Abstract
The magnitude of the cellular adaptive immune response is critical for the control of Mycobacterium tuberculosis infection in the chronic phase. In addition, the genetic background is equally important for resistance or susceptibility to tuberculosis. In this study, we addressed whether lung populations of dendritic cells, obtained from genetically different hosts, would play a role in the magnitude and function of CD4+ populations generated after M. tuberculosis infection. Thirty days post-infection, C57BL/6 mice, which generate a stronger interferon-γ (IFN-γ)-mediated immune response than BALB/c mice, exhibited a higher number and frequency of lung CD11c+ CD11b− CD103+ cells compared with BALB/c mice, which exhibited a high frequency of lung CD11c+ CD11b+ CD103− cells. CD11c+ CD11b− CD103+ cells, purified from lungs of infected C57BL/6 mice, but not from infected BALB/c mice, induced a higher frequency of IFN-γ-producing or interleukin-17 (IL-17)-producing CD4+ cells. Moreover, CD4+ cells also arrive at the lung of C57BL/6 mice faster than in BALB/c mice. This pattern of immune response seems to be associated with higher gene expression for CCL4, CCL19, CCL20 and CCR5 in the lungs of infected C57BL/6 mice compared with infected BALB/c mice. The results described here show that the magnitude of IFN-γ-producing or IL-17-producing CD4+ cells is dependent on CD11c+ CD11b− CD103+ cells, and this pattern of immune response is directly associated with the host genetic background. Therefore, differences in the genetic background contribute to the identification of immunological biomarkers that can be used to design human assays to predict progression of M. tuberculosis infection.
Keywords: dendritic cells, genetic background, T helper type 1 cells, T helper type 17 cells, tuberculosis
Introduction
Evidence collected from different models of experimental tuberculosis shows that the induction of the cellular immune response characterized by interferon-γ (IFN-γ) production is a strong protection correlate associated with the control of bacterial growth.1 Clinical findings clearly show that patients with tuberculosis produce IFN-γ, as determined by the secretion of this cytokine by peripheral blood mononuclear cells or by the detection of IFN-γ in lung biopsies.2–4 The remaining challenge is to decipher the factors that down-regulate the activation of IFN-γ-producing cells and IFN-γ-mediated biological effects. Given the importance of tuberculosis, which was declared to be a public health priority by the World Health Organization in 1993 and which was responsible for almost two million deaths worldwide in 2012,5 it is essential to more clearly understand key components that interfere with the magnitude of the immune response so as to develop novel vaccines and treatments based on immune therapies.
A hallmark of an efficient immune response that controls this infection could be the identification of cells and mediators in blood samples of PPD+ individuals. However, biological samples limit the ability to evaluate the peripheral versus local immune response in these studies.6 Although relevant, these studies do not provide all the necessary parameters to allow a full understanding of how the immune response controls the mycobacterial infection. Experimental models are still very useful tools to characterize the induced immune response during the infection and the modulation of the pro-inflammatory and anti-inflammatory responses. However, these experimental models are focused on progressive infection.7,8 An experimental model that mimics the latent infection would be helpful to provide a hallmark of protective immune response against Mycobacterium tuberculosis because latent state is believed to be maintained by the induction of an immune response that permits the bacillus persistence without symptoms.9 However, there is no mouse latent model that mimics the human latent state.10–12 Therefore, we have worked with an experimental model composed of two different mouse strains: C57BL/6 and BALB/c. This model allows the immune response to be studied from the perspective of the host. It is well established that the genetic background affects the magnitude of the inflammatory and cellular immune responses.13 Therefore, the magnitude of the cellular immune response following the infection of genetically different mouse strains may help to identify mechanisms associated with resistance to infection or lung injury and to predict biomarkers of this disease. C57BL/6 and BALB/c mice are considered more resistant to M. tuberculosis infection than DBA/2, CBA and C3H strains.14,15 However, C57BL/6 mice generate a robust cellular immune response characterized by interferon-γ (IFN-γ) production compared with the lower magnitude of cellular immune response observed in BALB/c mice.16,17 In previous work, our group established that M. tuberculosis-infected C57BL/6 mice produce higher levels of IFN-γ and interleukin-17 (IL-17) and lower levels of IL-10 along with a lower suppressor activity of regulatory T cells compared with infected BALB/c mice. This pattern of immune response seems to reflect the control of M. tuberculosis infection during the chronic phase (70 days) by C57BL/6 mice.18
It is well established that during active and progressive tuberculosis, T helper type 1 (Th1), Th2, Th17 and regulatory T cells participate in the lung immune response.18–26 The induction of a mycobacterial antigen-specific T-cell response depends on antigen-presenting cells and mediators delivered by these cells. Among the professional antigen-presenting cells, dendritic cells are the most efficient at priming naive T cells.27 During T-lymphocyte clonal expansion, dendritic cells also affect the ability to induce immunity or tolerance depending on the microenvironment.28,29 Accordingly, these cells play an important role in controlling the heterogeneity of the adaptive immune response mediated by CD4+ T lymphocytes.30
In the lung, dendritic cells are basically sorted into two populations: CD11c+ CD11b− CD103+ and CD11c+ CD11b+ CD103− cells. The expression of these markers also reflects the functional capacity of these cells.31,32 It has been determined that CD4+ T lymphocytes co-cultured with CD103+ dendritic cells express cytokines and chemokine receptors related to the Th1 and Th17 profiles, whereas those lymphocytes cultured with CD11b+ dendritic cells exhibited characteristics of the Th2 profile and regulatory T cells.33 In addition, CD11b+ dendritic cells, but not CD103+ cells, migrate to the lymph nodes and induce Th2 cells during experimental allergic inflammation.34
In this study, we addressed the question of whether dendritic cells of genetically different hosts would play a role in the magnitude of the adaptive response mediated by CD4+ cells. Our hypothesis is that mice with distinct genetic backgrounds have quantitative and functional differences in lung dendritic cell populations, which directly affect the magnitude of the immune response mediated by heterogeneous CD4+ populations after M. tuberculosis infection.
Methods
Animals
C57BL/6 and BALB/c female mice (6–8 weeks of age) were obtained from the breeding facility of the Medical School of Ribeirão Preto, University of São Paulo (FMRP-USP). All animals were maintained under barrier conditions in a Level III biosafety laboratory with free access to sterile food and water. This study was approved by the research ethics committee of FMRP-USP (protocol number 012/2010).
Bacteria and M. tuberculosis antigens
H37Rv M. tuberculosis (American Type Culture Collection 27294, Rockville, MD) was grown in 7H9 Middlebrook Broth (Difco Laboratories, Detroit, MI) at 37° for 7 days. The culture was harvested by centrifugation, and the cell pellet was resuspended in sterile PBS and vigorously agitated with glass spheres. The bacterial suspension was diluted in PBS and adjusted according to the number 1 McFarland scale. The viability of the bacteria in suspension was evaluated using fluorescein diacetate (Sigma, St Louis, MO) and ethidium bromide, as previously described.35 The M. tuberculosis antigens were obtained from the sonicated mycobacteria. Briefly, mycobacteria were grown as described previously and centrifuged at 3220 g for 20 min. The bacteria were heat-killed by incubation at 80° for 2 hr, and the suspension was sonicated at a frequency of 100 Hz with eight cycles of 5 min. The supernatant was collected and centrifuged at 5000 g for 30 min. A 0·22-μm filter was used to sterilize the supernatants. The protein concentration was quantified with the Coomassie Blue – Bradford Assay™ (OZ Biosciences, Marseille, France).
