MDSCs are also prenylation dependent, and thus, bisphosphonate sensitive. Furthermore, combination of the n-bisphosphonate zolendronic acid with a plasmid DNA vaccine encoding rat p185/Her-2 was shown to decrease circulating MDSC levels, enhance anti-p185 antibody levels and decrease tumor growth.20 These results have been recapitulated in other tumors models in which treatment with zolendronic acid increased IFNγ levels,decreased intratumoral MDSC levels and attenuated tumor growth.21
Strategies that promote MDSC maturation
One to 5 percent of MDSCs can form myeloid cell colonies and approximately one third of this population differentiates into mature, non-suppressive cells in the presence of specific cytokines.1 Stimulation of MDSC differentiation into mature myeloid cells without immunosuppressive functionalities is thus a promising strategy.
A compound well-known to have this stimulant effect is the all-trans retinoic acid (ATRA) metabolite of vitamin A. Various studies showed enhanced MDSC levels in the bone marrow and spleen of vitamin A deficient mice22 and in mice treated with a pan-retinoic-acid-receptor antagonist.23 In contrast, MDSC levels decreased upon ATRA administration, due to rapid stimulation of MDSC differentiation to DCs and macrophages in vitro and in vivo.5,24 ATRA activated the ERK1/2 kinase pathway leading to up-regulation of the reactive oxygen species (ROS) scavenger glutathione (GSH)25 and reduction of ROS levels in MDSCs.26 Importantly, Mirza et al. reported no significant toxicity in cancer patients receiving ATRA treatment.27 Furthermore, a vaccine targeting the H-2Db-restricted epitope of the human papillomavirus 16 (HPV-16) E7 protein in combinationwith ATRA decreased the growth of implanted C3 fibrosarcomas by 3-fold.24 A similar effect was observed in response to a combinatorial therapy in which p53-primed DCs were administered with ATRA to treat murine sarcomas expressing a mutant p53 gene. However, ATRA was also shown to induce the development of CD4+ regulatory T cells (Tregs), by upregulating expression of the T cell cell-fate regulatory transcription factor FoxP3.28 Thus, ATRA not an ideal candidate for MDSC depletion, as simultaneous Treg induction induced by ATRA treatment could further contribute to tumor development. These methods have not yet been widely investigated in clinical trials,25 however, more studies are warranted.
Another promoter of myeloid cell differentiation is 1α,25-dihydroxyvitamin D3. Testa et al. showed that vitamin D3 induced monocytic maturation in several leukemic cell lines.29 In contrast to such reports claiming vitamin D3 to be a direct promoter of myeloid cell maturation, others have suggested that vitamin D3 exerts its effects indirectly. For example, Serafini et al. showed that vitamin D3 efficiently reduced tumor growth when applied in mice with high levels of GM-CSF (Csf-2) activated MDSCs.3
Recently, treatment of immature myeloid cells with the flavonoid Icariin and its derivative 3, 5, 7-trihydroxy-4′-emthoxy-8-(3-hydroxy-3-methylbutyl)-flavone (ICT) has been reported to decrease MDSC percentages, likely due to differentiation to mature myeloid cells.30 This differentiation was shown to be induced by inhibition of S100A8/9 expression and the STAT3 and AKT signaling pathways. Upon ICT treatment, MDSCs produced levels of NO and ROS decreased simultaneously with increased CD8+ T cell IFNγ.30
Lastly, the novel Lentinus edodes-derived polysaccharide MPSSS was also found to induce MDSC differentiation, reducing MDSC levels and inhibiting tumor growth.31 Similarly, the naturally occurring antitumor compound curcumin that, as described above, is able to inhibit MDSC development from immature myeloid cells, has been suggested to also induce MDSC maturation.32
MDSC Expansion and Activation—Blockade Mechanisms
In healthy individuals, the bone marrow contains about 20–30% MDSCs, the spleen about 2–4% and the lymph nodes contain no MDSCs.1 In cancer patients a substantial increase in MDSCs in the periphery is often seen.4,5,27 MDSC enhancement, a process thought to occur in the bone marrow and the spleen,33 is influenced by 2 main types of factors: (i) promoters of MDSC expansion, and (ii) mediators involved in MDSC functional activation.
