Abstract
The insulin-like growth factor-1 receptor (IGF-1R) plays a crucial role in cellular growth, proliferation, transformation, and inhibition of apoptosis. A myriad of human cancer types have been shown to overexpress IGF-1R, including breast and pancreatic adenocarcinoma. IGF-1R signaling interferes with numerous receptor pathways, rendering tumor cells resistant to chemotherapy, anti-hormonal therapy, and epidermal growth factor receptor (EGFR, also known as HER-1) and v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2, (ERBB2, best known as HER-2) -targeted therapies. Targeting the IGF:IGF-1R axis with innovative peptide inhibitors and vaccine antibodies thus represents a promising therapeutic strategy to overcome drug resistance and to provide new avenues for individualized and combinatorial treatment strategies. In this study, we designed, synthesized, and characterized several B-cell epitopes from the IGF-1:IGF-1R axis. The chimeric peptide epitopes were highly immunogenic in outbred rabbits, eliciting high levels of peptide vaccine antibodies. The IGF-1R peptide antibodies and peptide mimics inhibited cell proliferation and receptor phosphorylation, induced apoptosis and antibody-dependent cellular cytotoxicity (ADCC), and significantly inhibited tumor growth in the transplantable BxPC-3 pancreatic and JIMT-1 breast cancer models. Our results showed that the peptides and antibodies targeting residues 56–81 and 233–251 are potential therapeutic and vaccine candidates for the treatment of IGF-1R-expressing cancers, including those that are resistant to the HER-2-targeted antibody, trastuzumab. Additionally, we found additive antitumor effects for the combination treatment of the IGF-1R 56-81 epitope with HER-1-418 and HER-2-597 epitopes. Treatment with the IGF-1R/HER-1 or IGF-1R/HER-2 combination inhibited proliferation, invasion, and receptor phosphorylation, and induced apoptosis and ADCC, to a greater degree than single agents.
Keywords: antibodies, epitopes, immunogenicity, peptide mimics, resistance, vaccine candidates
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; EGFR/HER-1, epidermal growth factor receptor; ERBB2/HER-2, v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2; IGF-1R, insulin-like growth factor-1 receptor; MVF, measles virus fusion protein; PBMC, peripheral blood mononuclear cell.
Introduction
The insulin-like growth factor-1 receptor (IGF-1R) has been broadly implicated as a key regulator of the proliferation, growth, differentiation, and development of several human malignancies.1 Overexpression of IGF-1R frequently occurs in multiple human tumor types,2 including breast,3 pancreatic,4 and colorectal 5 cancers. Of particular clinical relevance, IGF-1R signaling is has been implicated in the development of therapeutic resistance by interfering with numerous receptor signaling pathways.2,6 Consequently, IGF-1R is emerging as a major prospective molecular target for anticancer therapeutic strategies.
Over-expression of IGF-1R has been detected in ∼80% of breast cancers7 and is associated with poor prognosis in patients with early-stage mammary cancer.8 Human breast cancer lesions also show relatively higher levels of IGF-1R in comparison to those in normal breast tissue samples and further, higher levels of phosphorylated IGF-1R correlate with poor patient outcome.9,10 In addition, IGF-1R overexpression plays a key role in pancreatic cancer.11 Advanced-stage pancreatic cancer is associated with high plasma levels of IGF-1R, indicating a potential role for this receptor in pancreatic cancer progression.12 Importantly, IGF-1R is highly expressed in human pancreatic cancer samples, but is completely absent in the surrounding benign tissues.13
IGF-1R has emerged as a particularly important target in cancers that have developed resistance to epidermal growth factor receptor (EGFR/HER-1) and v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2, best known as HER-2) targeted therapies. Specifically, IGF-1R overexpression14 and/or crosstalk to HER-2 has been shown to facilitate the development of trastuzumab resistance.15-17 In fact, IGF-1R overexpression and increased signaling have been reported in cell lines and clinical samples derived from trastuzumab-resistant breast cancers.18 Similarly, crosstalk between IGF-1R and EGFR/HER-119,20 has been shown to foster the development of resistance to EGFR/HER-1 blockade.21 Many studies support targeting IGF-1R in combination with HER-1- or HER-2-targeted approaches. For example, a preclinical study indicated that combined treatment with IGF-1R- and HER-2-targeted therapies resulted in synergistic growth inhibition22 of breast cancers, findings that have encouraged the development of a novel HER-2:IGF-1R bispecific antibody.23 Further, we previously showed that IGF-1R-targeting improves trastuzumab sensitivity in cells that have acquired trastuzumab resistance.17 Thus, targeting the IGF-1R signaling axis is a promising strategy, partly because it has the potential to bypass multiple mechanisms that promote resistance to currently employed targeted therapies against breast cancer.
The discovery of the important role of IGF-1R in malignant disease development and progression has led to the evaluation of anti-IGF-1R-targeted antibodies in a dose-escalation Phase I trial,as well as a Phase II combination trial with paclitaxel and carboplatin.24,25 IGF-1R-targeted immunotherapeutic approaches are particularly attractive, as they may potentially elicit even stronger antitumor responses than traditional targeted approaches. A combinatorial multi-antigen vaccine targeting HER-2, IGF-1R and insulin growth factor binding protein 2 (IGFBP2) in a transgenic mouse model of adenocarcinoma (TgMMTV-neu) significantly blocked tumor progression and the development of palpable tumors in 65% of mice; this antitumor response was mainly due to the activation of T-cell responses, especially of the CD4 type.26 Through our own published work, we have reported novel platforms to stimulate the immune system and to block cancer-related signaling pathways, approaches that may facilitate the development of active immunotherapy against a wide variety of tumor types.27-30 Also, diminished expression of growth hormone receptor and IGF-1 deficiencies have been reported to be associated with cancer risk in humans.31
In this present study, we report the antitumor effects of 2 novel peptide vaccines and B-cell epitope mimics that specifically target the IGF-1R. We synthesized 4 peptides designed to interact with the contact sites between IGF-1 and IGF-1R on the basis of crystal structure and mutagenesis. In the case of the peptide mimics, the B-cell epitopes were synthesized alone , or linked with the measles virus T-cell epitope to produce a chimeric peptide vaccine. The peptide vaccines were immunogenic in outbred rabbits; antibodies (Abs) raised against the vaccine specifically bound to IGF-1R-expressing breast and pancreatic cancer cells and specifically recognized the recombinant human IGF-1R protein. Both the peptide mimics and the anti-peptide antibodies inhibited IGF-1R-dependent signaling pathways. Importantly, treatment with the B-cell epitopes as peptide inhibitors significantly reduced tumor growth in transplantable models of breast and pancreatic cancer. The 56–81 and 233–251 B-cell epitopes proved the best candidates, either as vaccines or peptide mimics, as they elicited the most significant inhibitory effects both in vitro and in vivo. These results illustrate the therapeutic potential of 2 novel epitopes that target IGF-1R in multiple cancer types, including trastuzumab-resistant breast cancer.
Our main objective was to select novel IGF-1R peptide inhibitors for use in multiple cancers and in combination with our novel EGFR- or HER-2-targeted inhibitors, which we previously reported.32–34 Thus, we evaluated the effects of combining agents targeting the IGF-1R 56-81 epitope with those antagonizing the HER-1 418 epitope in breast and pancreatic cancers. Our results showed a significant decrease in proliferation and receptor phosphorylation when these epitopes were used in combination. Combination treatment also increased the induction of antibody-dependent cellular cytotoxicity (ADCC) and increased apoptosis, as evident by increased caspase activity after treatment. Further, our combination approach targeting IGF-1R and HER-2 produced greater antitumor effects in both trastuzumab-sensitive (BT-474) and trastuzumab-resistant (JIMT-1) human breast cancer cells. These results strongly support further development of our novel IGF-1R peptide vaccine-induced antibodies and B-cell epitope peptide mimics, particularly in combination with HER1- and HER2-targeted therapies in cancer.
Results
Epitope selection, design, synthesis, and initial characterization
We identified 4 potential target sequences (Table 1 and Fig. S1) derived from the IGF-1R ligand-binding domain by the usage of computer algorithms to predict the antigenicity, immunogenicity and secondary structure profiles of the protein.35 We next examined the 3-dimensional structure of IGF-1R to further delineate the exact candidate epitopes for functional studies36 (Fig. S2). The 4 epitopes, IGF-IR-6-26, IGF-IR-26-42, IGF-IR-56-81, and IGF-IR-234-252, so named according to their epitope span,were chemically synthesized and characterized, as previously described.32 Based on initial characterization in vitro, we selected 2 of the peptide sequences, IGF-1R-56-81 and IGF-1R-234-252 for integration into an engineered recombinant peptide with a “promiscuous” Measles virus fusion (MVF) T-cell epitope (KLLSLIKGVIVHRLEGVE) with a 4-residue (GPSL) linker sequence. We subsequently examined the immunogenicity of these peptide mimics in rabbits and mice, as previously described.30 Furthermore, we developed vaccine antibodies against these peptides for functional testing in vitro. Our rationale for epitope design was based on the ligand-binding site of the receptor using crystallographic structures, mutagenesis studies, and models of the receptor-ligand complex.37 The other HER-family receptor epitopes included herein encompassed HER-1-418,32 HER-2-266,33 and HER-2-597,34 HER-1 and HER-2 targeting epitopes, previously synthesized and characterized for their respective antitumor properties in vitro and in vivo as potential cancer vaccine candidates.
Table 1.