Infection
Mice were anaesthetized by intraperitoneal injection of 100 μl of a saline solution containing 20% ketamine (Ketamina Agener, Embu-Guaçu, SP, Brazil) and 10% Xylazine (Dopazer® Laboratorios Calier S.A., Barcelona, Spain). Then, 1 × 105 bacilli/100 μl were administered by the intra-tracheal route.36
Processing of lung cells and colony-forming units assay
The lower and middle right lobes of the lungs were washed with sterile PBS, and each was placed in a Petri dish containing incomplete RPMI-1640 (Sigma) medium. The lungs were weighed, fragmented and transferred to a conical tube containing digestion solution: Liberase™ Research Grade (Roche, Indianapolis, IN) diluted (0·8 μg/ml) in incomplete RPMI-1640. Samples were incubated at 37° with agitation for 30 min. After incubation, the cells were dispersed using a 10-ml syringe and pelleted by centrifugation for 10 min at 400 g. The pellet was resuspended in 1 ml RPMI-1640/10% fetal bovine serum (FBS). For colony-forming unit (CFU) determination, serial dilutions (100, 1000, 10 000 and 100 000) of digested lungs were plated on supplemented 7H11 agar media (Difco, Becton, Dickinson and Company, Le Pont de Chaix, France). The CFU number was counted 28 days after incubation at 37°, and results are expressed as the log10 of CFU/g lung. For the cell culture experiments, lungs were digested as described above. Lung cells were resuspended in 4 ml of RPMI/10% FBS and then passed through a 100-μm Cell Strainer (BD Biosciences, Durham, NC), pelleted by centrifugation for 10 min at 400 g and resuspended in complete RPMI-1640 medium (10% FBS, 10 μg/ml gentamicin, 100 U/ml penicillin and 100 μg/ml streptomycin). Total cell counts were determined in a Countess™ automated cell counter (Invitrogen, Eugene, OR).
Flow cytometry
Lung cells were initially incubated for 40 min at 4° with Fc block (1 μg/1 × 106 cells; BD Biosciences, San Jose, CA), followed by incubation with monoclonal antibodies (0·5 μg/1 × 106 cells) for 30 min at 4° in total darkness. To characterize dendritic cell populations, the following monoclonal antibodies were used: anti-CD11c (clone HL3), anti-CD11b (clone M1/70), anti-CD103 (clone M290). Lymphocyte populations were identified with anti-CD4 (clone L3T4) and anti-CD8 (clone 53-6.7). After staining for cell surface molecules, Foxp3 (clone MF23), T-bet (clone: O4-46), (BD Biosciences or eBioscience, San Diego, CA) were stained using a Foxp3 Staining Buffer Set (eBioscience) based on the manufacturer's instructions.
For intracellular detection of cytokines, cells were fixed with 4% formaldehyde diluted in PBS and incubated for 11 min. Then, 1 ml PBS was added to the cell suspension, which was incubated for 18 hr at 4°. For permeabilization, the cell suspension was centrifuged at 350 g at 4° for 10 min and washed with PBS containing 1% FBS and 0·2% saponin. After washing, cells were incubated for 20 min at 4° with 10% rabbit serum to block the Fc receptor, followed by incubation with anti-CD4 (clone L3T4), anti-IFN-γ (cloneXMG1.2) and anti-IL-17 (clone TC11-18H10) for 30 min at 4° in total darkness.
Data on cells were acquired by flow cytometry using a BD FACSCanto II (BD Bioscience, Franklin Lakes, NJ). One hundred thousand events per sample were collected and analysed according to size, granularity and fluorescence intensity using FlowJo 7.6.1™ software (Tree Star, Inc., Ashland, OR).
Cytokine production
Concentrations of IFN-γ were determined in the left lung lobe homogenates by ELISA using the immunoassay kit BD OptEIA™ Set (BD Biosciences) according to the manufacturer's instructions.
Immunohistochemistry
The lungs were collected in Tissue Tek (Sakura, Torrance, CA) and frozen at −70°. Lung sections of 10-μm thickness were mounted on slides after removing Tissue Tek with acetone. Endogenous peroxidase activity was blocked with 3% of H2O2 in PBS for 30 min in the dark. Endogenous avidin and biotin were blocked with Blocking Kit SP 2001 (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. After that, lung sections were incubated with primary antibodies rat anti-mouse CD103 (BD Bioscience; clone M290, 1 μg/slide) and rat anti-mouse CD11b (BD Bioscience; clone M1/70, 7·8 μg/slide) overnight at 4°. Biotin goat anti-rat polyclonal antibody (0·125 μg/slide) was used for 45 min at room temperature, to detect the primary bound antibody, followed by incubation with Vectastain ABC Kit (Vector Laboratories) according to the manufacturer's instructions. Enzyme-linked antibody was revealed by reacting with 3,3′-diaminobenzidine/H2O2 for 1–5 min at room temperature. Finally, tissue sections were counterstained with haematoxylin.
CD4+ and CD8+ cell migration assay
At 30 days of infection, 1·5 × 107 spleen cells obtained from C57BL/6 or BALB/c mice were stained with 1·25 μm carboxyfluorescein succinimidyl ester (CFSE; (Invitrogen, Carlsbad, CA) diluted in PBS and incubated for 10 min at 37° in 5% CO2 in total darkness. Then, RPMI-1640 medium containing 5% FBS was added (nine times the initial volume) (Invitrogen), and the mixture was incubated for 10 min on ice to stop the reaction. The cells were centrifuged at 350 g at 4° for 10 min and washed again. Then, 5 × 106 CFSE-stained spleen cells from infected C57BL/6 or BALB/c mice were transferred intravenously to their counterpart day 30-infected mouse strain. Twenty-four hours after the cell transfer, the numbers of CD4+, CD8+ and Foxp3+ cells (cells from recipient mouse), and CD4+ CFSE+, CD8+ CFSE+ and CD4+ Foxp3+ CFSE+ cells (cell transfer) were evaluated in the lungs.
Polymerase chain reaction in real time
Total RNA was isolated and purified from lung using an illustra RNAspin Mini RNA Isolation Kit (GE Healthcare, Little Chalfont, UK) according to the manufacturer's instructions. The RNA was quantified in a NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific, Inc. Waltham, MA), and cDNA synthesis was performed using Reverse Transcriptase SuperScript™ II (Invitrogen, Life Biotechnologies™). Real-time PCR was performed using Maxima SYBR Green/ROX qPCR Master Mix (2×) (Thermo Fisher Scientific, Inc.) in the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). The samples were amplified using the following conditions: initial denaturation at 95° for 10 min, followed by 40 cycles at 95° for 15 seconds, an annealing phase at 58° for 30 seconds and an extension phase at 72° for 30 seconds. The samples were analysed using the Ct (Threshold Cycle) method. Gene expression was calculated as  , where ΔΔCt = ΔCt (sample) − ΔCt (calibrator) and ΔCt (sample) = Ct (target gene) − Ct (normalizer = β-actin). The following primer sequences were used: CCL19 (sense: GCTAATGATGCGGAAGACT, anti-sense: TGATAGCCCCTTAGTGTGGTGA), CCL21 (sense: GTGATGGAGGGGGACAGGACT, anti-sense: TCAGGCTTAGAGTGCTTC), and β-actin (sense: CCCTAGGCACCAGGGTGTGA, anti-sense: GCCATGTTCAATGGGGTACTTC). The primers for CCL4, CCL11, CCL20, CCL22, CCR3, CCR5 and CCR6 were kindly provided by Dr João Santana da Silva (FMRP-USP).