Strategies that block MDSC expansion
Mediators known to enhance the expansion of MDSCs are: cyclooxygenase-2 (COX2), prostaglandins, stem-cell factor (SCF),6 macrophage colony-stimulating factor (M-CSF), IL-6, granulocyte/macrophage colony-stimulating factor (GM-CSF) and vascular endothelial growth factor (VEGF). These mediators trigger activating pathways that involve Janus kinase (JAK) and signal transducer and activator of transcription (STAT).1 STAT3 is the transcription factor often considered to be the key mediator in regulation of MDSC expansion, as it promotes myelopoiesis and inhibits myeloid cell differentiation. STAT3 can operate through targeting genes encoding c-MYC, BCL-XL, cyclin D1 and survivin.1 Additionally, STAT3 induces expression of the inflammatory proteins S100A8 and S100A934 on the cell surface membrane of MDSCs. GM-CSF, another mediator of MDSC expansion, plays a role only in expansion of monocytic, but not granulocytic MDSCs.35 Thus, several studies aimed to directly neutralize these mediators in order to abrogate their MDSC expansion-promoting effects. Recently, stem cell factor (SCF)-mediated signaling of MDSCs was repressed by blocking the interaction with its receptor c-Kit. This inhibition resulted in decreased MDSC expansion and tumor angiogenesis.36
Other mediators involved in MDSC proliferation are VEGF, IL-6 and MMPs. Interestingly, during a clinical trial in which cancer patients were treated with antibodies neutralizing VEGF, (termed VEGF-traps), there was no change in MDSC levels.37 However, in another setting in which RCC (Caki-1) tumor-bearing animals were treated with the VEGF-specific blocking antibody avastin, MDSC levels in the peripheral blood were significantly reduced.38 Curcumin, thenaturally occurring antitumor compound that can inhibit MDSC formation, acts via STAT3 blockade, and abrogates MDSC expansion via its ability to inhibit MDSC-mediated IL-6 secretion.32 Inhibition of MMP-9, a downstream target of STAT3, lowered intratumoral MDSC levels and significantly delayed tumor growth.39 The mechanism by which STAT3 blockade leads to a reduction in MDSC expansion is not fully elucidated yet,40 however, it is not advisable to delete STAT3 with the purpose of MDSC targeting, as Welte et al. showed that transgenic mice with a STAT3 deletion in the bone marrow died within 2 months of a Crohn's disease-like inflammatory syndrome.41
Strategies that block MDSC activation
IFNγ, IL-13, IL-4 and transforming growth factor β (TGFβ), produced by stromal cells after tumor cell death, are molecules that promote MDSC activation, by inducing intracellular factors such as STAT1 and STAT6. In turn, these factors induce the expression of iNOS, arginase and suppressive cytokines, which are hallmarks of MDSC-mediated immunosuppressive activity.1 A possible intervention in the activation of MDSCs is to block IFNγ. Movahedi et al. found that an anti-IFNγ antibody largely abolished polymorphonuclear (PMN)-MDSC-mediated immune suppression.42 Another factor reported to participate in MDSC activation is TGFβ secreted by non-T and non-B cell splenocytes. Terabe et al indicated that these non-lymphoid splenocytes from tumor-bearing mice produce large amounts of CTL inhibitory TGFβ. This phenomenon has been identified as a response to IL-13 produced by CD1d restricted lymphocytes.43 More studies unraveling the mechanisms by which the TGFβ/TGFβR pathway regulates MDSC polarization toward an immunosuppressive phenotype are warranted.
Suppressive Activity of MDSCs—Blockade of Inhibitory Mechanisms
MDSCs have a remarkable ability to suppress both innate and adaptive immune responses1 while promoting tumor angiogenesis, cell invasion and metastasis.6 The immunosuppressive effects of MDSCs depend on direct cell-cell contact through membranous receptors or short-lived soluble mediators.1 Mechanisms and strategies that may counteract these effects of MDSCs are discussed below.