Sequences of IGF-IR peptide B-cell epitopes and chimeric peptide vaccines. The amino acid sequences of insulin growth factor receptor 1 (IGF-1R) peptides as well as the epidermal growth factor receptor (EGFR/HER-1) and v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2/HER-2) peptides, and the chimeric B-cell epitope vaccines engineered with the “promiscuous” T cell epitope of the measles virus fusion protein (MVF; italics) are shown. The B-cell epitopes were all acetylated (Ac) at the N-terminus and amidated (NH2) at the C- terminus. The flexible linker sequence GPSL was used in collinearly synthesizing the vaccines with MVF is underlined in the table and the molecular weights for each inhibitor is indicated.
| PEPTIDES | AMINO ACID SEQUENCE OF IGF-1R PEPTIDES AND CHIMERIC VACCINES | Mol.Wt (Da) |
|---|---|---|
| IGF-1R (6–26) | Ac-GIDIRNDYWWLKRLENCTVIE-NH2 | 2561 |
| IGF-1R (26–42) | Ac-EGYLHILLISKAEDYRSYRF-NH2 | 2515 |
| MVF-IGF-1R (56–81) | KLLSLIKGVIVHRLEGVE-GPSL-LLFRVAGLESLGDLFPNLTVIRGWKL-NH2 | 5267 |
| IGF-1R (56–81) | Ac-LLFRVAGLESLGDLFPNLTVIRGWKL-NH2 | 2969 |
| MVF-IGF-1R (233–251) | KLLSLIKGVIVHRLEGVE-GPSL-ACPPNTYRFEGWRCVDRDF-NH2 | 4670 |
| IGF-1R 233–251 | Ac-ACPPNTYRFEGWRCVDRDF-NH2 | 2372 |
| HER-1(418–435) | Ac-SLNITSLGLRSLKEISDG-NH2 | 1944 |
| MVF-HER-1(418–435) | KLLSLIKGVIVHRLEGVE-GPSL-SLNITSLGLRSLKEISDG-NH2 | 4242 |
| MVF-HER-2(597–626) | KLLSLIKGVIVHRLEGVE-GPSL-VARCPSGVKPDLSYMPIWKFPDEEGACQPL-NH2 | 5672 |
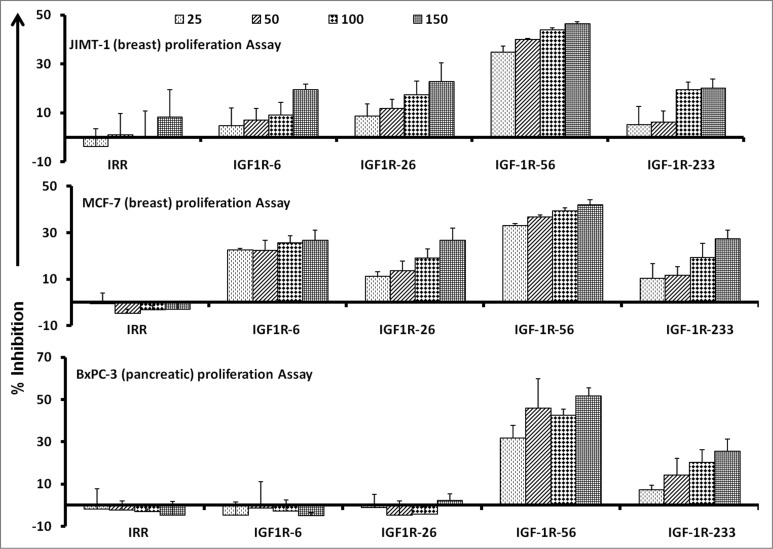
IGF-1R peptide mimics inhibit proliferation of breast and pancreatic cancer cells
To examine the functional activities of our IGF-1R targeting peptide constructs, we first evaluated the effects of the IGF-1R peptide mimics on the proliferation of human pancreatic (BxPC-3) and breast (MCF-7 and JIMT-1) cancer cells. Ligand binding to the IGF-1 receptor is known to activate intracellular signaling and subsequently increase cell proliferation. Thus, we set out to assess cancer cell proliferation (via MTT assay) in which cells were treated with the inhibitors at various concentrations and incubated for 3 d prior to the addition of the tetrazolium dye MTT. As shown in Figure 1, the IFG-1R peptide mimics successfully inhibit cancer cell proliferation. Results from the 3 different cancer cell lines showed that the IGF-1R-56 and IGF-1R-233 peptide mimics were the most consistent and significant inhibitors of proliferation. These particular IGF-1R targeting epitopes robustly inhibited the proliferation of all 3 cell lines in a dose-dependent manner, as shown in Figure 1. On the basis of these proliferation results, we decided to utilize these 2 epitopes to construct peptide vaccinescollinearly synthesized with a promiscuous T-helper epitope, as previously described.33
Figure 1.
IGF-1R peptide mimics inhibit proliferation of pancreatic and breast cancer cells. The indicated cancer cells were incubated with insulin growth factor receptor 1(IGF-1R) peptide mimics and irrelevant peptide (IRR) at various concentrations (ranging from 25–150 μg/mL) and incubated for 3 d prior to the addition of the tetrazolium dye MTT. The proliferation inhibition rate was calculated using the formula (OD normal untreated- OD peptide treated / OD normal untreated) × 100. Results are an average of 2 different experiments with each treatment performed in triplicate;error bars represent the mean ± S.D.; irrelevant peptide was used as the negative control.
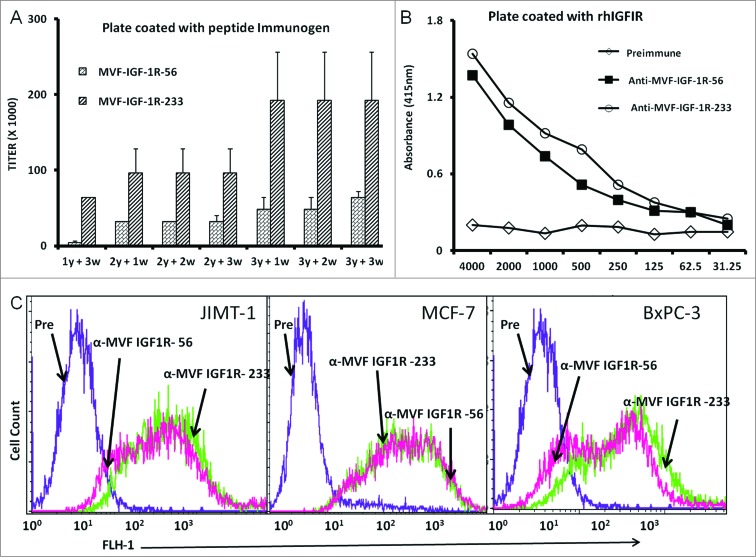
Immunogenicity of IGF-1R peptide vaccines in rabbits and cross-reactivity of vaccine antibodies to recombinant human IGF-1R
We next set out to evaluate the immune response elicited by administration of each of the 2 chimeric peptide vaccines constructs to outbred rabbits. Pairs of rabbits were immunized with the chimeric peptide vaccines emulsified with nor-muramyl dipeptide derivative (nor-MDP) as adjuvant in SEPPIC ISA 720 as the vehicle. The 2 vaccine constructs elicited high antibody production with titers greater than 100,000 in most cases. The IGF-1R-233 epitope exhibited the best immunogenicity stimulating the most extreme titers of anti-IGF-1R antibody (Fig. 2A). Antibody titers increased further after booster immunizations and remained high for the duration of the studies. These results demonstrated that the vaccine constructs were highly immunogenic and established immunological memory in the rabbits. Further, the vaccine antibodies were capable of binding to recombinant human IFG-1R (rhIGF-1R), as shown by ELISA assays (Fig. 2B). The binding of the recombinant peptides to rhIGF-1R appeared highly specific, as dilution of the antibodies was associated with a gradual decrease in binding. Furthermore, the pre-immune sera antibodies showed no binding, as expected.
Figure 2.
IGF-1R peptide vaccine is immunogenic in rabbits and generates antibodies that specifically interact with human IGF-1R. (A–C). Rabbits were immunized intramuscularly 3x per week over 3 week intervals with 1 mg of the indicated chimeric peptide vaccine emulsified with the adjuvant nor-muramyl dipeptide derivative (nor-MDP) in SEPPIC ISA 720 as the vehicle. (A) Antibody titers were defined as the reciprocal of the highest serum dilution that gives an absorbance of 0.2 after subtracting the presera levels. 1y +3w represent blood drawn 3 weeks after the first immunization while 3y +3w represent blood drawn 3 weeks after the third immunization. (B) Binding of vaccine antibodies to recombinant human IGF-1R and human cancer cell lines by ELISA analysis. Binding of purified vaccine antibodies to recombinant human IGF-1R protein was measured and specificity evaluated by dilution of the antibodies and a corresponding gradual decreased in binding. The antibodies from the presera showed no binding and were used as the negative control. In (A and B), results are an average of 2 different experiments with each treatment performed in duplicates. (C) Human cancer cell lines (JIMT-1, MCF-7 and BxPC-3) were incubated with peptide vaccine antibodies and the extent of cell binding was evaluated by immunofluorescence staining andcytofluorimetric analysis. A shift of the histogram to the right indicates increase in binding relative to pre-immune antibodies used as a negative control. Experiments were performed twice with similar results achieved and shown is a representative histogram from each treatment.
Cross-reactivity of vaccine antibodies in binding IGF-1R-expressing cells
We next set out to investigate the ability of the vaccine antibodies to recognize native IGF-1R on ovarian, pancreatic, and colon cancer cells. We used immunofluorescence staining and fluorescence cytofluorimmetric analysis to dissect the relative binding affinities of IGF-1R peptide mimic-induced vaccine antibodies. Antibodies generated against the 2 vaccine constructs were evaluated and showed high binding to the cell lines, whereas the pre-immune antibodies showed no binding (Fig. 2C).