, where ΔΔCt = ΔCt (sample) − ΔCt (calibrator) and ΔCt (sample) = Ct (target gene) − Ct (normalizer = β-actin). The following primer sequences were used: CCL19 (sense: GCTAATGATGCGGAAGACT, anti-sense: TGATAGCCCCTTAGTGTGGTGA), CCL21 (sense: GTGATGGAGGGGGACAGGACT, anti-sense: TCAGGCTTAGAGTGCTTC), and β-actin (sense: CCCTAGGCACCAGGGTGTGA, anti-sense: GCCATGTTCAATGGGGTACTTC). The primers for CCL4, CCL11, CCL20, CCL22, CCR3, CCR5 and CCR6 were kindly provided by Dr João Santana da Silva (FMRP-USP).
Co-culture of CD11c+ CD11b+ or CD11c+ CD11b− cells and CD4+ cells
CD11c+ CD11b+ and CD11c+ CD11b− cells were purified from the lungs of Day 30-infected C57BL/6 or BALB/c mice using an anti-Biotin MultiSort Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). CD4+ cells were purified from the spleens of infected C57BL/6 or BALB/c mice using a CD4 (L3T4) MicroBeads Kit (Miltenyi Biotec GmbH) according to the manufacturer's instructions. CD11c+ CD11b+ or CD11c+ CD11b− cells (1 × 105) were cultured in RPMI-1640 medium containing FBS 10%, 10 μg/ml gentamicin, 100 U/ml penicillin and 100 μg/ml streptomycin in the presence of CD4+ cells (2 × 105), with or without stimulation with M. tuberculosis antigens (10 μg/ml), at 37° and 5% CO2 for 48 hr. As a negative control, purified spleen CD4+ (2 × 105) cells were left non-stimulated. After 42 hr cell cultures received an additional polyclonal stimulus with PMA (100 ng/ml) and ionomycin (500 ng/ml) (Sigma-Aldrich, St Louis, MO). At the same time, a protein transport inhibitor containing monensin (BD GolgiStop™, 4 μl for every 6 ml of cell culture; BD Biosciences) was added before the last 6 hr of incubation at 37° in 5% CO2.
Statistical analysis
The data were analysed with prism software. The statistical significance between two groups was estimated using a two-tailed Student's t-test. The data from experiments with three or more groups were analysed using one-way analysis of variance with the Bonferroni test. The data are expressed as the mean ± standard deviation (SD). Values of P 0·05 were considered significant.
Results
The profile of cellular immune response of C57BL/6 mice was more closely associated to infection control than that of BALB/c mice
A previous study by our group showed that although at 30 days post-infection there is no difference in lung CFU between BALB/c and C57BL/6 mice, these mouse strains exhibit a contrasting difference regarding the production of IFN-γ, IL-17 and IL-10.18 Figure1(a) shows that at 30 days of infection, the recovery of bacilli from the lungs of BALB/c and C57BL/6 mice was similar, despite the significant ex vivo production of IFN-γ, which was higher in the lungs of C57BL/6 mice than in those of BALB/c mice (Fig.1b). Because we observed no difference in the number of lung CD4+ cells from infected BALB/c and C57BL/6 mice (Fig.1c), we assessed the expression of T-bet transcription factor that is the signature of Th1 CD4+ cells. We found a significant increase in the expression of T-bet in the lungs of infected C57BL/6 and BALB/c mice compared with their control counterparts (Fig.1d). However, CD4+ T-bet+ cell number was significantly higher in the lungs of infected C57BL/6 mice compared with those of infected BALB/c mice (Fig.1d). These results show that C57BL/6 mice, but not BALB/c mice, generated a profile of cellular immune response associated with infection control, characterized by higher production of IFN-γ and a greater number of lung CD4+ T-bet+ cells.
Figure 1.
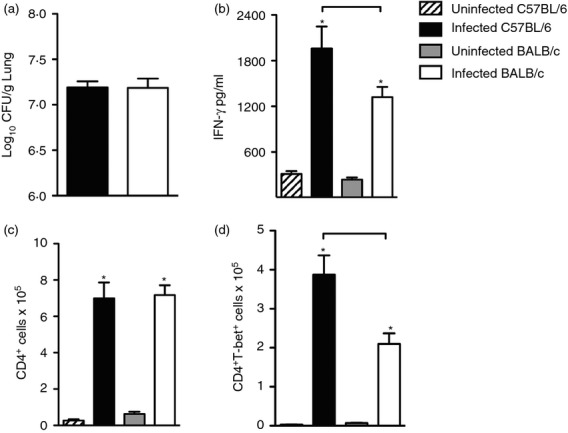
Infected C57BL/6 mice generate a T helper type 1 (Th1) cell-mediated immune response at a higher magnitude than BALB/c mice. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with 1 × 105 Mycobacteirum tuberculosis bacilli by the intra-tracheal route or left uninfected (hatch and grey bars). Thirty days after infection, colony-forming unit (CFU) counts (a), interferon-γ (IFN-γ) (b), CD4+ cell number (c) and T-bet expressing CD4+ cells (d) were determined in the lungs. Viable bacilli were determined by plating serial dilutions of digested lungs. IFN-γ was detected in the lung homogenates by immune enzymatic assay. T-bet was evaluated by flow cytometry. Data are presented as the mean ± SD. Data are representative of two independent experiments (n = 8 to n = 10 mice/group). *P < 0·05 comparing infected and respective uninfected groups. Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
More CD4+ and CD8+ cells migrated to the lungs of C57BL/6 mice than BALB/c mice
Next, we assessed whether the migration capacity of lymphocytes also differed between mouse strains. Spleen cells of Day 30-infected C57BL/6 and BALB/c mice were labelled with CFSE and transferred intravenously to the respective mouse strain with the same period of infection. Twenty-four hours after cell transfer, CD4+, CD8+ and CD4+ Foxp3+ cells, positive or negative for CFSE, were analysed in the lungs (Fig.2a). A representative analysis performed to evaluate these cell populations is depicted in the Supporting information (Fig. S1). We found no difference in the number of CD4+ cells, CD4+ Foxp3+ cells and CD8+ cells (which represented the cells from recipient mice) in the lungs of both strain of infected mice (Fig.2b–d). When we assessed the number of CFSE+ cells, which represented those that have migrated to the lung following cell transfer, we found a significant increase in the number of CD4+ CFSE+ cells (Fig.2e) and CD8+ CFSE+ cells (Fig.2g) in the lung of infected C57BL/6 mice compared with BALB/c mice. There was no difference in the number of CD4+ Foxp3+ CFSE+ cells (Fig.2f). To ascertain whether the increased influx of CD4+ and CD8+ cells to the lungs of infected C57BL/6 mice was a consequence of a higher number of these cell populations in the spleens of C57BL/6 mice compared with BALB/c mice, we quantified the number of CD4+, CD4+ Foxp3+ and CD8+ cells in the spleens of infected and uninfected mouse strains. There was no difference in the number of CD4+, CD4+ Foxp3+ and CD8+ cells in the spleens of infected C57BL/6 and BALB/c mice compared with uninfected counterparts of the same strain (Fig.3a–c). However, we observed an increase in the number of spleen CD4+ and CD8+ cells obtained from infected BALB/c mice compared with the number of these cells in the spleens of infected C57BL/6 mice (Fig.3a,c). This experimental evidence suggests that during the infection with M. tuberculosis, the influx of CD4+ and CD8+ cells into the lungs of C57BL/6 mice is higher than in BALB/c mice and is not dependent on the number of primed spleen cells.
