Abstract
Monocytes, key components of the immune system, are a heterogeneous population comprised of classical monocytes (CD16−) and non-classical monocytes (CD16+). Monocytes are short lived and undergo spontaneous apoptosis, unless stimulated. Dysregulation of monocyte numbers contribute to the pathophysiology of inflammatory diseases, yet the contribution of each subset remains poorly characterized. Protein kinase C (PKC) family members are central to monocyte biology; however, their role in regulating lifespan and immune function of CD16− and CD16+ monocytes has not been studied. Here, we evaluated the contribution of PKCδ and PKCε in the lifespan and immune response of both monocyte subsets. We showed that CD16+ monocytes are more susceptible to spontaneous apoptosis because of the increased caspase-3, -8 and -9 activities accompanied by higher kinase activity of PKCδ. Silencing of PKCδ reduced apoptosis in both CD16+ and CD16− monocytes. CD16+ monocytes express significantly higher levels of PKCε and produce more tumour necrosis factor-α in CD16+ compared with CD16− monocytes. Silencing of PKCε affected the survival and tumour necrosis factor-α production. These findings demonstrate a complex network with similar topography, yet unique regulatory characteristics controlling lifespan and immune response in each monocyte subset, helping define subset-specific coordination programmes controlling monocyte function.
Keywords: apoptosis, heterogeneous monocyte population, inflammation, protein kinase Cδ, protein kinase Cε
Introduction
Monocytes are key cells of the innate immune system responsible for the initiation, progression and resolution of inflammation, pathogen clearance, wound healing and tissue homeostasis.1 Monocytes constitute a heterogeneous population classified into two main groups: classical monocytes or CD14+ CD16− cells (referred to hereafter as CD16−) accounting for 90% of all circulating monocytes, expressing CD14, a lipopolysaccharide (LPS) co-receptor and non-classical or CD14+ CD16+ cells (referred hereafter as CD16+) that express CD14 and the receptor for the low-affinity immunoglobulin FcγRIII or CD16.2 Monocyte heterogeneity is also found in mice.3 The distinct monocyte subsets seem to reveal diverse functional roles, such as recruitment to inflammatory lesions and immunoregulatory function. Monocytes originate in the bone marrow and circulate in the bloodstream for 24–48 hr before undergoing apoptosis in the absence of survival stimuli.4,5 Controlling proper monocyte numbers constitutes a central homeostatic process for the regulation of the immune system. Hence, dysregulation of monocyte numbers is implicated in the pathophysiology of inflammatory diseases, including atherosclerosis, arthritis and multiple sclerosis.6–8 Clinical studies revealed increased numbers of CD16+ monocytes in rheumatoid arthritis, tuberculosis and sepsis.7,9,10 Yet, our understanding of the monocyte function and lifespan is mainly based on the knowledge of CD16− monocytes. Differences in oxidant-induced apoptosis and glucocorticoid-induced apoptosis in CD16+ and CD16− subsets have been recently reported.11,12 The increased expression of glucocorticoid receptors on CD16+ may suggest subset-specific mechanisms involved in the regulation of monocyte numbers. Our previous work showed that a complex network of survival and apoptotic factors controls the dynamic behaviour of monocyte lifespan.13 Furthermore, timely activation of apoptosis in monocytes is controlled by negative and positive regulators.12,14–16 We found that caspase-3, a member of the conserved cysteine-aspartate-specific proteases, is essential for monocyte apoptosis.4 Activation of caspase-3 is mediated by the extrinsic or intrinsic pathways, through caspase-8 and caspase-9, respectively,17 however the specific contribution of these caspases in monocyte apoptosis remains poorly defined. Caspase-3 activation promotes the cleavage of multiple proteins, including poly-ADP ribose polymerase (PARP), resulting in cellular disassembly during cell death. Differentiation factors18,19 or inflammatory stimuli20 block the apoptotic programme, which can be reactivated by anti-inflammatory molecules.11,21 Protein kinase C isoform δ (PKCδ), a member of the PKC-family, phosphorylates caspase-3, thereby acting as a positive activator of monocyte apoptosis. PKCδ-deficient mice showed exacerbated atherosclerosis with increased macrophage numbers, which are resistant to apoptosis, highlighting the importance of PKCδ in the execution of cell death.22,23 The 11 human PKC isoforms are classified based on their structure and co-factor requirements into three groups: classical including PKCα, βI, βII, and γ require calcium, 1,2-diacylglycerol (DAG) and phosphatidylserine (PS), novel PKC (δ, ε, η, and θ), that require DAG and PS and atypical PKC (ζ and λ/ι) which are calcium and DAG independent.24 PKC play central roles in monocyte behaviour, including differentiation, apoptosis and immune response.16,24–28 PKCε-deficient mice showed impaired nuclear factor (NF-κB) activity, resulting in decreased tumour necrosis factor-α (TNF-α) production when treated with LPS. However, how different PKC isoforms contribute to the heterogeneous behaviour of both monocyte subsets has not been evaluated.