Strategies that inhibit nutrient depletion
MDSCs can deplete nutrients needed for the synthesis of lymphocyte-activating proteins.6 For instance, MDSCs can deplete L-arginine44 and L-cysteine,45 2 amino acids required for T-cell differentiation. To do so, they need arginase-1 (ARG1) and inducible nitric oxide synthase-2 (iNOS2), for which L-arginine is a substrate. MDSCs highly express these enzymes as a result of cytokine stimulation, including IL-10, IFNγ, and TNFα. ARG1 converts L-arginine into urea and L-ornithine and iNOS2 metabolizes it into nitric oxide (NO) and L-citrulline. These conversions result in a down-regulation of the ζ-chain of the T cell receptor (TCR) complex, thus disturbing the normal signal transmission required for activation. Also, NO produced by MDSCs interferes with T cell JAK-STAT signaling proteins required for various T-cell effector functions, thus inhibiting MHC Class II expression and inducing T-cell apoptosis.25
Several attempts have been made to inhibit this MDSC-mediated nutrient depletion. Firstly, methods were applied to inhibit the upregulation of ARG1 expression. COX2 stimulates production of prostaglandin E2 (PGE2), which, in some tumor models, was suggested to up-regulate ARG1 expression in MDSCs.1 Indeed, blockade of PGE2 synthesis in tumor-bearing mice and humans resulted in improved antitumor T-cell responses.46 This was confirmed by studies reporting a downregulation of ARG1 expression in MDSCs after administration of COX2 inhibitors, followed by enhanced antitumor T-cell responses and immunotherapy efficacy.46,47 Furthermore, the use of the COX2 inhibitor celecoxib reduced levels of tumor-infiltrating MDSCs and potentiated dendritic cell-based immunotherapy.48
Phosphodiesterase 5 (PDE5) inhibitors such as sildenafil (Viagra) and tadalafil (Cialis) act likewise as COX inhibitors. They down-regulate the expression of ARG1 and iNOS in MDSCs,49 by inhibiting the degradation of cyclic guanosine monophosphate (cGMP). This downregulation resulted in MDSC inhibition, induction of antitumor immune responses and delay of tumor progression. Particularly, CD8+ T-cell tumor infiltration, activation and proliferation were enhanced. The mechanism by which immunosuppressive MDSC functions were attenuated was through a decrease in levels of IL-4α receptor as well as those of the effector molecules ARG1 and iNOS. PDE-5 inhibitors have a good safety profile and are already used for treatments of erectile dysfunctions, pulmonary hypertension and cardiac hypertrophy.49 Currently, a Phase II clinical trial is ongoing in which head and neck squamous cell carcinoma (HNSCC) patients are treated with tadalafil combined with the conventional HNSCC therapy (clinicaltrials.gov ID NCT01697800).
Another compound well known for its inhibitory effect on ARG1 activity is N-hydroxy-L-arginine (NOHA). In vitro studies have reported that NOHA inhibits ARG1 function, and thus MDSC suppressive capacities. Concomitantly, NOHA inhibited MDSC-mediated Treg expansion. It is widely known that Tregs are detrimental for tumor control, as clonal expansion of antigen-specific natural Tregs translates into de novo stimulation of FoxP3+ Tregs.50 When these FoxP3+ Tregs are generated, the activation and expansion of tumor antigen-specific T cells are abolished.25
Strategies that block the induction of oxidative stress
The second type of suppressive mechanisms involves MDSC induction of oxidative stress via production of ROS and reactive nitrogen species (RNS). These reactive species, which are the result of cooperative activities of NADPH oxidase, ARG1, iNOS and TGFβ,6 cause loss of TCR ζ-chain expression and desensitization of the TCR.51 In vitro studies revealed a complete abrogation of the MDSC suppressive effect when ROS production was repressed.25
Several ROS inhibitors that block MDSC-induced oxidative stress have been examined. Nitroaspirin countered ARG1 and iNOS activity in splenic MDSCs.52 A comparable agent, N-acetyl cysteine (NAC), reduced ROS production and increased the extracellular pool of cysteine.53 ARG1 and iNOS production was also inhibited by CpG oligodeoxynucleotides (ODN), which additionally induced antitumor type 1 macrophage differentiation.54-56 ROS levels could also be reduced by synthetic triterpenoids, such as bardoxolone methyl (CDDO-Me), via upregulation of antioxidant genes.57 In higher concentrations CDDO-Me also inhibited STAT3. Moreover, CDDO-Me therapy decreased MDSC-mediated ROS production, enhanced T-cell function and reduced murine tumor growth.58 This compound was tested in a Phase I clinical trial with pancreatic cancer patients receiving gemcitabine, resulting in significantly enhanced T-cell responses (Clinical Trial No. RTA 402-C-0702).