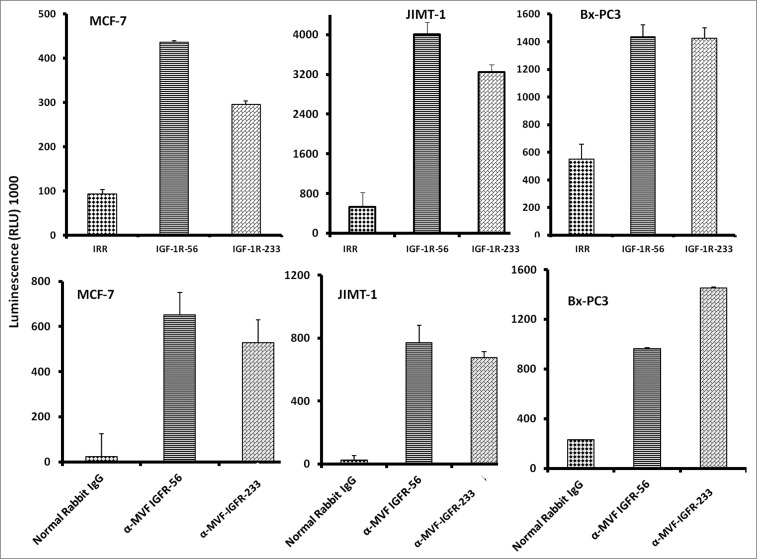
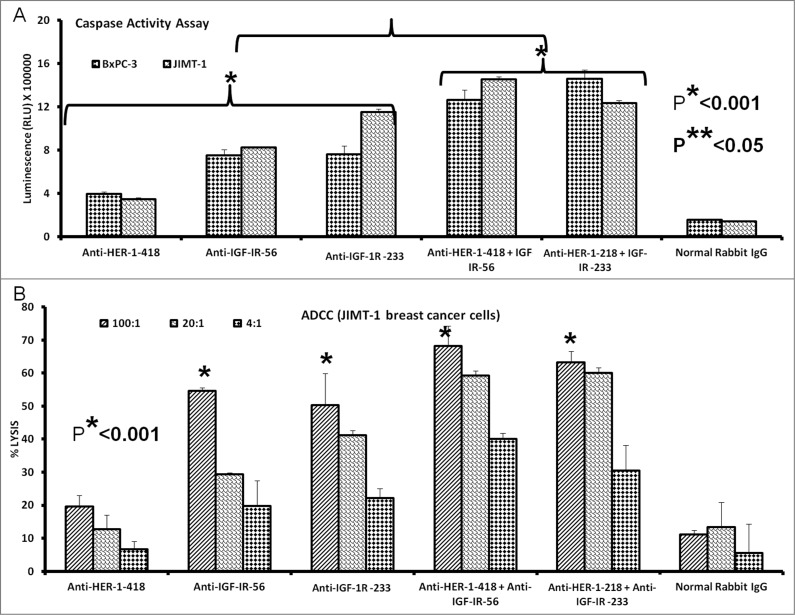
Apoptosis determination by caspase activity assay
We next evaluated whether the IGF-1R peptide mimics and/or the vaccine antibodies were capable of inducing apoptosis of cancer cells via a caspase activation assay. Caspase activity was measured after IGF-1R peptide mimics and/or the vaccine treatment using the Caspase-Glo assay. Breast cancer cells (MCF-7, JIMT-1, and BxPC-3) in exponential growth phase were seeded in 96-well plates and the following day, cells were treated with peptide mimics and/or vaccine antibodies as inhibitors and incubated for an additional day. After treatment, caspase 3/7 release was determined using the Caspase-Glo reagent as a measure of apoptotic induction We found that the IGF-1R inhibitors solicited a significant increase in the amount of caspase activity in treated cells as compared to either of the negative controls (irrelevant peptide and normal rabbit IgG). IGF-1R peptide mimics or vaccine antibodies increased caspase release more than 10-fold (Fig. 3), clearly indicative of increased apoptosis.
Figure 3.
IGF-1R peptide mimics and vaccine antibodies are capable of eliciting cancer cell apoptosis. Apoptosis was evaluated by measuring caspase activity after treatment with 150 μg/mL peptide vaccine antibodies. Cells were plated in 96-well plates and treated with inhibitors for 24 h prior to the addition of the caspase reporter (Casp-GLO) reagent. caspase activity with a luminometer. Normal rabbit IgG was used as a negative control. Results showed that the antibodies and peptide mimics increased caspase activity, indicating induction of apoptosis. Results shown are averages of 3 different experiments with each treatment performed in duplicates and error bars represent the mean ± S.D.
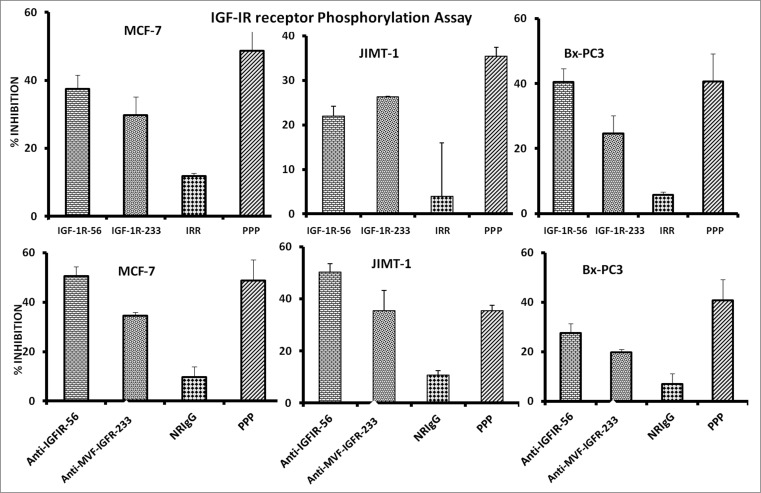
Downregulation of IGF-1R expression and phosphorylation after treatment with IGF-1R peptide mimics and vaccine anti-peptide antibodies
Considering that receptor phosphorylation is a key marker of IGF-1R activation, we next evaluated the effects of the peptide mimics and anti-peptide antibodies on IGF-1R phosphorylation in MCF-7, JIMT-1, and BxPC-3 cells. Phosphorylated levels of IGF-1R were measured via a sandwich ELISA method with anti-human-phospho-IGF-1R. Treatment significantly inhibited receptor phosphorylation in all 3 cell lines, indicating that the peptide mimics and vaccine antibodies reduced the activation status of IGF-1R (Fig. 4).
Figure 4.
IGF-1R peptide mimics and vaccine antibodies Inhibits of IGF-1R phosphorylation in breast (MCF-7 and JIMT-1) and pancreatic (BxPC-3) cancer cells. The indicated cancer cells were treated with the various inhibitors or controls (see below) at a concentration of 100 μg/mL and incubated for 1 h before stimulating with 50 ng/mL IGF-1 ligand for 10 min. Treated ells were then lysed in RIPA lysis buffer, and receptor phosphorylation was measured using human phospho-IGF-1R ELISA kits from R&D Systems. Cyclolignan PPP is an IGF-1R tyrosine kinase inhibitor (TKI) and was used as a positive control. Irrelevant peptide (IRR) and normal rabbit IgG (NrIgG) were used as negative controls. Results are an average of 2 different experiments with each treatment performed in triplicates and error bars represent the mean ± S.D.
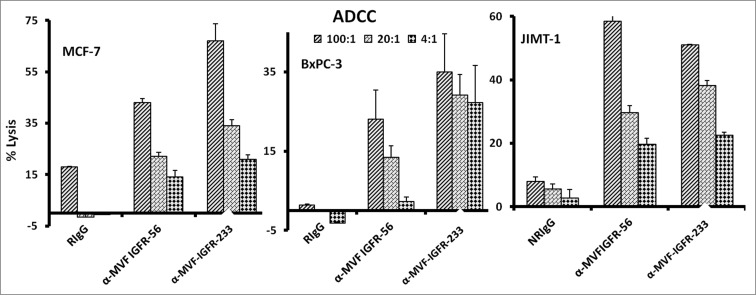
Vaccine antibodies induce ADCC of cancer cells
One major mechanism of immunologic action of antibodies is to induce targeted ADCC. This antibody-mediated phenomenon results from immunoglobulin Fc regions interacting with Fc receptors expressed on the surface of peripheral blood mononuclear cells (PBMCs), which subsequently attracts them to specific (in this case IGF-1R expressing) cellular targets. With this in mind, we hypothesized that active immunization with the MVF-IGF-1R peptides would elicit the production of high-affinity antibodies that would be capable of inducing ADCC of cancer cells. To address this possibility, we next measured the ability of the anti-peptide antibodies to mediate ADCC in vitro using MCF-7, JIMT-1, and BxPC-3 breast cancer cells as targets and PBMCs from normal human donors as effector cells. using a bioluminescence cytotoxicity assay kit (aCella-TOXTM), as previously described.38 We found that cancer cell lysis was, indeed,significantly increased following treatment with our vaccine antibodies. The effects increased as the effector to target cell ratio increased, with the greatest effects being achieved at an effector to target cell ratio of 100:1, as shown in Figure 5. These findings indicate that the novel vaccine antibodies against IGF-1R are capable of effectively stimulating human PBMCs to lyse cancer cells.
Figure 5.
Vaccine antibodies induce ADCC of cancer cells. Target cells (MCF-7, JIMT-1, and BxPC-3) were plated in the presence of human peripheral blood mononuclear cells (PBMCs) at an effector to target ratio of 100:1, 20:1, and 4:1 in triplicates, incubated with 100 μg/mL of the purified anti-peptide rabbit Abs or controls, and incubated for 2–4 hours at 37°C. Antibody dependent cell cytotoxicity (ADCC) was measured by a non –radioactive assay using the aCella-TOX reagent according to manufacturer's instructions. Results are an average of 2 different experiments with each treatment performed in triplicates and error bars represent the mean ± S.D.
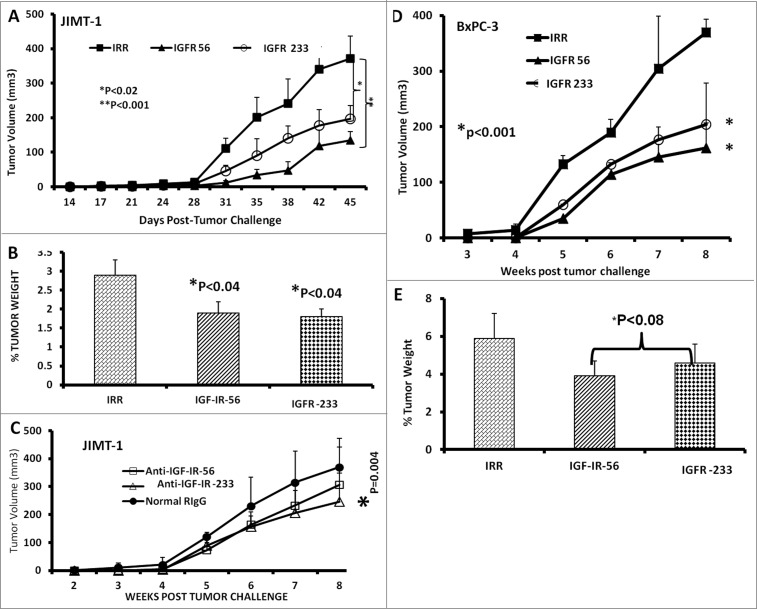
Therapy with IGF-1R peptide mimics prevents breast and pancreatic tumor growth in vivo in transplantable cancer mouse models
Next, we tested the in vivo effects of the peptide mimics. Transplantable breast and pancreatic mouse models were established by injecting BxPC-3 pancreatic or JIMT-1 breast cancer cells subcutaneously into the flanks of severe combined immunodeficiency disease (SCID) host mice. After tumor challenge, the mice were treated intravenously with the peptide mimics starting at d 0 (day of tumor challenge) and weekly for a total of 7 weeks. Tumor growth was monitored twice weekly. At the end of the treatment, all mice were euthanized,tumors extracted and weighed, and the percentage of tumor weight was calculated. Both of the IGF-1R peptide mimics significantly inhibited tumor growth of JIMT-1 cells (IGF-1R 56, *p<0.001; IGF-1R 234,*p<0.02) (Fig. 6A). In addition, the percentage of JIMT-1 tumor weight decreased after treatment with these epitopes (Fig. 6B). The anti-peptide antibody IGF-1R-234 also demonstrated significant (*p < .004) inhibition of tumor growth in the JIMT-1 transplantable mouse model (Fig. 6C). In the BxPC-3 transplantable mouse model, both IGF-1R peptide mimics significantly (*p < 0.001) inhibited tumor growth and development (Fig. 6D) and tended to reduce (*P < 0.08) tumor weights(Fig. 6E).