Figure 2.
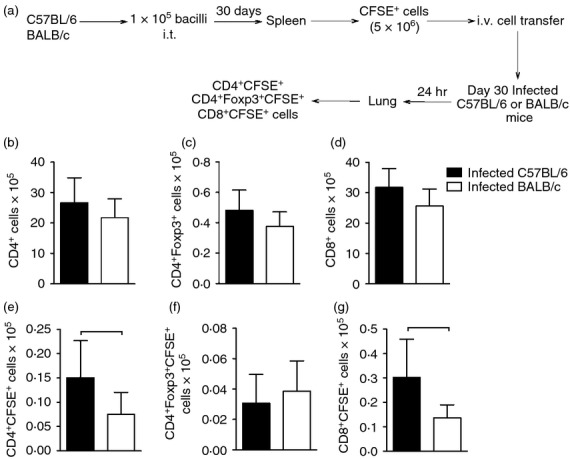
CD4+ and CD8+ cells migrate to the lungs of C57BL/6 mice in higher numbers than in BALB/c mice. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with Mycobacterium tuberculosis as described in Fig.1. Thirty days after infection, spleen cells were stained with CFSE and adoptively transferred (5 × 106 cells) by the intravenous route to Day 30-infected mice. Twenty-four hours after the cell transfer, lung cell suspensions were obtained, stained with anti-CD4, anti-CD8, and anti-Foxp3 and evaluated by flow cytometry (a). Cells from receptor mice: CD4+ (b), CD4+ Foxp3+ (c), CD8+ (d). Cell migration: CD4+ CFSE+ (e), CD4+ Foxp3+ CFSE+ (f), CD8+ CFSE+ (g). Data are presented as the mean ± SD. Data are representative of two independent experiments (n = 6 to n = 9 mice/group). Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
Figure 3.
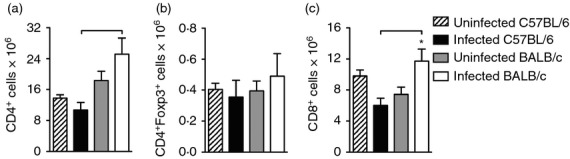
Number and frequency of CD4+, CD4+ Foxp3+ and CD8+ cells in the spleens of infected C57BL/6 and BALB/c mice. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with Mycobacterium tuberculosis or left uninfected (hatch and grey bars) as described in Fig.1. Thirty days after infection, spleen cell suspensions were stained with anti-CD4, anti-CD8 and anti-Foxp3, and evaluated by flow cytometry. The absolute number of CD4+ (a), CD4+ Foxp3+ (b) and CD8+ (c) cells was analysed. Data are presented as the mean ± SD (n = 4 or n = 5 mice/group). *P < 0·05 comparing infected and respective uninfected groups. Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
Infected C57BL/6 mice exhibited higher expression of CCR5, CCL4 and CCL20 than BALB/c mice
Because we found differences between infected C57BL/6 and BALB/c mice in the production of IFN-γ and in the T-bet-expressing CD4+ cell population in the lung, and observed that the migration of both CD4+ and CD8+ cells was more pronounced in the lungs of infected C57BL/6 mice, we assessed gene expression for chemokine and chemokine receptors associated with recruitment of Th1, Th2, Th17 and regulatory T cells.
CCL4 binds to the CCR5 receptor and preferentially induces the migration of Th1 lymphocytes. CCR5 gene expression was higher in the lungs of infected C57BL/6 mice compared with infected BALB/c mice (Fig.4a). The lungs of both infected strains exhibited significantly increased CCL4 expression compared with uninfected counterparts. In addition, CCL4 gene expression was higher in the lungs of infected C57BL/6 mice than in infected BALB/c mice (Fig.4b). In an attempt to establish an association with Th17 migration, we evaluated CCR6 and CCL20 gene expression. We found no difference in the expression of CCR6 between infected strains (Fig.4c). However, the CCR6 ligand, CCL20, was significantly increased in the lungs of infected C57BL/6 mice compared with infected BALB/c mice (Fig.4d). The analysis of gene expression for CCR4 and CCL22 was used as a marker for regulatory T cell migration, while gene expression for CCR3 and CCL11 was used as a marker for Th2 migration. There was no difference in gene expression for CCR4, CCL22, CCR3 or CCL11 in the lungs of either strain (Fig.4e–h).
Figure 4.
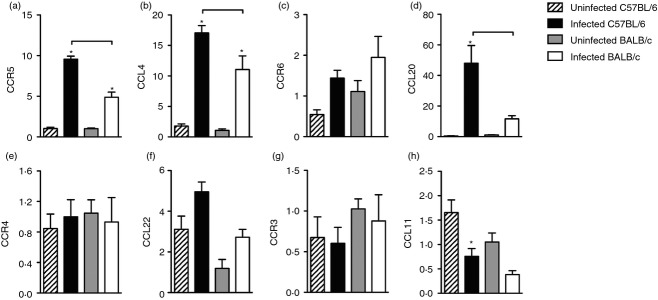
Infected C57BL/6 mice exhibited higher expression of CCR5, CCL4 and CCL20 than BALB/c mice. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with Mycobacterium tuberculosis or left uninfected (hatch and grey bars) as described in Fig.1. Thirty days after infection, gene expression for CCR5 (a), CCL4 (b), CCR6 (c), CCL20 (d), CCR4 (e), CCL22 (f), CCR3 (g) and CCL11 (h) was evaluated by real-time PCR. Data are presented as the mean ± SD. *P < 0·05 comparing infected and respective uninfected groups. Data are representative of one representative experiment repeated twice (n = 3–4 mice/group). Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
These collective data show that the influx of CD4+ cells into the lungs of infected C57BL/6 mice is associated with a predominant population of IFN-γ-producing CD4+ cells and suggest that their migration is dependent on CCR5 and CCL4.