Lastly, root extracts of the plant Withaferin somnifera (WRE) has been investigated because of its tumor growth reducing properties.59 Withaferin A (WA), its most abundant constituent, shows antitumor effects via its antioxidant properties when tested against cultured and xenografted tumor cells. Sinha et al. investigated the effects of WA on MDSCs and found that WA indeed reduced MDSC-mediated immune suppression, thus making it an interesting compound for anti-MDSC therapy.60
Strategies that reverse blockade of lymphocyte trafficking and viability
Various studies have shown that MDSCs can also influence lymphocyte tumor trafficking and viability. When expressed on the plasma membrane of MDSCs, disintegrin and ADAM metallopeptidase domain-containing protein 17 (ADAM17) downregulate CD62L (L-selectin) expression on the surface of naïve T cells,6 thus limiting T-cell recirculation to lymph nodes.61 As a result, T cells do not encounter tumor antigens presented by APCs in the lymph nodes, and as a result, are not activated.27 Other means by which MDSCs mediate immunosuppression include decreased effector CD8+ T-cell migration to tumors,62 T-cell apoptosis,63 and interference with natural killer (NK) cell function. MDSCs prevent NK cell production of IFNγ, a cell-cell contact dependent phenomenon involving the NK cell activation receptor NKG2D and membrane-bound TGFβ.25
Local MDSCs at the Tumor Site
MDSC-induced immunosuppression generates notorious hallmarks of cancer development, of which angiogenesis is crucial. After migrating to tumors, MDSCs release factors that promote blood vessel formation. Also, they generate MMPs, such as MMP-9, which release matrix-bound VEGF and recruit pericytes that can form new blood vessels. That MDSCs directly stimulate the process of tumor development was demonstrated by the correlation between the inhibition of MDSC tumor migration and decreased tumor angiogenesis.25
Strategies that deplete intra-tumoral MDSCs
More than a decade ago, treatment of tumor-bearing mice with monoclonal anti-Gr-1 antibody (clone RB6–8C5) resulted in enhanced CD8+ T-cell function and a delay in tumor progression in vivo.64 However, this antibody also targets neutrophils, thus lacking the necessary specificity for clinical use.3
Specific targeting of MDSCs can be achieved with an engineered RNA aptamer.65 This compound is specific for mouse and human IL4Rα, a membranous receptor that is upregulated on the surface of MDSCs present in tumor-bearing mice66 and cancer patients.67 Treatment with this RNA aptamer leads to intratumoral MDSC apoptosis, enhanced T-cell infiltration and delayed tumor growth. Notably, treatment with the aptamer alone did not cause complete tumor regression. However, because the aptamer was able to engage the receptor, this method remains an interesting potential therapeutic agent.65
Another way to target MDSCs is with chemotherapeutic drugs like gemcitabine. Gemcitabine is a nucleoside analog that effectively lowers MDSC levels in the spleen, thus improving antitumor responses induced by immunotherapy. This decrease is due to gemcitabine-induced MDSC cell death through apoptosis and necrosis.68 Le et al. reported significant inhibition of tumor growth and decrease in splenic MDSC levels after gemcitabine treatment when started 5 d after tumor inoculation. This treatment also resulted in expansion of splenic T cells and a boost of their IFNγ secretion after stimulation with the proper tumor antigen in vitro. Furthermore, MDSC suppression was also revealed in bone marrow and blood harvested 24 and 48 h after gemcitabine treatment.69
The pyrimidine analog 5-fluorouracil (5-FU) is another cytostatic drug with MDSC-specific cytotoxicity. Five-FU administration to tumor-bearing mice increased survival, possibly due to enhanced cytotoxic T-cell activation. As compared to gemcitabine, 5-FU induced a more potent apoptosis-mediated MDSC depletion in vitro and in vivo.70 However, 5-FU treatment was not curative in this tumor model because of Nlrp3 inflammasome induction, which led to MDSC-derived IL-1β secretion and angiogenesis.70 As a solution, combinatorial treatment of 5-FU together with anti-IL-1β could be a successful therapeutic approach.71
In a recent study performed by Qin H. et al., efficient depletion of both intratumoral granulocytic and monocytic murine MDSC subsets was achieved with the help of a new therapeutic peptibody that specifically targets MDSCs without affecting other proinflammatory immune cells, such as DCs.72 The peptibody was generated by genetically fusing sequences encoding mouse MDSC-binding peptides, (and previously identified by phage display), with those encoding the Fc portion of mouse immunoglobulin G2b (IgG2b). Intravenous peptibody administration completely depleted intratumoral, intrasplenic and circulating MDSC subsets. When intravenously administered to EL4 tumor-bearing mice for a 2-week period, peptibody treatment induced superior tumor growth inhibition as compared to anti-Gr1 monoclonal antibody treatment. Although the mechanism of action of this peptibody is thought to be through the S100 family of proteins expressed on the surface of MDSCs, more studies are warranted to completely elucidate this mechanism. Nevertheless, this novel approach to deplete MDSCs seems very promising, as it offers the advantage of specifically targeting both MDSC subsets.