Figure 6.
IGF-1R peptide mimic therapy inhibits breast and pancreatic tumor growth. Effects of IGF-1R peptide mimics in transplantable mouse models of breast (A–C) and pancreatic (D and E) cancer. Balb/c SCID host mice (n=5 per group) at the age of 5–6 weeks old were injected subcutaneously with 5 × 10 6 human cancer cells (JIMT-1 and BxPC-3). From the day of tumor challenge (day 0), the mice were treated intravenously with 200 μg of the indicated peptide mimic or 500 μg of peptide vaccine antibody weekly until week 7. The percentages of tumor weights after treatment are shown; error bars represent the mean ± S.D.
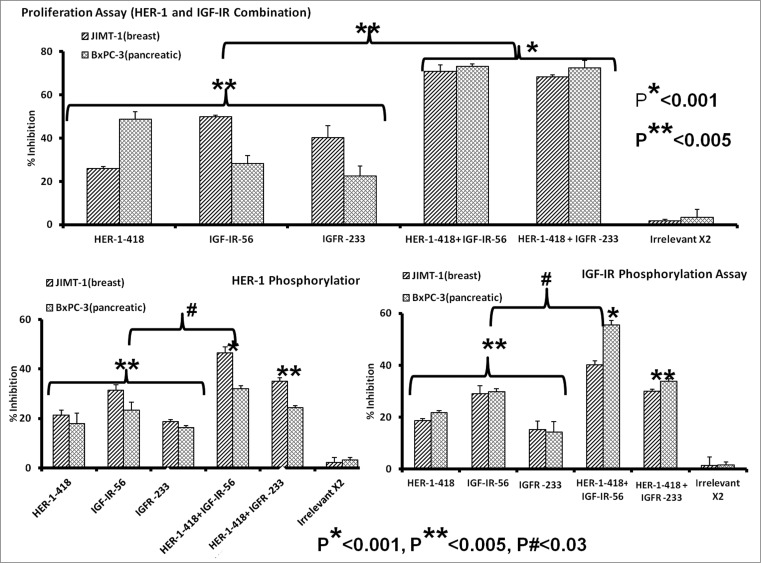
Combined HER-1 & IGF-1R peptide mimic treatment potently inhibits cancer cell proliferation and receptor phosphorylation
There is considerable evidence of crosstalk between EGFR/HER-1 and IGF-1R in breast and pancreatic cancer cells. HER-1 expression has been associated with poor patient outcome in aggressive pancreatic cancers39,40 and decreased overall survival.41-43 IGF-1R is expressed in 50–60% of pancreatic cancers,44,45 and, together with HER-1, is believed to be a key player in the oncogenesis of many types of cancers. Co-targeting HER-1 and IGF-1R has been previously observed to elicit robust antitumor responses against the MDA-MB-468 breast cancer cell line.46 Furthermore, we recently demonstrated antitumor effects of HER-1 peptide vaccines and peptide mimics in breast cancer cells both in vitro and in vivo.32 Combination treatment of JIMT-1 and BxPC3 cancer cells with HER-1-418 and either IGF-IR-56 or IGF-1R-234 peptides inhibited cell proliferation to a greater degree (*p < 0.001) than did single peptides (**p < 0.005) (Fig. 7A). Combined treatment was also a superior inhibitor of HER-1 (Fig. 7B) and IGF-1R (Fig. 7C) phosphorylation compared to that of single-agent peptide treatments (#p < 0.03). These results indicate that co-targeting HER-1 and IGF-1R with these new peptide mimics is a potentially beneficial approach for inhibiting breast and pancreatic cancers.
Figure 7.
Combination therapy with IGF-1R and HER-1 peptide mimics inhibits cancer cell proliferation and IGF-1R receptor phosphorylation. (A) Breast (5,000/well) and pancreatic (4,000/well) cancer cells were plated in a 96-well plate, treated with a combination of epidermal growth factor receptor (EGFR,/ HER-1) and IGF-IR peptide mimics as inhibitors and subject to MTT proliferation assay. (B and C) HER-1 and IGF-1R phosphorylation was measured using human phospho-HER-1/IGF-1R ELISA kits (R&D systems) after combination peptide mimic treatment of 1 x 106 cancer cells in a 6-well plate. Each inhibitor was used at a concentration of 200 μg, which was determined after a dose-dependent titration from 12.5 μg to 200 μg. Results are an average of 2 different experiments with each treatment performed in triplicates and error bars represent the mean ± S.D.; *p < 0.001; **p < 0.005; #p < 0.03.
Combination treatment with HER-1 and IGF-1R vaccine antibodies increases apoptosis and ADCC of human cancer cells
Combination treatment with peptide vaccine antibodies showed greater induction of apoptosis in breast and pancreatic cancer cells relative to monotherapy. Cancer cells (JIMT-1 and BxPC-3) in exponential growing phase were treated with peptide vaccine antibodies alone or in combination. Apoptosis was evaluated as previous by measuring caspase 3/7 release.We observed a significant increase in the amount of caspases released after vaccine antibody treatment, with the greatest caspase activity observed in response to combinatorial treatment of both JIMT-1 and BxPC-3 cancer cells with HER-1 and IGF-1R anti-peptide vaccine antibodies (*p < 0.001 in both cases) (Fig. 8A). We also evaluated the effects of combination treatment with HER-1 and IGF-1R vaccine antibodies on ADCC of JIMT-1 breast cancer cells (Fig. 8B). Treatment with the peptide antibodies induced ADCC, with potentiated ADCC observed in response to combinations of HER-1 and IGF-1R anti-peptide vaccine antibodies.
Figure 8.
Induction of cancer cell apoptosis and ADCC by combination treatment with HER-1 and IGF-1R peptide vaccine antibodies. Cancer cells treated in vitro with HER-1 and IGF-1R peptide vaccine antibodies were monitored for cell death induction via apoptosis assay and antibody dependent cellular cytotoxicity (ADCC) assay. (A) Breast (JIMT-1) and pancreatic (BxPC3) cancer cells apoptosis was evaluated by measuring caspase activity after combination treatment with 100 μg/mL each HER-1 and IGF-1R peptide vaccine antibodies . Cells were plated in 96-well plates and treated with inhibitors for 24 h prior to adding Caspase-GLO reagent and read with a luminometer. (B) Target cells (JIMT-1) were incubated with 100 μg of single or combination treatments of the peptide vaccine antibodies and assayed in the presence of human peripheral blood mononuclear cells (PBMCs) at an effector to target ratio of 100:1, 20:1, or 4:1 in triplicates. Lysis was measured using the acella-TOX kit according to manufacturer's instructions. Normal rabbit IgG was used as negative controls Results are an average of 2 different experiments with each treatment performed in triplicates;error bars represent the mean ± S.D.; *p < 0.001; **p < 0.05.
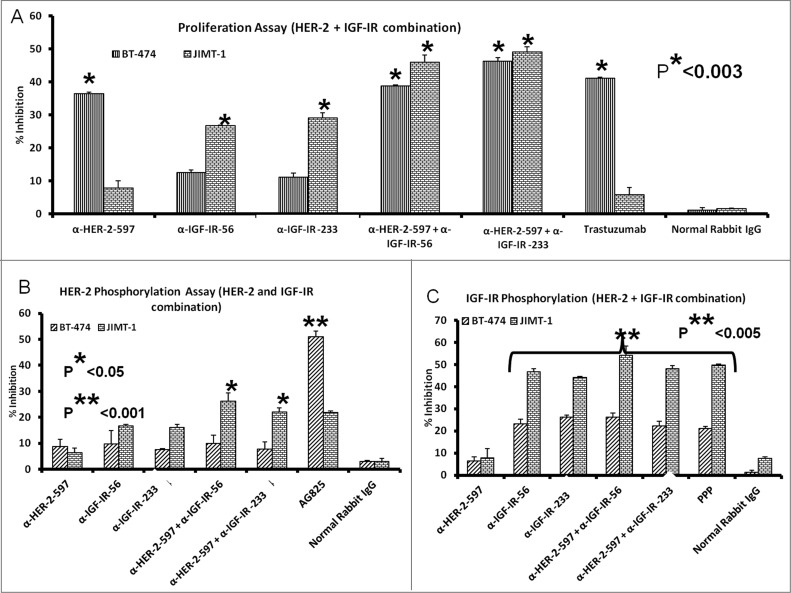
Combined HER-2 and IGF-1R anti-peptide antibody treatment in trastuzumab-resistant (JIMT-1) and trastuzumab-sensitive (BT-474) human breast cancer cells inhibits proliferation and receptor phosphorylation
Human breast cancer cells were treated with the anti-IGF-1R antibodies or a novel anti-HER2 antibody (HER2-597 anti-peptide vaccine antibody) alone or in combination. The anti-HER-2 antibody had a minimal effect on the proliferation of trastuzumab-resistant JIMT-1 cells but showed increased effects on the trastuzumab-sensitive BT-474 cell line, similar to that of the HER-2-targeted antibody, trastuzumab (Fig. 9A). Most importantly, combination treatment with the either the HER-2 or IGF-1R targeting antibodies elicited superior anti-proliferative effects in both the trastuzumab sensitive and resistant cell lines (*p < 0.003). As a measure of receptor activation, we also evaluated the effects of treatment with the anti-peptide antibodies on IGF-1R and HER-2 phosphorylation in JIMT-1 and BT-474 breast cancer cells. Protein lysates were obtained after treatment with single or combinations of antibodies to measure phosphorylated levels of the receptors using a sandwich ELISA method and human-phospho-HER-2/IGF-1R ELISA kits. Single agents and combination treatments inhibited HER-2 (Fig. 9B) and IGF-1R phosphorylation (Fig. 9C). Overall, our data demonstrate that antibodies raised against these novel peptide mimics inhibit receptor phosphorylation and proliferation of breast cancer cells, including those that are resistant to another HER2-targeted antibody.
Figure 9.