Infected C57BL/6 mice exhibited a higher number of lung CD103+ cells while infected BALB/c mice exhibited a higher number of lung CD11b+ cells
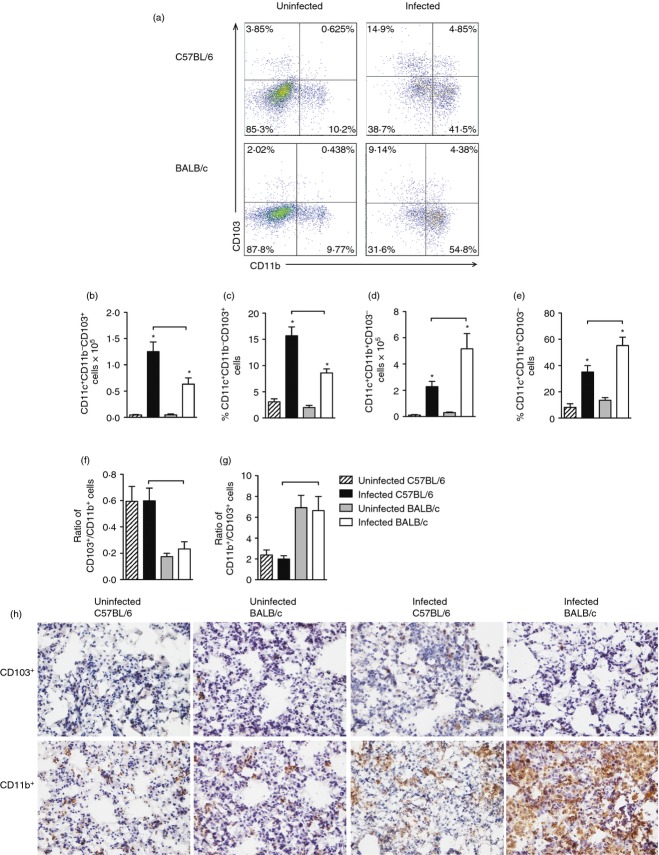
Considering the different magnitude of IFN-γ-producing cells, we next evaluated the lung dendritic cell populations because these antigen-presenting cells induce and drive the differentiation of CD4+ cells.30 Two populations of lung dendritic cells were evaluated: CD11c+ CD103+ and CD11c+ CD11b+. Figure5(a) shows a representative analysis of these populations, initially gated on CD11c+ cells. Infected C57BL/6 and BALB/c mice had a significant increase in the number and frequency of CD11c+ CD103+ dendritic cells (CD11c+ CD11b− CD103+) compared with uninfected counterparts (Fig.5b,c). Moreover, the number and the frequency of CD11c+ CD103+ cells obtained from lungs of infected C57BL/6 mice was significantly greater than those found in the cells of infected BALB/c mice (Fig.5b,c).
Figure 5.
Infected C57BL/6 mice exhibited a higher number of lung CD103+ cells while infected BALB/c mice exhibited a higher number of lung CD11b+ cells. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with Mycobacterium tuberculosis or left uninfected (hatch and grey bars) as described in Fig.1. Thirty days after infection, CD11c+ CD11b− CD103+ cells and CD11c+ CD11b+ CD103− cells were evaluated by flow cytometry (a). The absolute number and frequency of CD11c+ CD11b− CD103+ cells (b, c) and CD11c+ CD11b+ CD103− cells (d, e), and ratio of CD11c+ CD11b− CD103+/CD11c+ CD11b+ CD103− cells (f) and CD11c+ CD11b+ CD103−/CD11c+ CD11b− CD103+ cells (g) were analysed. Data are presented as the mean ± SD. Data shown are representative of two independent experiments (n = 7 to n = 9 mice/group). *P < 0·05 comparing infected and respective uninfected groups. Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice. Immunohistochemistry was performed to visualize the distribution of CD103+ and CD11b+ cells (both in brown label) in the lung parenchyma (h). Frozen sections of lungs from uninfected C57BL/6 or BALB/c mice and Day 30-infected C57BL/6 or BALB/c mice were evaluated. Magnification: ×400.
A significantly higher number and frequency of CD11c+ CD11b+ cells (CD11c+ CD11b+ CD103−) were found in the lungs of infected C57BL/6 and BALB/c mice compared with their uninfected counterparts (Fig.5d,e). Furthermore, the number and frequency of CD11c+ CD11b+ cells was higher in the lungs of infected BALB/c mice compared with infected C57BL/6 mice (Fig.5d,e).
In addition, we observed that the ratio of the CD103+/CD11b+ cell number (CD11c+ CD11b− CD103+/CD11c+ CD11b+ CD103−) was significantly higher in infected C57BL/6 compared with infected BALB/c mice (Fig.5f), while the ratio of CD11b+/CD103+ cell number (CD11c+ CD11b+ CD103−/CD11c+ CD11b− CD103+) was significantly higher in infected BALB/c mice compared with infected C57BL/6 mice (Fig.5g). To visualize the distribution of CD103+ cells and CD11b+ cells in the lung parenchyma, we performed an immunohistochemical analysis. We confirmed that CD103+ cells had a sparse distribution in the lungs of uninfected mice compared with CD11b+ cells (Fig.5h). Mycobacterium tuberculosis infection clearly increased the population of lung CD11b+ cells, which was more evident in infected BALB/c mice compared with infected C57BL/6 mice (Fig.5h). We observed a slight increase in the population of CD103+ cells in the lungs of infected C57BL/6 mice compared with uninfected C57BL/6 mice (Fig.5h). This was expected considering the density of CD103+ cells compared with CD11b+ cells.
Therefore, while infected C57BL/6 mice displayed a higher number and frequency of lung CD11c+ CD103+ cells, infected BALB/c mice displayed a higher number and frequency of lung CD11c+ CD11b+ cells.
Next, we analysed gene expression of CCL19 and CCL21 in the lung homogenates because the migration of dendritic cells to the draining lymph nodes depends on CCL19 and CCL21 chemokines. We found a significant increase of CCL19 gene expression in the lungs of infected C57BL/6 mice compared with the uninfected group and with infected BALB/c mice (Fig.6a). There was no difference in CCL21 gene expression between infected C57BL/6 and BALB/c mice (data not shown).
Figure 6.
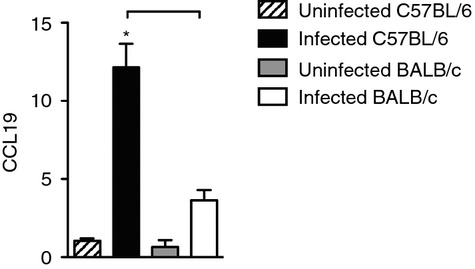
Infected C57BL/6 mice exhibited higher CCL19 gene expression than BALB/c mice. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with Mycobacterium tuberculosis or left uninfected (hatch and grey bars) as described in Fig.1. Thirty days after infection, gene expression for CCL19 was evaluated by real-time PCR. Data are presented as the mean ± SD. Data are representative of one experiment performed twice (n = 3 to n = 4 mice/group). *P < 0·05 comparing infected and respective uninfected groups. Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
CD103+ cells of infected C57BL/6 mice induced a higher frequency of IFN-γ-producing or IL-17-producing CD4+ cells than CD103+ cells of BALB/c mice
Because infected C57BL/6 mice secreted more IFN-γ and have quantitatively more lung tissue CD11c+ CD103+ cells than infected BALB/c mice, we hypothesized that CD11c+ CD103+ cells would be a key factor in the activation of IFN-γ-producing CD4+ cells. In an attempt to assess the functional ability of CD11c+ CD103+ cells, we performed a co-culture assay. Initially, we purified lung CD11c+ CD11b− and CD11c+ CD11b+ cells from infected C57BL/6 and BALB/c mice and confirmed that in the CD11c+ CD11b− population, CD103+ cells predominated (Fig.7a). In contrast, in the lung CD11c+ CD11b+ population, CD103− cells predominated (Fig.7a). Next we co-cultured either CD11c+ CD11b− (CD103+) or CD11c+ CD11b+ (CD11b+) cells with purified CD4+ cells (either stimulated or not with M. tuberculosis antigens) obtained from spleens of infected BALB/c or C57BL/6 mice and determined the frequency of cytokine-producing CD4+ cells. Figure7(b) shows a representative analysis of the frequency of IFN-γ-producing CD4+ cells or IL-17-producing CD4+ cells in the cell co-culture from C57BL/6 or BALB/c mice. Data collected from co-culture experiments showed that lung CD11c+ CD11b− (CD103+) cells of infected C57BL/6 mice induced a significant increase in the frequency of IFN-γ-producing CD4+ cells and IL-17-producing CD4+ cells after re-stimulation with M. tuberculosis antigens compared with CD11c+ CD11b− (CD103+) cells of infected BALB/c mice (Fig.7c,d). In addition, the CD11c+ CD11b− (CD103+) cells of infected C57BL/6 or BALB/c mice, in the presence of M. tuberculosis antigens, also induced significant production of IFN-γ by CD4+ cells compared with respective co-culture in the absence of M. tuberculosis antigens (Fig.7c,d). When we analysed the population of CD11c+ CD11b+ (CD11b+), we did not find differences in the ability of dendritic cells to induce IFN-γ-producing or IL-17-producing CD4+ cells in different experimental conditions or between mouse strains (Fig.7e,f). Experimental controls for evaluation of IFN-γ-producing or IL-17-producing CD4+ cells are represented in the Supporting information (Fig. S2).