MDSC-targeting compounds that also counteract tumor-promoting phenomena
Some of the compounds reported to target MDSC development, expansion or activation can also counteract non-MDSC related tumor-promoting phenomena. For example, the tyrosine kinase inhibitor sunitinib is also a reported inhibitor of tumor angiogenesis, through blockade of VEGF 1–3 receptors. Another dual acting compound able to inhibit angiogenesis is withaferin A.59,60 However, while some of these compounds alone induce tumor regression due to their multiple tumor-targeting mechanisms, studies focus nowadays on combinatorial therapies with superior antitumor effects.
Combination Therapies
New studies focused on combining MDSC targeting methods with various immunotherapies are emerging. Most commonly, MDSC-targeting compounds employed in multi-therapy approaches are chemotherapeutics already approved for clinical use. In a study combining a DNA-vaccine with a Pseudomonas-derived neurotoxin, MDSC depletion was induced by administration of IL-13 linked to the respective neurotoxin (IL-13-PE). Addition of this neurotoxin to the DNA vaccine against IL-13 receptor led to a significantly greater decrease in tumor growth of IL-13 receptor positive MCA307 sarcomas, as compared to vaccination alone.73 Combination of the nucleoside analog gemcitabine with IFNβ treatment led to an enhancement of antitumor immune activity superior to that observed in either treatment alone.68 A multimodal therapy regimen composed of gemcitabine, a HER-2/neu vaccine and monoclonal antibodies against anti-glucocorticoid tumor necrosis factor receptor related protein (GITR) managed to break self-tolerance and induced strong antitumor immunity in a tolerogenic HER2/neu mouse tumor model.74 Similarly, a significant increase in tumor-specific T-cell responses and a prolonged antitumor effect was observed in various tumor models after combined treatment of ATRA with 2 different cancer vaccines.24
Recent studies suggest that combination of sunitinib with various immune-based cancer therapies may effectively enhance their efficacy (Table 2).75,76 Despite these promising preclinical results, combination of the TroVax vaccine with sunitinib in a Phase III clinical trial did not lead to enhanced survival, as compared to sunitinib alone.77 This can be explained by the fact that sunitinib might not be able to directly enhance intratumoral vaccine-induced T-cell infiltration. In our hands, administration of sunitinib followed by therapeutic immunization with a viral vector based vaccine directed against human papilloma virus led to a stable decrease of intratumoral, intrasplenic and circulating MDSC levels and a concomitant increase of total and antigen-specific CD8+ T cells. Additionally, this multi-therapy induced a regression of tumor growth, thereby leading to 75% of mice exhibiting tumor-free survival in a pre-clinical model of HPV-induced cancer (Table 2, unpublished data).
Table 2.
Summary of combination therapies aimed at effective MDSC targeting to potentiate antitumor therapeutic immunization
| Combined therapies | Results in pre-/clinical settings | Refs. |
|---|---|---|
| Zolendronic acid + plasmid DNA vaccine encoding rat p185/Her-2 | - significant decrease in MMP-9 expression in tumor stroma - reduced MDSC expansion in bone marrow and peripheral blood - enhanced anti-p185 antibody levels - impaired tumor growth in (FVB × BALB-neuT)F1 mice |
20 |
| ATRA + peptide vaccine containing an H-2Db-restricted epitope of the HPV-16 E7 protein / p53-transduced DC vaccine | - reduced number of immature myeloid cells (due to differentiation) - increased CD8-mediated T cell responses in fibrosarcoma or sarcoma tumor-bearing mice - synergy with different cancer vaccines in reducing tumor growth |
24 |
| IL13-PE immunotoxin + IL13Rα2-targeted DNA vaccine | - prolonged survival and inhibition of tumor growth in established 4T1 breast carcinoma and MCA304 sarcoma tumor models | 73 |
| Gemcitabine + IFNβ / Her-2/neu and anti-GITR mAb | - specific depletion of intrasplenic MDSCs - increase in antitumor activity of CD8+ T cells and activated NK cells - induction of antitumor immunity in a tolerogenic murine tumor model |
74 |
| Sunitinib + SFVeE6,7 viral vector immunization / low-dose local tumor irradiation / DC vaccine / adoptive T cell therapy | - Decreased intrasplenic, intratumoral and circulating MDSC levels - Increased infiltration of total and antigen-specific T cells in tumors - Enhanced antigen-specific CTL to MDSC ratio and reduced MDSC to Treg ratio - Synergistically induced tumor regression and increased survival |
(our unpublished data)75,76 |
*Abbreviations: MMP9, metalloproteinase 9; HPV-16, human papillomavirus 16; DC, dendritic cell; IL13, interleukin 13; IFNβ, interferon β; GITR, anti-glucocorticoid tumor necrosis factor receptor; mAb, monoclonal antibody; NK cells, natural killer cells; SFVeE6,7, Semliki Forest virus E6,7; CTL, cytotoxic T lymphocyte; Treg, regulatory T cell.