IGF-1R and HER2 antibody combination treatment inhibits proliferation and IGF-1R and HER-2 receptor phosphorylation in trastuzumab-resistant breast cancer cells. (A) MTT proliferation assay using combination treatment with v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2/HER-2) and IGF-1R antibodies (200 μg each) as inhibitors in JIMT-1 trastuzumab-resistant (plated at 4,000 cells/well) and BT-474 trastuzumab-sensitive (plated at 5,000 cells/well) breast cancer cells in a 96-well plate. (B and C) HER-2 and IGF-1R receptor phosphorylation was measured in 1 x 106 cancer cells per 6-well plate after combination treatment with IGF-1R and HER-2 antibodies (200 μg each) using human phospho-HER-2/IGF-1R ELISA kitsResults are an average of 2 different experiments with each treatment performed in duplicates and error bars represent the mean ± S.D.. p < 0.003, for proliferation, *p < 0.05 for HER-2 phosphorylation and **p < 0.005 for IGF-1R receptor phosphorylation.
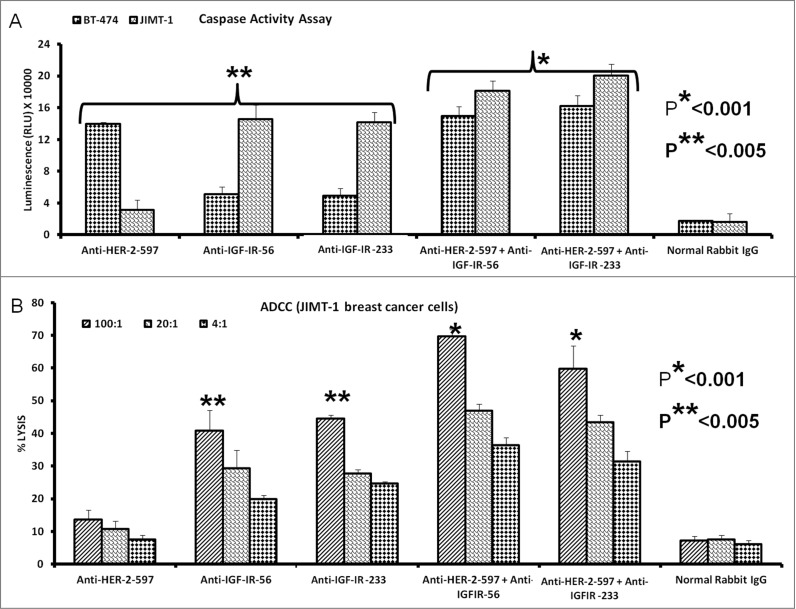
Combination treatment with HER-2 and IGF-1R vaccine antibodies potentiates breast cancer cell apoptosis and ADCC
We evaluated the effects of combination treatment with HER-2 and IGF-1R peptide vaccine antibodies on the induction of apoptosis in breast cancer cells by measuring the release of caspase enzymes after treatment. Human breast cancer cells (BT-474 and JIMT-1) were treated with the vaccine antibody combination as described above. As shown in Figure 10A combination treatment with anti-HER-2 and anti-IGF-1R peptide vaccine antibodies affected both trastuzumab-sensitive (BT-474) and resistant (JIMT-1) cells. Specifically, single treatment with the HER-2-targeted peptide antibody caused significant induction of apoptosis in the BT-474 breast cancer cells (**p < 0.005) but had little effect on the JIMT-1 trastuzumab-resistant breast cancer cells. Interestingly, combination treatment with the anti-HER-2 antibody and either of the IGF-1R targeting antibodies slightly increased apoptosis as compared to IGF-1R single treatments in the JIMT-1 cells (*p < 0.001), indicating that HER-2 and IGF-1R may cooperatively sustain cell survival in trastuzumab-resistant cells. In support of this concept, Figure 10B shows that the combination of HER-2 and IGF-1R antibodies increased tumor cell lysis mediated by ADCC in JIMT-1 breast cancer cells (*p < 0.001).
Figure 10.
Combination treatment with HER-2 and IGF-1R vaccine antibodies potentiates breast cancer cell apoptosis and ADCC. Induction of apoptosis and ADCC by combination treatment with HER-2 and IGF-1R peptide vaccine antibodies. (A) Apoptosis was evaluated by measuring caspase activity after combination treatment with 100 μg/mL each with anti-HER-2 and IGF-1R peptide vaccine antibodies () in both trastuzumab-sensitive (BT-474) and resistant (JIMT-1) breast cancer cells. Cells were plated in 96-well plates and treated with inhibitors for 24 h before adding caspase (Caspase-GLO) reagent and subsequent luminescence read with a luminometer. Normal rabbit IgG was used as negative controls. (B) Combination treatment with vaccine antibodies induces ADCC of cancer cells. Target cells (JIMT-1) were incubated with 100 μg of single or combination treatments of the peptide vaccine antibodies and assayed in the presence of human peripheral blood mononuclear cells (PBMCs) at an effector to target ratio of 100:1, 20:1, and 4:1 in triplicates. Effects of combination treatment of HER-2 and IGF-1R are shown; tumor cell lysis was measured as described in Figure 9. Results are an average of 2 different experiments and error bars represent the mean ± S.D.
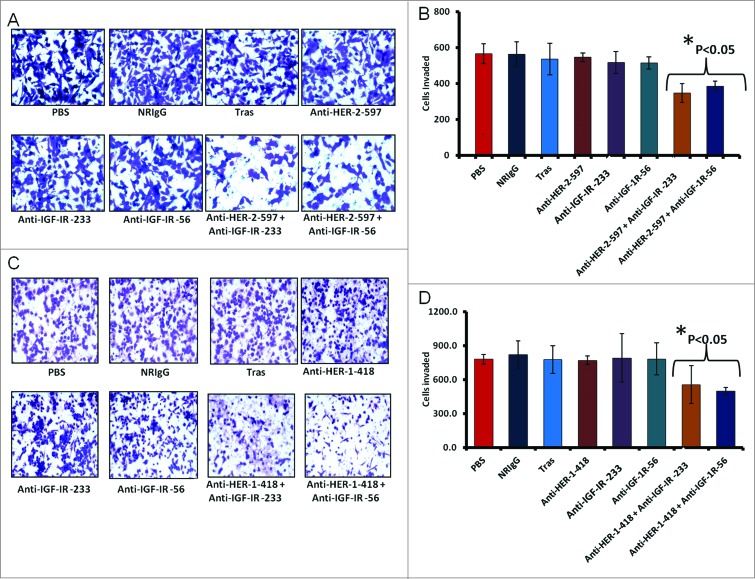
Combination treatment significantly inhibits cellular invasion in vitro
We further evaluated the effects of combination treatment on cellular invasion using trastuzumab-resistant JIMT-1 breast cancer cells treated for 24 hours with single agents or combinations of the HER-2 or IGF-1R antibodies. Single treatments did not significantly affect cellular invasiveness, with similar numbers of invasive cells detected relative to trastuzumab and control treated groups (normal Rabbit IgG and PBS) (Fig. 11A, B). In contrast, the combination of HER-2 and IGF-1R peptide mimics significantly (p < 0.05) inhibited the invasive potential of JIMT-1 cells (Fig. 11B). Anti-invasive effects were also observed following combination treatment with HER-1 and IGF-1R epitopes (Fig. 11C) with statistically significant effects were statistically significant (p < 0.05) compared to the control groups (Fig. 11D).
Figure 11.
Co-targeting HER-2 and IGF-1R suppresses invasiveness of trastuzumab-resistant breast cancer cells. (A and B) JIMT-1 cells were pretreated for 48 h with the indicated inhibitors before being seeded in Boyden matrigel-coated chambers with 10% FBS. After 24 h, invasion was measured by taking photos and counting the number of invaded cells in 10 random fields; results represent the average of triplicates per group. Representative photos (A) and quantitation of the invasive cells under the different treatment conditions (B). (C and D) Similar inhibition of invasive capabilities of JIMT-1 cells were observed via co-targeting HER-1 and IGF-1R. Boyden chamber migration and invasion assay was performed as described above; photographs (C) and quantitative results of invasive cell number are shown in (D). Results are an average of 2 different experiments and error bars represent the mean ± S.D.. Statistical analysis demonstrated that combination treatment with HER-2 and the IGF-1R vaccine antibodies or HER-1 and IGF-1R vaccine antibodies significantly (p < 0.05) inhibited invasion.
Discussion
In the present study, we sought to develop novel agents targeting the IGF-1R axis, an established oncogenic target in many cancers, including breast, colon, and pancreatic tumors.1 A principal rationale to prospectively tackle this receptor protein is its role in resistance to targeted therapies, such as trastuzumab.47 There are currently no approved IGF-1R-targeted agents available clinically, such that the development of therapies with the potential to block IGF-1R signaling are consequently a priority. Peptides as therapeutic tools are becoming increasingly popular due to their efficacy and non-toxic nature, as compared to other treatment modalities.29 We previously reported on novel peptides that target various HER family receptors. Here, we extended those studies using our peptide immunotherapeutic strategies to develop IGF-1R inhibitors for the treatment of solid tumors.
We thus designed peptide mimics targeting the ligand-binding domain of IGF-1R, identifying 4 B-cell epitopes, and synthesized the peptides. We tested the inhibitory effects of the 4 synthetic peptide mimics on the proliferation of breast and pancreatic cancer cells. Of note, the IGF-1R is overexpressed and highly implicated in the growth and metastasis of diverse human cancer cells. Therefore, blocking the receptor should prevent proliferation of cells that are dependent upon IGF-1R signaling. Indeed, we observed decreased proliferation after treatment with the peptide mimics. Two of the peptides (IGF-1R-56–81 and IGF-1R-234–252) significantly inhibited proliferation of all 3 cell lines used in this study. We decided to use these 2 epitopes to produce our peptide vaccines and evaluated their immunogenicity in rabbits. In the course we found that both of the 2 peptide vaccines were immunogenic in rabbits, and further, that the vaccine antibodies were able to specifically bind recombinant human IGF-1R. In order for the vaccine antibodies to attenuate cancer cell growth, they should be able to recognize the native IGF-1R on the surface of malignant cells. Vaccine antibodies after immunization showed a high rate of binding, as indicated by a shift to the right of the binding profile histogram obtained by flow cytometry.i]In contrast, the pre-immune sera did not bind, indicating that immunization produced IGF-1R-specific antibodies.
A major hallmark of cancer is the evasion of apoptosis.Naturally, effective anticancer agents generally induce apoptosis. Because apoptosis is associated with increased caspase activities, we evaluated the apoptotic effects of our peptide mimics and vaccine antibodies via increased caspase activity. After treatment, we observed increased caspase activity, presumably reflecting endogenous increased apoptosis. In addition, the peptide mimics and peptide vaccine antibodies significantly reduced phosphorylated levels of the receptor targets (IGF-1R, HER-2, HER-1), confirming that the intended molecular targets displayed reduced activation status, i.e., had been inhibited.