Figure 7.
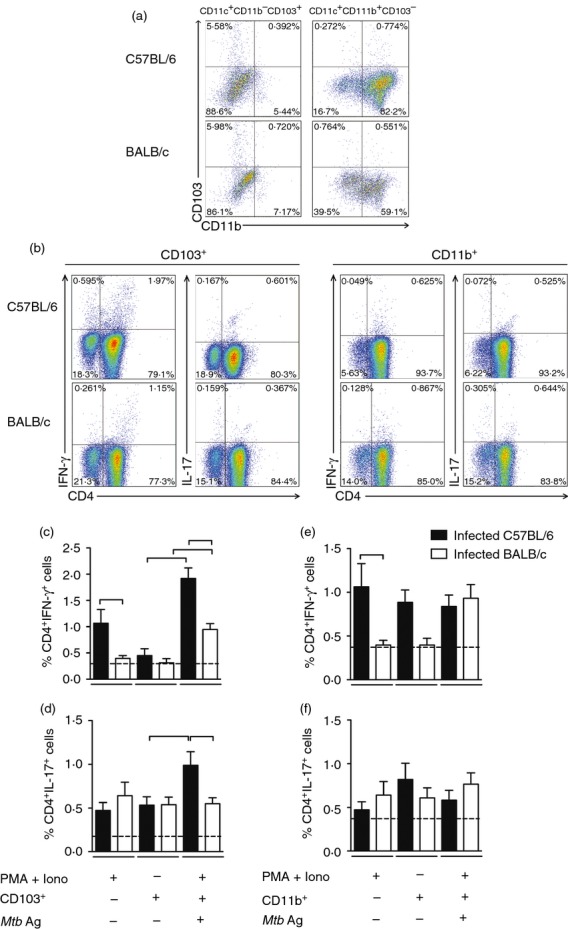
CD103+ cells of infected C57BL/6 mice induced a higher frequency of interferon-γ (IFN-γ) -producing CD4+ cells and interleukin-17 (IL-17) -producing CD4+ cells than CD103+ cells of BALB/c mice. C57BL/6 (black bars) and BALB/c (white bars) mice were infected with Mycobacterium tuberculosis as described in Fig.1. Thirty days after infection, lung CD11c+ CD11b+ cells or CD11c+ CD11b− cells were purified, stained with anti-CD103 or anti-CD11b and the frequency of CD11c+ CD11b+ cells and CD11c+ CD11b− cells expressing the CD103 molecule was analysed by flow cytometry (a). Purified lung CD11c+ CD11b− CD103+ cells (CD103+) or CD11c+ CD11b+ CD103− cells (CD11b+) (1 × 105 cells) were co-cultured with 2 × 105 purified spleen CD4+ cells and re-stimulated or not with M. tuberculosis antigens (Mtb Ag). As a positive control, co-cultures were stimulated with PMA and ionomycin. As a negative control, purified spleen CD4+ cells were left non-stimulated (dashed line). After 48 hr of co-culture, the frequency of CD4+ IFN-γ+ and CD4+ IL-17+ cells was evaluated by flow cytometry. Representative analysis showing the frequency of CD4+ IFN-γ+ and CD4+ IL-17+ cells (b). Frequency of CD4+ IFN-γ+ and CD4+ IL-17+ cells in the co-culture with CD103+ cells (c, d). Frequency of CD4+ IFN-γ+ and CD4+ IL-17+ cells in the co-culture with CD11b+ cells (e, f). Data are presented as the mean ± SD. Data are representative of three independent experiments (n = 7 to n = 9 mice/group). Bars show the difference (P < 0·05) between co-cultures of infected C57BL/6 and BALB/c mice under the same stimulation or between co-cultures of infected C57BL/6 or BALB/c mice under different stimulation.
Discussion
In the present study, we evaluated the role of lung dendritic cell populations in the magnitude of the adaptive immune response of M. tuberculosis-infected mouse strains with different genetic backgrounds. We show that the difference in the magnitude of the cellular immune response is associated with a higher number of CD11c+ CD11b− CD103+ cells in the lungs of infected C57BL/6 mice compared with infected BALB/c mice. This cell population (CD11c+ CD11b− CD103+) purified from the lungs of infected C57BL/6 mice stimulated a significant increase in the frequency of IFN-γ-producing or IL-17-producing CD4+ cells compared with BALB/c mice. The higher magnitude of cellular immune response observed in infected C57BL/6 mice seems to also be associated with a more intense migration of CD4+ cells to the lungs. Moreover, the results suggest that the recruitment of CD4+ cell populations is dependent on CCR5, CCL4 and CCL20.
The genetic background of an animal is important for the generation of an immune response that can increase either the resistance or the susceptibility to M. tuberculosis infection. Although C57BL/6 mice are resistant and BALB/c mice have an intermediate resistance to M. tuberculosis infection,15 the former generate a stronger Th1 immune response, while BALB/c mice generate a Th2-driven immune response.16,17 Previous study by our group has shown that C57BL/6 mice exhibit higher IFN-γ and IL-17 production, lower IL-10 production, and greater suppressive function of regulatory T cells followed by lower lung bacterial growth when compared with BALB/c mice.18 Because the induction of a T-cell-mediated immune response is dependent on signals released by antigen-presenting cells and many of those signals drive the differentiation of CD4+ cells, it is reasonable to suppose that dendritic cells directly affect the magnitude of the CD4+ cell response during M. tuberculosis infection. While CD103+ dendritic cells play a tolerogenic role in the gut, CD11b+ dendritic cells induce a pro-inflammatory immune response.37–39 Because regulatory T cells are associated with progression of M. tuberculosis infection and suppression of IFN-γ production, we hypothesized that a higher number of CD103+ dendritic cells would be found in the lungs of infected BALB/c mice and that a higher number of CD11b+ dendritic cells would be found in the lungs of C57BL/6 mice. However, we found the opposite: a higher number and frequency of CD11c+ CD103+ cells in the lungs of infected C57BL/6 compared with infected BALB/c mice. Therefore, depending on the microenvironment, an equivalent population of dendritic cells may play different roles. Sung et al.40 showed that CD103+ dendritic cells purified from airways secreted higher concentrations of IL-12 than CD11b+ dendritic cells. In addition, Furuhashi et al.33 showed that CD4+ lymphocytes from ovalbumin-specific T-cell receptor transgenic mice (DO11.10) co-cultured with lung CD103+ dendritic cells secreted significant levels of IFN-γ and IL-17, whereas in the presence of lung CD11b+ dendritic cells there was preferential IL-4, IL-6 and IL-10 production. Consequently, the higher number of CD103+ dendritic cells in the lungs of infected C57BL/6 mice may represent a key factor for the higher magnitude of adaptive response (based on IFN-γ and IL-17 production) previously reported by us and other groups.16–18 Indeed, we confirmed this hypothesis when we showed that lung CD103+ dendritic cells from M. tuberculosis-infected C57BL/6 mice, but not from BALB/c mice, induced a higher frequency of IFN-γ-secreting or IL-17-secreting CD4+ cells. Recently, Kim et al.41 reported that lung CD103+ dendritic cells induce effector CD8+ cells while the absence of these cells encourages the generation of central memory CD8+ cells.