In the last decade, several studies have reported that local tumor irradiation can enhance immunotherapy efficacy, either by increasing localized APC uptake of tumor antigens or by enhancing intratumoral migration of immunization-induced antigen-specific CTLs.78,79 Also, addition of sunitinib to low-dose local tumor irradiation led to improved survival in a mouse glioma model.80 These promising results, make the multi-therapy approach of sunitinib, local tumor irradiation and therapeutic immunization constitute an interesting option to analyze. However, a very careful dose scheduling and treatment succession of these therapies must be employed, combined with stringent monitoring of ongoing studies. Meticulous orchestration of the timing and dosage of such treatments is necessary and justified since sunitinib has been previously reported to inhibit T-cell priming when administered simultaneously with therapeutic immunization, an effect that does not occur upon sequential administration.81
Concluding Remarks
Current knowledge indicates MDSC-mediated immune suppression as a main hallmark of cancer development. Given that many promising MDSC targeting agents are already FDA-approved, their implementation in bi- or multi-therapy regimens that include cancer immunotherapy is warranted.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Dutch Cancer Society; Grant number: KWF 2009–4549.
References
- 1. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 2004; 172:989-99; PMID: [DOI] [PubMed] [Google Scholar]
- 3. Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 2006; 16:53-65; PMID:; http://dx.doi.org/ 10.1016/j.semcancer.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 4. Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007; 13:721s-6s; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2197 [DOI] [PubMed] [Google Scholar]
- 5. Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001; 166:678-89; PMID: [DOI] [PubMed] [Google Scholar]
- 6. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58:49-59; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mantovani A. The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol 2010; 40:3317-20; PMID:; http://dx.doi.org/ 10.1002/eji.201041170 [DOI] [PubMed] [Google Scholar]
- 9. Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 2008; 111:5457-66; PMID:; http://dx.doi.org/ 10.1182/blood-2008-01-136895 [DOI] [PubMed] [Google Scholar]
- 10. Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol 2009; 9:470-81; PMID:; http://dx.doi.org/ 10.1016/j.coph.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 11. Sansone P, Bromberg J. Targeting the interleukin-6/jak/stat pathway in human malignancies. J Clin Oncol 2012; 30:1005-14; PMID:; http://dx.doi.org/ 10.1200/JCO.2010.31.8907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148-57; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1332 [DOI] [PubMed] [Google Scholar]
- 13. Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010; 70:3526-36; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kodera Y, Katanasaka Y, Kitamura Y, Tsuda H, Nishio K, Tamura T, Koizumi F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res 2011; 13:R66; PMID:; http://dx.doi.org/ 10.1186/bcr2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 2008; 14:6674-82; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-5212 [DOI] [PubMed] [Google Scholar]
- 16. Bill MA, Fuchs JR, Li C, Yui J, Bakan C, Benson DM, Jr, Schwartz EB, Abdelhamid D, Lin J, Hoyt DG, et al. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol Cancer 2010; 9:165, 4598-9-165; PMID:; http://dx.doi.org/ 10.1186/1476-4598-9-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm 2012; 27:495-503; PMID:; http://dx.doi.org/ 10.1089/cbr.2012.1219 [DOI] [PubMed] [Google Scholar]
- 18. Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest 1996; 97:2692-6; PMID:; http://dx.doi.org/ 10.1172/JCI118722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002; 109:625-37; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res 2007; 67:11438-46; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother 2012; 61:1373-85; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, De Luca LM, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 2000; 95:3349-56; PMID: [PubMed] [Google Scholar]
- 23. Walkley CR, Yuan YD, Chandraratna RA, McArthur GA. Retinoic acid receptor antagonism in vivo expands the numbers of precursor cells during granulopoiesis. Leukemia 2002; 16:1763-72; PMID:; http://dx.doi.org/ 10.1038/sj.leu.2402625 [DOI] [PubMed] [Google Scholar]
- 24. Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 2003; 63:4441-9; PMID: [PubMed] [Google Scholar]