Antibody therapy usually induces ADCC due to interactions between the Fc regions with receptors on PBMCs, resulting in recruitment of immune cells to specific antibody-bound cellular targets. The Fab regions of the antibodies that we developed are specific for cell surface receptors expressed on cancer cells Thus, we hypothesized that the vaccine antibodies would induce ADCC of the targeted cancer cells. We evaluated these effects using a non-cytotoxicity luminescence assay. As predicted, the vaccine antibodies caused ADCC of all 3 cancer cell lines. The effects were highly dependent on the effector to target ratio, with a higher amount of effector cells causing more significant cancer cell lysis.
The antitumor effects of the peptide mimic inhibitors were next evaluated in 2 independent mouse models of transplantable human cancer (breast and pancreatic). One of these was a breast adenocarcinoma model of trastuzumab resistance. Resistance to trastuzumab has been shown to be mediated in part by increased IGF-1R signaling,17,48 indicating that resistance to HER-2-targeted agents, such as trastuzumab, may be overcome by blocking IGF-1R. JIMT-1 trastuzumab-resistant breast cancer cells were derived from a patient with HER2-overexpressing breast cancer and are intrinsically resistance to trastuzumab.49 Implicating the IGF-1R signaling axis as a key mediator of drug resistance phenomena, IGF-1R is known to be hyper-phosphorylated in this cell line. Combinatorial targeting of IGF-1R and HER-2 increased apoptosis and induced ADCC in this drug-resistant cell line. Furthermore, combination treatment with the IGF-1R inhibitors and either HER-1 or HER-2 inhibitors significantly reduced invasion of JIMT-1 breast cancer cells. Of particular clinical relevance, upon treatment of JIMT-1 xenograft tumors with the 2 most effective IGF-1R peptide mimics, we observed strong antitumor effects in vivo. Both peptide mimics significantly (p<0.001) inhibited tumor growth and development, such that the tumor burden after treatment was significantly (p<0.05) reduced. The vaccine antibody to the IGF-1R-234 peptide epitope also significantly inhibited JIMT-1 tumor growth. The IGF-1R-56-induced antibody showed diminished effects in vivo, despite displaying strong anti-proliferative effects in vitro. This discrepancy highlights the critical contributions of the tumor environment in situ on the anticancer effects of these peptide inhibitors, the differential responses to peptide vaccines versus the antibodies generated in response to the vaccines, and the need to test novel agents in vivo to faithfully recapitulate the complexity and bona fide nature of human tumors.
Independent studies have corroborated our findings, similarly showing that a combination approach concurrently targeting HER-2 and IGF-1R with a novel bispecific antibody displayed superior antitumor effects in mice than single targeting agents.50 Co-targeting HER-2 and IGF-1R with trastuzumab and heat-induced expression of dominant-negative IGF-1 receptor (486/STOP) has been shown to synergistically inhibited human breast cancer cells.16 Further, synergistic antitumor effects were reported with the combination of trastuzumab and the IGF-1R kinase inhibitor NVP-AEW541 preferentially in human breast cancer cells that are HER-2-dependent.51 These studies are consistent with our results and provide further evidence supporting a combinatorial strategy to simultaneously target IGF-1R and HER-2, particularly those occurring in breast cancers patients that have developed resistance to trastuzumab.
The second xenograft model that we tested in vivo was the BxPC-3 transplantable pancreatic cancer model. Similar to the JIMT-1 breast cancer model, tumor growth and development of BxPC-3 pancreatic cancer cells were significantly reduced by both IGF-1R epitopes. The 2 epitopes also significantly inhibited endpoint tumor weight in the treated mice (Pp < 0.04), demonstrating the potent anticancer effects of our novel IGF-1R peptide epitopes against both pancreatic and breast cancer cells in vitro and in vivo. Therefore, the 2 novel epitopes, namely IGF-1R-56–81 and 234–252, represent potential therapeutic candidates for targeting IGF-1R-expressing cancers.
Regardless of their initial response, often including tumor regression, most cancers eventually develop resistance to targeted anticancer agents. Nevertheless, this drawback can potentially be overcome by coincidently targeting multiple receptor signaling pathways. With this in mind, we evaluated 2 novel combination approaches targeting (i) HER-1 and IGF-1R in breast and pancreatic cancers and (ii) HER-2 and IGF-1R in trastuzumab-sensitive and -resistant breast cancer cells. We demonstrated that combination treatment with HER-1 and IGF-IR peptide mimics enhanced growth inhibition and downregulated receptor phosphorylation in breast and pancreatic cancer cells. These independent studies reflect similar results to those reported for gefitinib, a small-molecule EGFR/HER-1 kinase inhibitor. In this study, combination with the small-molecule IGF-1R kinase inhibitor AG1024, additive/synergistic effects on human breast cancer cells were achieved.52 Similarly, co-inhibition of EGFR and IGF-1R using the IGF-1R tyrosine kinase inhibitor AG1024 and the EGFR tyrosine kinase inhibitor AG1478 sensitized human breast cancer cells that were EGFR- and IGF-1R-dependent to radiation therapy.46 In addition to breast cancers, human pancreatic cancers are highly dependent on HER family receptors and IGF-1R. Afatinib, an EGFR/HER-1 small-molecule kinase inhibitor, synergistically inhibited the growth of human pancreatic cancer cells in combination with NVP-AEW541, an IGF-1R kinase inhibitor.53 These results are highly consistent with the antitumor effects we observed with our HER-1 and IGF-1R combinations of peptides or antibodies.
Our previous publications have focused heavily on the development of novel HER-2 vaccines 54–57 and state-of-the-art therapies based on blockade of receptor/ ligand interactions, such as those targeting the B7 ligand family interactions with the CD28 costimulatory receptor.58–60 We have also developed effective inhibitors of vascular endothelial growth factor signaling (VEGF/VEGFR2) 61 that diminish tumor angiogenesis. Furthermore, we have shown that co-targeting HER-2 and VEGF signaling produced superior antitumor effects in vitro and in vivo.62 We further validated combinatorial therapies targetingHER-2 and VEGF,63 and demonstrated that immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel elicited superior anti-neoplastic effects in transplantable and transgenic mouse models of human breast cancer.29 We recently translated our vaccine studies into a phase 1 clinical trial 38 and are presently conducting a new FDA-approved, NCI-funded phase 1/11b trial (NCT01376505) with an improved vaccine combination. These strategies possess the combined advantages of inhibiting multiple distinct receptors (HER-1:HER-2; HER-2:HER-2; and HER-2:HER3) to potentially overcome mechanisms of resistance to conventional HER-targeted therapies.64,65 The work presented here further builds on our immunotherapeutic approach targeting the HER family of receptors and provides strong rationale for co-targeting IGF-1R along with HER-1 or HER-2 via immunotherapeutic peptide or peptide-induced antibodies. These agents offer a novel strategy for treating pancreatic and breast cancers, as well as other types of cancers, including those that have progressed despite traditional receptor-targeted therapies.
Materials and Methods
Peptide selection, design, and synthesis
We identified candidate epitope sequences derived from the IGF-1R-ligand binding domain based on the ligand-binding site of the receptor using crystallographic structures, mutagenesis studies, and models of the complex of receptor-ligand interactions.37 Peptide synthesis was performed as previously described.66 Briefly, synthesis was performed on a solid-phase peptide synthesizer using Fmoc chemistry; crude peptides were purified by reverse-phase HPLC, after which characterization was done by mass spectrometry. Amino acid sequences and molecular weights of the different peptide mimics and peptide vaccines are shown in Table 1.
Animals
Balb/c SCID mice and New Zealand white rabbits were purchased from Charles River Laboratories; all animal care and use was in accordance with ULAR institutional guidelines.
Rabbit immunization and immunogenicity of peptide vaccines
Rabbits were immunized with 1 mg of peptide vaccine epitopedissolved in water and emulsified in Montanide ISA720 vehicle, as previously described.33 Rabbits were immunized intramuscularly 3 times at 3-week intervals using nor-MDP as adjuvant. Blood was collected weekly from the rabbits through the auricular artery; the blood was used to measure the immunogenicity of the peptide vaccines by measuring the antibody titers via ELISA. Plates were coated overnight with 100 μL of 2 μg/mL IGF-1R peptide vaccines and recombinant human IGF-1R protein. After incubation, plates were washed twice in PBS buffer before adding the substrate; binding was detected with a spectrophotometer at 415 nm. Peptide vaccine antibodies were purified by affinity chromatography using a protein A/G column and the concentration was measured by Coomassie protein assay.
Cell lines and inhibitors
The human breast cancer cell line MCF-7 and pancreatic cancer cell line BxPC-3 were purchased from the American Type Culture Collection (Manassas, VA) and maintained according to the supplier's instructions. JIMT-1 human breast cancer cells 67 were purchased from DSMZ (Germany) and were grown in regular Dulbecco's modified Eagle's medium (DMEM). These cells express high levels of the IGF-1R protein. All growth media, FBS, and other supplements were obtained from Invitrogen. The IGF-1R tyrosine receptor inhibitor PPP was purchased from Santa Cruz Biotechnology, Inc.
Proliferation or MTT cell growth inhibition assay
MTT proliferation assay was performed basically as previously described.29 Human cancer cells (MCF-7, JIMT-1, and BxPC-3) were plated in a 96-well plate and incubated overnight at 37°C before changing growth media to low serum media (1% FBS) and incubating for another 24 h. The low serum media was then aspirated, and new low serum media with the inhibitors and controls were added and incubated for 3 d. After 3 d of incubation, MTT was added to the plates and incubated for 2 h before adding extraction buffer. The plate was then incubated overnight and read the following day using a spectrophotometer at 570 nm. The percentage inhibition was calculated using the following formula: ODUNTREATED – ODTREATED / ODUNTREATED × 100.
IGF-1R receptor phosphorylationa Assay
Breast and pancreatic cancer cells that express high levels of the IGF-1R were plated on a 6-well plate and incubated overnight at 37°C. The next day, the inhibitors and controls were added to the wells in binding buffer (RPMI, 1% BSA, 0.002% NaN3) at a concentration of 100 μg/mL and incubated for 1 h before stimulating with 50 ng/mL IGF-1 ligand () for 10 min. The binding buffer was then aspirated, and cells were washed once with cold PBS before adding RIPA lysis buffer (Santa Cruz, CA). The cells were lysed by incubating at 4°C on a rotator for about 2 hours, after which the protein lysates were collected, and cell debris was removed by centrifugation at 10,000 rpm for 5 minutes. Phosphorylated levels of the IGF-1R were measured using a very sensitive, IGF-1R-specific sandwich ELISA, Human-Phospho IGF-1R ELISA (R&D Systems, MN). The ELISA measurement was performed according to the manufacturer's recommendations. The percentage inhibition was calculated using the formula: ODUNTREATED – ODTREATED / ODUNTREATED × 100.