Compared with other infections that affect the airways, the adaptive immune response induced against M. tuberculosis is defined by a postponed onset. Among the multiple factors that contribute to a delayed adaptive response are an arrest in the dissemination of bacilli to the secondary lymphoid organs and a delay in the migration of dendritic cells to these organs.42–45 We observed increased gene expression for CCL19, a chemokine that drives the migration of dendritic cells from peripheral organs to draining lymph nodes, in the lungs of infected C57BL/6 mice compared with infected BALB/c mice, suggesting that the lung lymphoid vessels of C57BL/6 mice exhibit an environment suitable for the dendritic cell migration.
Most of the studies aimed at evaluating the role of dendritic cells during the infection with M. tuberculosis were based on the analysis of CD11c+ cells. This emphasizes that most current studies evaluated a single lung dendritic cell population. In the first of these studies, the authors showed that CD11c+ dendritic cells purified from the lung of C57BL/6 mice, and expanded and infected in vitro with M. tuberculosis, secreted IL-12p40 and induced IFN-γ-producing CD4+ cells.27 An increase in the number of CD11c+ dendritic cells was described in the lungs of BALB/c mice 14 and 21 days post-infection.46 In addition, the bacillus aggregates were more numerous in the lung macrophages than in the CD11c+ cells from infected BALB/c mice.46 Mycobacterial infection seems to induce dendritic cell maturation, as demonstrated by experiments showing that dendritic cells obtained from bacillus Calmette–Guérin-infected BALB/c mice stimulated with ovalbumin were more efficient than dendritic cells from uninfected mice in inducing activation of ovalbumin-specific CD4+ cells.47 Recently, a study showed that the progression of M. tuberculosis infection was associated with an increase in the number of CD103+ cells in the lung of resistant C57BL/6 mice compared with susceptible DBA/J2-infected mice.48 These authors attributed the generation of IFN-γ-producing CD4+ Foxp3+ cells to the presence of these CD103+ cells. To some extent, these findings confirm previous studies33 and corroborate the data presented here.
In conclusion, our study shows that the genetic background affects the number and the functional capacity of CD11c+ CD103+ cells obtained from the lungs of infected C57BL/6 and BALB/c mice. Although there is no difference in the restriction of bacterial growth 30 days post-infection, infected C57BL/6 mice have a higher frequency and number of lung CD11c+ CD103+ cells. These cells generate IFN-γ- or IL-17-producing CD4+ cells that migrate faster, most likely dependent on CCL4 and CCL20, respectively, to the lungs of infected C57BL/6 mice compared with infected BALB/c mice. These findings associate the pattern of protective adaptive immune response generated by infected C57BL/6 mice with lung CD11c+ CD11b− CD103+ cells and suggest that the magnitude of IFN-γ- and IL-17-mediated responses are determinant for the restriction of bacterial load at the chronic phase (70 days) of the infection.11 Therefore, differences in the genetic background contribute to the identification of immunological biomarkers that can be used to design human assays to predict progression of M. tuberculosis infection. Further studies to evaluate the populations of dendritic cells from PPD+ and PPD− individuals may be useful for the identification of immunological biomarkers.
Acknowledgments
C.A.S. performed the experiments and analysed the data; T.B.B. performed the experiments, analysed the data and wrote the paper; A.F.G. and R.Q.P. performed the experiments, and V.L.D.B. designed the experiments, analysed the data and wrote the paper.
Author's contributions
The research leading to these results has received funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (grants 11/09702-2 and 09/18519-7) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Disclosures
The authors declare that they have no competing interests to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Flow cytometry representative analysis and frequency of CD4+, CD4+ Foxp3+ and CD8+ cells that migrate to the lungs of infected C57BL/6 and BALB/c mice. Lung cells were first gated by size and granularity, following the gate on CD4+, or CD4+ Foxp3+ or CD8+ cells (cells from recipient mice). In addition, CFSE+ cells were evaluated within CD4+, CD4+ Foxp3+ and CD8+ gated cells (cell transfer) (a). Frequency of CD4+ (b), CD4+ CFSE+ (c), CD4+ Foxp3+ (d), CD4+ Foxp3+ CFSE+ (e), CD8+ (f) and CD8+ CFSE+ cells (g). Data are presented as the mean ± SD. Data are representative of two independent experiments (n = 6 to n = 9 mice/group). Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
Figure S2. Control plots for evaluation of interferon-γ (IFN-γ) or interleukin-17 (IL-17) -producing CD4+ cells. Lung cells were left unstained and autofluorescence was delimited. Markers used to define IFN-γ+- or IL-17+-producing CD4+ cells were defined based on Fluorescence minus one (FMO) controls (a). Representative analysis showing the frequency of CD4+ IFN-γ+ and CD4+ IL-17+ cells that were left non-stimulated (b). Purified spleen CD4+ cells were left non-stimulated and co-cultured, during 48 hr, with CD11c+ CD11b− CD103+ cells (CD103+) or CD11c+CD11b+ CD103− (CD11b+) obtained from infected C57BL/6 or BALB/c mice.
References
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AVS, Lalvani A. Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–25. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon-γ production in patients with tuberculosis. Clin Infect Dis. 1997;25:617–20. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- Rueda CM, Marín ND, García LF, Rojas M. Characterization of CD4 and CD8 T cells producing IFN-γ in human latent and active tuberculosis. Tuberculosis. 2010;90:346–53. doi: 10.1016/j.tube.2010.09.003. [DOI] [PubMed] [Google Scholar]
- WHO. 2013. Global Tuberculosis Report.