- 25. Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: targets for therapy. Oncoimmunology 2013; 2:e24117; PMID:; http://dx.doi.org/ 10.4161/onci.24117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res 2007; 67:11021-8; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2593 [DOI] [PubMed] [Google Scholar]
- 27. Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 2006; 66:9299-307; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma J, Liu Y, Li Y, Gu J, Liu J, Tang J, Wang J, Ryffel B, Shen Y, Brand D, et al. Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J Leukoc Biol 2014; 95:275-83; PMID:; http://dx.doi.org/ 10.1189/jlb.0513297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Testa U, Masciulli R, Tritarelli E, Pustorino R, Mariani G, Martucci R, Barberi T, Camagna A, Valtieri M, Peschle C. Transforming growth factor-beta potentiates vitamin D3-induced terminal monocytic differentiation of human leukemic cell lines. J Immunol 1993; 150:2418-30; PMID: [PubMed] [Google Scholar]
- 30. Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY, Wei S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol 2011; 11:890-8; PMID:; http://dx.doi.org/ 10.1016/j.intimp.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu H, Tao N, Liu X, Li X, Tang J, Ma C, Xu X, Shao H, Hou B, Wang H, et al. Polysaccharide from lentinus edodes inhibits the immunosuppressive function of myeloid-derived suppressor cells. PLoS One 2012; 7:e51751; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0051751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, Liu A, Wang TC, Yang CS. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012; 5:205-15; PMID:; http://dx.doi.org/ 10.1158/1940-6207.CAPR-11-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010; 70:3526-36; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007; 81:28-37; PMID:; http://dx.doi.org/ 10.1189/jlb.0306170 [DOI] [PubMed] [Google Scholar]
- 35. Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 2012; 72:876-86; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 2008; 111:219-28; PMID:; http://dx.doi.org/ 10.1182/blood-2007-04-086835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, Sosman JA, Gabrilovich DI. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res 2007; 13:4840-8; PMID:; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 38. Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 2008; 181:346-53; PMID: [DOI] [PubMed] [Google Scholar]
- 39. Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res 2007; 67:11438-46; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005; 11:1314-21; PMID:; http://dx.doi.org/ 10.1038/nm1325 [DOI] [PubMed] [Google Scholar]
- 41. Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A 2003; 100:1879-84; PMID:; http://dx.doi.org/ 10.1073/pnas.0237137100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008; 111:4233-44; PMID:; http://dx.doi.org/ 10.1182/blood-2007-07-099226 [DOI] [PubMed] [Google Scholar]
- 43. Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1 d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med 2003; 198:1741-52; PMID:; http://dx.doi.org/ 10.1084/jem.20022227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004; 64:5839-49; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0465 [DOI] [PubMed] [Google Scholar]
- 45. Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 2010; 70:68-77; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol 2007; 7:140-51; PMID:; http://dx.doi.org/ 10.1016/j.intimp.2006.09.021 [DOI] [PubMed] [Google Scholar]
- 47. Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 2005; 65:3044-8; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-4505 [DOI] [PubMed] [Google Scholar]
- 48. Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. celecoxib influences MDSC function. BMC Cancer 2010; 10:464, 2407-10-464; PMID:; http://dx.doi.org/ 10.1186/1471-2407-10-464; 10.1186/1471-2407-10-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006; 203:2691-702; PMID:; http://dx.doi.org/ 10.1084/jem.20061104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res 2010; 70:99-108; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 2007; 13:828-35; PMID:; http://dx.doi.org/ 10.1038/nm1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A 2005; 102:4185-90; PMID:; http://dx.doi.org/ 10.1073/pnas.0409783102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007; 12:230-8; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol 2012; 188:1592-9; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1101304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdorfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol 2002; 169:3892-9; PMID: [DOI] [PubMed] [Google Scholar]