Antibody-dependent cellular cytotoxicity assay
The ADCC assay was performed basically as previously described38 using effector PBMCs derived from normal human donors and enriched using density-gradient centrifugation in Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ). The cells were washed twice in RPMI 1640 with 5% FCS and then serially diluted in 96-well plates to give effector to target ratios of 100:1, 10:1, and 4:1. The following day 1 × 106 target cells (human breast and pancreatic cancer cells) were treated with 100 μg/mL total Protein A/G purified anti-peptide rabbit Abs or Controls. The cells were then incubated for 2–4 h at 37°C, after which cell death was measured by a non –radioactive assay using the a Cella-TOX (Promega) reagent kit according to the instructions from the manufacturer.
Caspase activity assay for apoptosis
Apoptosis was measured using the caspase activity assay (Caspase-GLO; Promega) as previously described.66 Cancer cells were plated in 96-well microtiter plates at 5 × 103 cells/well and incubated overnight at 37°C. Growth media containing peptides or anti-peptide antibody were added to the wells. The plates were incubated for 2 h at 37°C, and the Caspase-GLO reagent was then added. Apoptosis was evaluated by measuring the amount of luminescence (as readout of caspase activity) using a luminometer. The percentage of increased release of caspases was calculated using the formula (ODUNTREATED - ODTREATED)/ODUNTREATED × 100. All experimental treatments were performed in triplicate.
Flow cytometry
Binding of the peptide vaccine antibodies to the human breast (JIMT-1 and MCF-7) and pancreatic (BxPC-3) cancer cells were performed basically as previously described.61 The cells were grown to about 80% confluence before trypsinization followed by resuspension in 10 mL of appropriate growth media. The cells were then centrifuged at 10,000g and then resuspended to 1 × 107 cells/mL. Staining was performed using 1 × 106 cells (100 μL) incubated with blocking buffer containing sodium azide for 30 min (to prevent non-specific binding) followed by the rabbit peptide vaccine antibodies. aSince the antibodies were raised in rabbit hosts, binding was measured using the secondary goat-anti-rabbit IgG antibody coupled to Alexa Flour 488. The tubes were then washed twice in 1 mL ice cold PBS and the samples were then spun at 1,700 rpm for 5 min. After washing, the samples were then resuspended in PBS containing 1% formaldehyde before analysis using a Coulter ELITE flow cytometer (Beckman Coulter). A total of 10,000 cells were analyzed, and single-parameter histograms were drawn after gating selection of healthy cells through light scattered assessment using the FLOWJO data analysis software (The Ohio State University analytical Core Facility).
IGF-1R peptide treatments in transplantable mouse models of breast and pancreatic cancers
Balb/c SCID mice (n = 5) at the age of 5–6 weeks were challenged subcutaneously with human cancer cells 5 × 10 6 (JIMT-1 and BxPC-3). From the day of tumor challenge (day 0), the mice were treated intravenously with 200 μg of each peptide mimic weekly until week 7. Tumor growth was monitored twice weekly using Vernier calipers. Tumor sizes and weights were analyzed using Stata's cross-sectional estimating linear equation model, which allows one to specify repeated measurements.
Invasion chamber assays
JIMT1 cells at 1 × 105 cells/mL were plated in serum-free media in BD BioCoat Matrigel Invasion Chambers (BD Biosciences; Franklin Lakes, NJ) () with 0.75 mL of chemoattractant (culture media containing 10% FBS) in the wells. Invasion chambers were treated as follows for 24 hours: PBS, control rabbit IgG, 20 μg/mL trastuzumab , and 400 μg/mL of either anti-Her-2-597 (), anti-IGF-1R-234, anti-IGF-1R-56-81, or combinations of anti-Her-2-597 plus anti-IGF-1R-234, or anti-Her-2-597 plus anti-IGF-1R-56-81. Treatments were added directly to chambers. Non-invading cells were removed from the interior surface of the membrane by scrubbing gently with a dry cotton-tipped swab. Each insert was then transferred into 100% methanol for 10 min followed by crystal violet staining for 20 min. Membranes were then washed in water and allowed to air dry completely before being separated from the chamber. Membranes were mounted on slides with permanent mounting medium Permount (Fisher Scientific). Multiple photographs of each sample were taken at 20× magnification, with triplicates performed per treatment group. The number of cells was counted in each field; the sum total of the fields was calculated for each sample.
Statistical analysis
Five different experimental treatments comparing the difference in mice tumor volume over time was studied using the XTGEE (cross-sectional generalized estimating equations) model. Various comparisons of untreated, control and experimentally treated groups were analyzed using random-effects maximum likelihood regression. This model uses the between and within mouse variance when estimating standard errors for repeated measures over time on the same mouse and includes terms for treatment group, day, and their interaction. Treatment by time interaction was used to calculate the differences in the slopes for each group. The log-transformed model comparing the different treatment groups to the control group was used to test for significance and if the overall model p-value from the log likelihood test for each experiment was significant (p-value ≤ 0.05), then the regression line slopes for each treatment was further tested to see if they differed significantly from the untreated group.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Rita Nahta is a Glenn Breast Cancer Research Scholar at the Winship Cancer Institute of Emory University
Funding
This work was supported by funding from NIH R01 CA 84356 (PTPK), OSU Peptide Research Fund (PTPK) and R01CA157754 (RN).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev 2005; 16:407-20; PMID:; http://dx.doi.org/ 10.1016/j.cytogfr.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 2. Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer 2004; 4:505-18; PMID:; http://dx.doi.org/ 10.1038/nrc1387 [DOI] [PubMed] [Google Scholar]
- 3. Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998; 351:1393-6; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(97)10384-1 [DOI] [PubMed] [Google Scholar]
- 4. Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res 1995; 55:2007-11; PMID: [PubMed] [Google Scholar]
- 5. Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Nat Cancer Inst 1999; 91:620-5; PMID:; http://dx.doi.org/ 10.1093/jnci/91.7.620 [DOI] [PubMed] [Google Scholar]
- 6. Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci 2000; 57:1050-93; PMID:; http://dx.doi.org/ 10.1007/PL00000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papa V, Gliozzo B, Clark GM, McGuire WL, Moore D, Fujita-Yamaguchi Y, Vigneri R, Goldfine ID, Pezzino V. Insulin-like growth factor-I receptors are overexpressed and predict a low risk in human breast cancer. Cancer Res 1993; 53:3736-40; PMID: [PubMed] [Google Scholar]
- 8. Taunk NK, Goyal S, Moran MS, Yang Q, Parikh R, Haffty BG. Prognostic significance of IGF-1R expression in patients treated with breast-conserving surgery and radiation therapy. Radiother Oncol 2010; 96:204-8; PMID:; http://dx.doi.org/ 10.1016/j.radonc.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 9. Resnik JL, Reichart DB, Huey K, Webster NJ, Seely BL. Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res 1998; 58:1159-64; PMID: [PubMed] [Google Scholar]
- 10. Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 2008; 68:10238-46; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2755 [DOI] [PubMed] [Google Scholar]
- 11. Hakam A, Fang Q, Karl R, Coppola D. Coexpression of IGF-1R and c-Src proteins in human pancreatic ductal adenocarcinoma. Dig Dis Sci 2003; 48:1972-8; PMID:; http://dx.doi.org/ 10.1023/A:1026122421369 [DOI] [PubMed] [Google Scholar]
- 12. Xu JW, Wang TX, You L, Zheng LF, Shu H, Zhang TP, Zhao YP. Insulin-like growth factor 1 receptor (IGF-1R) as a target of MiR-497 and plasma IGF-1R levels associated with TNM stage of pancreatic cancer. PLoS One 2014; 9:e92847; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0092847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol 2003; 34:803-8; PMID:; http://dx.doi.org/ 10.1016/S0046-8177(03)00291-0 [DOI] [PubMed] [Google Scholar]
- 14. Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 2001; 93:1852-7; PMID:; http://dx.doi.org/ 10.1093/jnci/93.24.1852 [DOI] [PubMed] [Google Scholar]
- 15. Balana ME, Labriola L, Salatino M, Movsichoff F, Peters G, Charreau EH, Elizalde PV. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene 2001; 20:34-47; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1204050 [DOI] [PubMed] [Google Scholar]
- 16. Camirand A, Lu Y, Pollak M. Co-targeting HER2/ErbB2 and insulin-like growth factor-1 receptors causes synergistic inhibition of growth in HER2-overexpressing breast cancer cells. Med Sci Monit 2002; 8:BR521-6; PMID: [PubMed] [Google Scholar]
- 17. Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 2005; 65:11118-28; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3841 [DOI] [PubMed] [Google Scholar]
- 18. Nahta R. Deciphering the role of insulin-like growth factor-I receptor in trastuzumab resistance. Chemoth Res Pract 2012; 2012:648965; PMID:; http://dx.doi.org/ 10.1155/2012/648965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia 2008; 13:485-98; PMID:; http://dx.doi.org/ 10.1007/s10911-008-9107-3 [DOI] [PubMed] [Google Scholar]
- 20. Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer 2003; 107:873-7; PMID:; http://dx.doi.org/ 10.1002/ijc.11487 [DOI] [PubMed] [Google Scholar]
- 21. Zahorowska B, Crowe PJ, Yang JL. Combined therapies for cancer: a review of EGFR-targeted monotherapy and combination treatment with other drugs. J Cancer Res Clin Oncol 2009; 135:1137-48; PMID:; http://dx.doi.org/ 10.1007/s00432-009-0622-4 [DOI] [PubMed] [Google Scholar]
- 22. Chakraborty AK, Liang K, DiGiovanna MP. Co-targeting insulin-like growth factor I receptor and HER2: dramatic effects of HER2 inhibitors on nonoverexpressing breast cancer. Cancer Res 2008; 68:1538-45; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5935 [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Zhang Y, Zhang Y, Li J, Tsao SW, Zhang MY. Superior antitumor activity of a novel bispecific antibody cotargeting human epidermal growth factor receptor 2 and type I insulin-like growth factor receptor. Mol Cancer Ther 2014; 13:90-100; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0558 [DOI] [PubMed] [Google Scholar]