- Brighenti S, Andersson J. Local immune responses in human tuberculosis: learning from the site of infection. J Infect Dis. 2012;205(Suppl. 2):S316–24. doi: 10.1093/infdis/jis043. [DOI] [PubMed] [Google Scholar]
- Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- Morais Fonseca D, Rosada RS, Paula MO, Wowk PF, Franco LH, Soares EG, Silva CL, Bonato VLD. Experimental tuberculosis: designing a better model to test vaccines against tuberculosis. Tuberculosis. 2010;90:135–42. doi: 10.1016/j.tube.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Gomez JE, McKinney JD. Mycobacterium tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Barry CE, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- Radaeva TV, Nikonenko BV, Mischenko VV, Averbakh MM, Apt AS. Direct comparison of low-dose and Cornell-like models of chronic and reactivation tuberculosis in genetically susceptible I/St and resistant B6 mice. Tuberculosis. 2005;85:65–72. doi: 10.1016/j.tube.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Kramnik I, Dietrich WF, Demant P, Bloom BR. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2000;97:8560–5. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- Chackerian AA, Behar SM. Susceptibility to Mycobacterium tuberculosis: lessons from inbred strains of mice. Tuberculosis. 2003;83:279–85. doi: 10.1016/s1472-9792(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Arko-Mensah J, Rahman MJ, Dégano IR, Chuquimia OD, Fotio AL, Garcia I, Fernandez C. Resistance to mycobacterial infection: a pattern of early immune responses leads to a better control of pulmonary infection in C57BL/6 compared with BALB/c mice. Vaccine. 2009;27:7418–27. doi: 10.1016/j.vaccine.2009.06.110. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Ryan L, LaCourse R, North RJ. Differences in the ability to generate type 1 T helper cells need not determine differences in the ability to resist Mycobacterium tuberculosis infection among mouse strains. J Infect Dis. 2009;199:1790–6. doi: 10.1086/599092. [DOI] [PubMed] [Google Scholar]
- Paula MO, Fonseca DM, Wowk PF, Gembre AF, Fedatto PF, Sérgio CA, Silva CL, Bonato VLD. Host genetic background affects regulatory T-cell activity that influences the magnitude of cellular immune response against Mycobacterium tuberculosis. Immunol Cell Biol. 2011;89:526–34. doi: 10.1038/icb.2010.116. [DOI] [PubMed] [Google Scholar]
- da Fonseca DM, Silva CL, Wowk PF, Paula MO, Ramos SG, Horn C, Marchal G, Bonato VLD. Mycobacterium tuberculosis culture filtrate proteins plus CpG oligodeoxynucleotides confer protection to Mycobacterium bovis BCG-primed mice by inhibiting interleukin-4 secretion. Infect Immun. 2009;77:5311–21. doi: 10.1128/IAI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsh CS. A role for CD4+ CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surcel HM, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guérin infection. J Immunol. 2007;178:3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Orme IM. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun. 2001;69:1127–33. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Turner OC, Turner J, Marietta P, Brooks JV, Orme IM. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect Immun. 2001;69:1722–8. doi: 10.1128/IAI.69.3.1722-1728.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroza-González A, García-Romo GS, Aguilar-León D, et al. In situ analysis of lung antigen-presenting cells during murine pulmonary infection with virulent Mycobacterium tuberculosis. Int J Exp Pathol. 2004;85:135–45. doi: 10.1111/j.0959-9673.2004.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S, Shaler CR, Xing Z. Pulmonary mucosal dendritic cells in T-cell activation: implications for TB therapy. Expert Rev Respir Med. 2011;5:75–85. doi: 10.1586/ers.10.81. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–24. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, et al. Mouse CD11bhigh lung dendritic cells have more potent capability to induce IgA than CD103+ lung dendritic cells in vitro. Am J Respir Cell Mol Biol. 2012;46:773–80. doi: 10.1165/rcmb.2011-0329OC. [DOI] [PubMed] [Google Scholar]
- Furuhashi K, Suda T, Hasegawa H, et al. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am J Respir Cell Mol Biol. 2012;46:165–72. doi: 10.1165/rcmb.2011-0070OC. [DOI] [PubMed] [Google Scholar]
- Raymond M, Rubio M, Fortinzr G, Shalaby KH, Hammad H, Lambrecht BN, Sarfati M. Selective control of SIRP-α-positive airway dendritic cell trafficking through CD47 is critical for the development of TH2-mediated allergic inflammation. J Allergy Clin Immunol. 2009;124:1333–42. doi: 10.1016/j.jaci.2009.07.021. .el. [DOI] [PubMed] [Google Scholar]
- Bonato VL, Gonçalves ED, Soares EG, Santos Júnior RR, Sartori A, Coelho-Castelo AA, Silva CL. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-γ recovery and reduction of lung injury. Immunology. 2004;113:130–8. doi: 10.1111/j.1365-2567.2004.01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato VL, Goncalves ED, Santos RR, Silva CL. Genetic aspects and microenvironment affect expression of CD18 and VLA-4 in experimental tuberculosis. Scand J Immunol. 2002;56:185–94. doi: 10.1046/j.1365-3083.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–50. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- Kapina MA, Rubakova EI, Majorov KB, Logunova NN, Apt AS. Capacity of lung stroma to educate dendritic cells inhibiting mycobacteria-specific T-cell response depends upon genetic susceptibility to tuberculosis. PLoS ONE. 2013;8:e72773. doi: 10.1371/journal.pone.0072773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- Sung SS, Fu SM, Rose CE, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (αE)- β7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–72. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8+ T cell differentiation by a CD24-dependent mechanism. Immunity. 2014;40:400–13. doi: 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–9. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–93. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow GM, Cooper A, Reiley W, Chatterjee M, Woodland DL. Early T-cell responses in tuberculosis immunity. Immunol Rev. 2008;225:284–99. doi: 10.1111/j.1600-065X.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–15. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Romo GS, Pedroza-González A, Aguilar-León D, Orozco-Estevez H, Lambrecht BN, Estrada-Garcia I, Flores-Romo L, Hernández-Pando R. Airways infection with virulent Mycobacterium tuberculosis delays the influx of dendritic cells and the expression of costimulatory molecules in mediastinal lymph nodes. Immunology. 2004;112:661–8. doi: 10.1046/j.1365-2567.2004.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis MM, Fulton SA, Reba SM, Liu Y, Harding CV, Boom WH. Modulation of pulmonary dendritic cell function during mycobacterial infection. Infect Immun. 2008;76:671–7. doi: 10.1128/IAI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leepiyasakulchai C, Ignatowicz L, Pawlowski A, Källenius G, Sköld M. Failure to recruit anti-inflammatory CD103+ dendritic cells and a diminished CD4+ Foxp3+ regulatory T cell pool in mice that display excessive lung inflammation and increased susceptibility to Mycobacterium tuberculosis. Infect Immun. 2012;80:1128–39. doi: 10.1128/IAI.05552-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow cytometry representative analysis and frequency of CD4+, CD4+ Foxp3+ and CD8+ cells that migrate to the lungs of infected C57BL/6 and BALB/c mice. Lung cells were first gated by size and granularity, following the gate on CD4+, or CD4+ Foxp3+ or CD8+ cells (cells from recipient mice). In addition, CFSE+ cells were evaluated within CD4+, CD4+ Foxp3+ and CD8+ gated cells (cell transfer) (a). Frequency of CD4+ (b), CD4+ CFSE+ (c), CD4+ Foxp3+ (d), CD4+ Foxp3+ CFSE+ (e), CD8+ (f) and CD8+ CFSE+ cells (g). Data are presented as the mean ± SD. Data are representative of two independent experiments (n = 6 to n = 9 mice/group). Bars show the difference (P < 0·05) between infected C57BL/6 and BALB/c mice.
Figure S2. Control plots for evaluation of interferon-γ (IFN-γ) or interleukin-17 (IL-17) -producing CD4+ cells. Lung cells were left unstained and autofluorescence was delimited. Markers used to define IFN-γ+- or IL-17+-producing CD4+ cells were defined based on Fluorescence minus one (FMO) controls (a). Representative analysis showing the frequency of CD4+ IFN-γ+ and CD4+ IL-17+ cells that were left non-stimulated (b). Purified spleen CD4+ cells were left non-stimulated and co-cultured, during 48 hr, with CD11c+ CD11b− CD103+ cells (CD103+) or CD11c+CD11b+ CD103− (CD11b+) obtained from infected C57BL/6 or BALB/c mice.