- 56. Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, Hammerling GJ. NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol 2001; 167:5247-53; PMID: [DOI] [PubMed] [Google Scholar]
- 57. Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 2007; 7:357-69; PMID: [DOI] [PubMed] [Google Scholar]
- 58. Konopleva M, Zhang W, Shi YX, McQueen T, Tsao T, Abdelrahim M, Munsell MF, Johansen M, Yu D, Madden T, et al. Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-overexpressing breast cancer cells. Mol Cancer Ther 2006; 5:317-28; PMID: [DOI] [PubMed] [Google Scholar]
- 59. Muralikrishnan G, Dinda AK, Shakeel F. Immunomodulatory effects of withania somnifera on azoxymethane induced experimental colon cancer in mice. Immunol Invest 2010; 39:688-98; PMID:; http://dx.doi.org/ 10.3109/08820139.2010.487083 [DOI] [PubMed] [Google Scholar]
- 60. Sinha P, Ostrand-Rosenberg S. Myeloid-derived suppressor cell function is reduced by withaferin A, a potent and abundant component of withania somnifera root extract. Cancer Immunol Immunother 2013; 62:1663-73; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 2009; 183:937-44; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0804253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 2011; 208:1949-62; PMID:; http://dx.doi.org/ 10.1084/jem.20101956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging tim-3 functions in antimicrobial and tumor immunity. Trends Immunol 2011; 32:345-9; PMID:; http://dx.doi.org/ 10.1016/j.it.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci U S A 1995; 92:6254-8; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res 2012; 72:1373-83; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2772 [DOI] [PubMed] [Google Scholar]
- 66. Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 2006; 116:2777-90; PMID:; http://dx.doi.org/ 10.1172/JCI28828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol 2009; 182:6562-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0803831 [DOI] [PubMed] [Google Scholar]
- 68. Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713-21; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0883 [DOI] [PubMed] [Google Scholar]
- 69. Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol 2009; 9:900-9; PMID:; http://dx.doi.org/ 10.1016/j.intimp.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 70. Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70:3052-61; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3690 [DOI] [PubMed] [Google Scholar]
- 71. Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19:57-64; PMID:; http://dx.doi.org/ 10.1038/nm.2999 [DOI] [PubMed] [Google Scholar]
- 72. Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 2014; 20:676-81; PMID:; http://dx.doi.org/ 10.1038/nm.3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nakashima H, Terabe M, Berzofsky JA, Husain SR, Puri RK. A novel combination immunotherapy for cancer by IL-13Ralpha2-targeted DNA vaccine and immunotoxin in murine tumor models. J Immunol 2011; 187:4935-46; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1102095; 10.4049/jimmunol.1102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res 2007; 67:7477-86; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-4639 [DOI] [PubMed] [Google Scholar]
- 75. Bose A, Taylor JL, Alber S, Watkins SC, Garcia JA, Rini BI, Ko JS, Cohen PA, Finke JH, Storkus WJ. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer 2011; 129:2158-70; PMID:; http://dx.doi.org/ 10.1002/ijc.25863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009; 69:2514-22; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, McDonald M, Eastty S, Shingler WH, de Belin J, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res 2010; 16:5539-47; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2082 [DOI] [PubMed] [Google Scholar]
- 78. Draghiciu O, Walczak M, Hoogeboom BN, Franken KL, Melief KJ, Nijman HW, Daemen T. Therapeutic immunization and local low-dose tumor irradiation, a reinforcing combination. Int J Cancer 2014; 134:859-72; PMID:; http://dx.doi.org/ 10.1002/ijc.28418 [DOI] [PubMed] [Google Scholar]
- 79. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174:7516-23; PMID: [DOI] [PubMed] [Google Scholar]
- 80. D'Amico R, Lei L, Kennedy BC, Sisti J, Ebiana V, Crisman C, Christensen JG, Gil O, Rosenfeld SS, Canoll P, et al. The addition of sunitinib to radiation delays tumor growth in a murine model of glioblastoma. Neurol Res 2012; 34:252-61; PMID:; http://dx.doi.org/ 10.1179/1743132812Y.0000000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer 2012; 130:1948-59; PMID:; http://dx.doi.org/ 10.1002/ijc.26219 [DOI] [PMC free article] [PubMed] [Google Scholar]