- 24. Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751871 in patients with refractory solid tumors. Clin Cancer Res 2007; 13:5834-40; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1118 [DOI] [PubMed] [Google Scholar]
- 25. Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, Blakely LJ, Eisenberg PD, Langer CJ, Blumenschein G Jr., et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol 2009; 27:2516-22; PMID:; http://dx.doi.org/ 10.1200/JCO.2008.19.9331 [DOI] [PubMed] [Google Scholar]
- 26. Disis ML, Gad E, Herendeen DR, Lai VP, Park KH, Cecil DL, O'Meara MM, Treuting PM, Lubet RA. A multiantigen vaccine targeting neu, IGFBP-2, and IGF-IR prevents tumor progression in mice with preinvasive breast disease. Cancer Prev Res (Phila) 2013; 6:1273-82; PMID:; http://dx.doi.org/ 10.1158/1940-6207.CAPR-13-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foy KC, Miller MJ, Moldovan N, Carson Iii WE, Kaumaya PT. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. Oncoimmunology 2012; 1:1048-60; PMID:; http://dx.doi.org/ 10.4161/onci.20708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foy KC, Miller MJ, Moldovan N, Bozanovic T, Carson Iii WE, Kaumaya PT. Immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel causes superior antineoplastic effects in transplantable and transgenic mouse models of human breast cancer. Oncoimmunology 2012; 1:1004-16; PMID:; http://dx.doi.org/ 10.4161/onci.21057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J Biol Chem 2011; 286:13626-37; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.216820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem 2011; 286:13612-25; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.216812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 2011; 3:70ra13; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PT. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J Immunol 2013; 191:217-27; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1300231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allen SD, Garrett JT, Rawale SV, Jones AL, Phillips G, Forni G, Morris JC, Oshima RG, Kaumaya PT. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol 2007; 179:472-82; PMID:; http://dx.doi.org/ 10.4049/jimmunol.179.1.472 [DOI] [PubMed] [Google Scholar]
- 34. Garrett JT, Rawale S, Allen SD, Phillips G, Forni G, Morris JC, Kaumaya PT. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. J Immunol 2007; 178:7120-31; PMID:; http://dx.doi.org/ 10.4049/jimmunol.178.11.7120 [DOI] [PubMed] [Google Scholar]
- 35. Lairmore MD, Lal RB, Kaumaya PT. Evaluation of immunodominant epitopes of human T-lymphotropic virus type 1 (HTLV-I) using synthetic peptides. Biomed Peptides, Proteins Nucleic Acids: Struct, Syn Biol Act 1995; 1:117-22; PMID: [PubMed] [Google Scholar]
- 36. Garrett TP, McKern NM, Lou M, Frenkel MJ, Bentley JD, Lovrecz GO, Elleman TC, Cosgrove LJ, Ward CW. Crystal structure of the first three domains of the type-1 insulin-like growth factor receptor. Nature 1998; 394:395-9; PMID:; http://dx.doi.org/ 10.1038/28668 [DOI] [PubMed] [Google Scholar]
- 37. Epa VC, Ward CW. Model for the complex between the insulin-like growth factor I and its receptor: towards designing antagonists for the IGF-1 receptor. Protein Eng Des Sel 2006; 19:377-84; PMID:; http://dx.doi.org/ 10.1093/protein/gzl022 [DOI] [PubMed] [Google Scholar]
- 38. Kaumaya PT, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, Lamb T, Mani A, Kane Y, Balint CR, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol 2009; 27:5270-7; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.22.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277-300; PMID:; http://dx.doi.org/ 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 40. Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US oncology research network. Cancer Control 2008; 15:308-13; PMID: [DOI] [PubMed] [Google Scholar]
- 41. Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, Solomides C, Peiper SC, Witkiewicz AK. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer 2012; 118:3484-93; PMID:; http://dx.doi.org/ 10.1002/cncr.26661 [DOI] [PubMed] [Google Scholar]
- 42. Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res 1998; 18:4613-9; PMID: [PubMed] [Google Scholar]
- 43. Sherwood ER, Van Dongen JL, Wood CG, Liao S, Kozlowski JM, Lee C. Epidermal growth factor receptor activation in androgen-independent but not androgen-stimulated growth of human prostatic carcinoma cells. Br J Cancer 1998; 77:855-61; PMID:; http://dx.doi.org/ 10.1038/bjc.1998.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol 2003; 163:1001-11; PMID:; http://dx.doi.org/ 10.1016/S0002-9440(10)63460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishiwata T, Bergmann U, Kornmann M, Lopez M, Beger HG, Korc M. Altered expression of insulin-like growth factor II receptor in human pancreatic cancer. Pancreas 1997; 15:367-73; PMID:; http://dx.doi.org/ 10.1097/00006676-199711000-00006 [DOI] [PubMed] [Google Scholar]
- 46. Li P, Veldwijk MR, Zhang Q, Li ZB, Xu WC, Fu S. Co-inhibition of epidermal growth factor receptor and insulin-like growth factor receptor 1 enhances radiosensitivity in human breast cancer cells. BMC Cancer 2013; 13:297; PMID:; http://dx.doi.org/ 10.1186/1471-2407-13-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inno A, Di Salvatore M, Cenci T, Martini M, Orlandi A, Strippoli A, Ferrara AM, Bagala C, Cassano A, Larocca LM, et al. Is there a role for IGF1R and c-MET pathways in resistance to cetuximab in metastatic colorectal cancer? Clin Colorectal Cancer 2011; 10:325-32; PMID:; http://dx.doi.org/ 10.1016/j.clcc.2011.03.028 [DOI] [PubMed] [Google Scholar]
- 48. Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer 2004; 108:334-41; PMID:; http://dx.doi.org/ 10.1002/ijc.11445 [DOI] [PubMed] [Google Scholar]
- 49. Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J, Elenius K, Isola J. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 2004; 3:1585-92; PMID: [PubMed] [Google Scholar]
- 50. Chen C, Zhang Y, Li J, Tsao SW, Zhang MY. Superior antitumor activity of a novel bispecific antibody cotargeting human epidermal growth factor receptor 2 and type I insulin-like growth factor receptor. Mol Cancer Ther 2014; 13:90-100; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0558 [DOI] [PubMed] [Google Scholar]
- 51. Esparis-Ogando A, Ocana A, Rodriguez-Barrueco R, Ferreira L, Borges J, Pandiella A. Synergic antitumoral effect of an IGF-IR inhibitor and trastuzumab on HER2-overexpressing breast cancer cells. Ann Oncol 2008; 19:1860-9; PMID:; http://dx.doi.org/ 10.1093/annonc/mdn406 [DOI] [PubMed] [Google Scholar]
- 52. Camirand A, Zakikhani M, Young F, Pollak M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res 2005; 7:R570-9; PMID:; http://dx.doi.org/ 10.1186/bcr1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ioannou N, Seddon AM, Dalgleish A, Mackintosh D, Modjtahedi H. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF-IR inhibitor, NVP-AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer 2013; 13:41; PMID:; http://dx.doi.org/ 10.1186/1471-2407-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dakappagari NK, Douglas DB, Triozzi PL, Stevens VC, Kaumaya PT. Prevention of mammary tumors with a chimeric HER-2 B-cell epitope peptide vaccine. Cancer Res 2000; 60:3782-9; PMID: [PubMed] [Google Scholar]
- 55. Dakappagari NK, Pyles J, Parihar R, Carson WE, Young DC, Kaumaya PT. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. J Immunol 2003; 170:4242-53; http://dx.doi.org/ 10.4049/jimmunol.170.8.4242 [DOI] [PubMed] [Google Scholar]
- 56. Dakappagari NK, Sundaram R, Rawale S, Liner A, Galloway DR, Kaumaya PT. Intracellular delivery of a novel multiepitope peptide vaccine by an amphipathic peptide carrier enhances cytotoxic T-cell responses in HLA-A*201 mice. J Pept Res 2005; 65:189-99; PMID:; http://dx.doi.org/ 10.1111/j.1399-3011.2005.00212.x [DOI] [PubMed] [Google Scholar]
- 57. Dakappagari NK, Lute KD, Rawale S, Steele JT, Allen SD, Phillips G, Reilly RT, Kaumaya PT. Conformational HER-2/neu B-cell epitope peptide vaccine designed to incorporate two native disulfide bonds enhances tumor cell binding and antitumor activities. J Biol Chem 2005; 280:54-63; PMID:; http://dx.doi.org/ 10.1074/jbc.M411020200 [DOI] [PubMed] [Google Scholar]
- 58. Srinivasan M, Wardrop RM, Gienapp IE, Stuckman SS, Whitacre CC, Kaumaya PT. A retro-inverso peptide mimic of CD28 encompassing the MYPPPY motif adopts a polyproline type II helix and inhibits encephalitogenic T cells in vitro. J Immunol 2001; 167:578-85; PMID:; http://dx.doi.org/ 10.4049/jimmunol.167.1.578 [DOI] [PubMed] [Google Scholar]
- 59. Srinivasan M, Gienapp IE, Stuckman SS, Rogers CJ, Jewell SD, Kaumaya PT, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis using peptide mimics of CD28. J Immunol 2002; 169:2180-8; http://dx.doi.org/ 10.4049/jimmunol.169.4.2180 [DOI] [PubMed] [Google Scholar]
- 60. Allen SD, Rawale SV, Whitacre CC, Kaumaya PT. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J Peptide Res: Off J Am Peptide Soc 2005; 65:591-604; PMID:; http://dx.doi.org/ 10.1111/j.1399-3011.2005.00256.x [DOI] [PubMed] [Google Scholar]
- 61. Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J Biol Chem 2011; 286:13612-25; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.216812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J Biol Chem 2011; 286:13626-37; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.216820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Foy KC, Miller MJ, Moldovan N, Carson WE, Kaumaya PTP. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. OncoImmunology 2012; 1(7):1048-1060; doi: 10.4161/onci.20708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kaumaya PT. Could precision-engineered peptide epitopes/vaccines be the key to a cancer cure? Future Oncol 2011; 7:807-10; PMID:; http://dx.doi.org/ 10.2217/fon.11.60 [DOI] [PubMed] [Google Scholar]
- 65. Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol 2012; 8:961-87; PMID:; http://dx.doi.org/ 10.2217/fon.12.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PT. Peptide vaccines and peptidomimetics of EGFR (HER-1) ligand binding domain inhibit cancer cell growth in vitro and in vivo. J Immunol 2013; 191:217-27; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1300231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koninki K, Barok M, Tanner M, Staff S, Pitkanen J, Hemmila P, Ilvesaro J, Isola J. Multiple molecular mechanisms underlying trastuzumab and lapatinib resistance in JIMT-1 breast cancer cells. Cancer Lett 2010; 294:211-9; PMID:; http://dx.doi.org/ 10.1016/j.canlet.2010.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.