Our findings showed that CD16+ monocytes are more susceptible to spontaneous apoptosis than CD16− cells. A higher PKCδ kinase activity accompanied by an earlier increase of caspase activity was found in CD16+ monocytes. Silencing experiments demonstrated that PKCδ is a positive regulator of apoptosis, whereas PKCε contributes to monocyte survival. Inhibition of PKCδ expression showed that this kinase is dispensable for the immune response in both subsets. In contrast, PKCε played a central role in the immune response and its higher expression in CD16+ cells may help to explain the ability of CD16+ monocytes to produce higher levels of TNF-α during LPS stimulation. Collectively, these results suggest distinct roles of PKCε and PKCδ in the immunobiology and lifespan of monocytes, providing a novel understanding of the molecular networks that regulate the behaviours of specific monocyte subsets.
Materials and methods
Reagents and antibodies
Isoform-specific PKC antibodies including PKCα (C-20), PKCβI (C16), PKCβII (C18) PKCγ (C19), PKCδ (C-20), PKCε (C-15), PKCθ (C-18), PKCη (C15), PKCζ (C20) and PKCι/λ (H-76) were obtained from Santa Cruz (Santa Cruz, CA). The anti-inactive-caspase-3 antibody was purchased from BD Biosciences (San Jose, CA) and the anti-active-caspase-3 and anti-histone 2B (H2B) antibodies were obtained from Cell Signaling (Danvers, MA). The anti-β-tubulin antibody was from Millipore (Billerica, MA). Secondary antibodies linked to horseradish peroxidase and enhanced chemiluminescence were purchased from Amersham Biosciences (Arlington Heights, IL). Recombinant PKC proteins (rPKC), used as controls, including rPKCα, rPKCβI, rPKCβII, rPKCγ, rPKCδ, rPKCε, rPKCθ, rPKCη, rPKCζ and rPKCι/λ were obtained from Invitrogen (Grand Island, NY).
Monocyte isolation and cell culture
Peripheral blood mononuclear cells were isolated from healthy donors (American Red Cross) by Histopaque-1077 gradient (Sigma, St. Louis, MO) centrifugation as previously described.16 CD16− and CD16+ monocyte subpopulations were isolated using the CD16+ monocyte isolation kit (Miltenyi Biotec, Auburn, CA) following the manufacturer's instructions. Briefly, peripheral blood mononuclear cells were resuspended in MACS buffer (PBS, 0·5% BSA and 2 mm EDTA) and incubated with FcR blocking reagent and non-monocyte depletion cocktail to remove CD56+ CD16+ cells and CD56+ CD14+ cells by magnetic cell sorting. Flow through aliquots were incubated with anti-CD16 antibody-coated magnetic microbeads (80 μl beads/1 × 108 cells) for 15 min at 4° and purified by magnetic sorting. Samples containing CD16+ or CD16− cells were incubated with anti-CD14 antibody-coated magnetic microbeads (16 μl beads/1 × 107 cells) for 15 min at 4° and purified by magnetic sorting to obtain the CD14+ CD16− (CD16−) and CD14+ CD16+ (CD16+) monocyte subsets. Purity was assessed by flow cytometry using anti-CD14-allophycocyanin and anti-CD16-phycoerythrin antibodies (BD Biosciences) reaching routinely 95% and 85% pure CD16− and CD16+ monocyte subsets, respectively. Monocytes (0·5 × 106 cells/ml) were cultured in non-adherence polypropylene tubes for different lengths of time in serum-free RPMI-1640 (Invitrogen) at 37° in 5% CO2.
Cell lysates and immunoblotting
Cells were lysed with Nonidet P-40 lysis buffer for 2 hr as previously described.15,29 Five micrograms of lysates were used to detect most PKC and 50 μg to assess PKCγ, PKCι/λ, inactive and active-caspase-3 protein expression by immunoblot. Relative expression of PKC isoforms (intensity/mm2) was calculated by densitometry as follows: (density of PKC isoform)/(density of β-tubulin).
Caspase activity assays
The caspase-9, -8 and -3 activity assays were performed using 20–50 μm of LEHD-, IETD- or DEVD-AFC tetrapeptide substrates (MP Biomedicals, Santa Ana, CA) as previously described.16,30 Release of free 7-amino-4-trifluoromethyl coumarin (AFC) was determined using a Cytofluor 4000 fluorometer (Perseptive Company, Framingham, MA; filters: excitation; 400 nm, emission; 508 nm).
In vitro kinase assays
In vitro kinase assays were performed as previously described.29 Briefly, 50 μg of lysates were immunoprecipitated for 12 hr at 4° with anti-PKCδ or IgG isotype control antibodies followed by 1 hr incubation with protein G-agarose beads (Invitrogen). Immunoprecipitates were incubated for 1 hr at 37° with a kinase assay buffer containing 2 μCi of [γ-32P]-ATP (Perkin Elmer, Waltham, MA), 0·5 mm ATP, 200 μg/ml PS, 20 μg/ml DAG and 1 μg H2B as exogenous substrate (Roche Applied Science, Indianapolis, IN). Reactions were separated by SDS–PAGE and phosphorylated H2B was visualized by autoradiography and the same membrane was immunoblotted with an anti-PKCδ and anti-H2B antibodies.
SiRNA-transfection and flow cytometry
Monocytes (1 × 106 cells/ml) were transfected with 6 pmol of PKCε-small interfering RNA (siRNA) (sense, 5′-AAGCCCCUAAAGACAAUGAAGTT-3′; Dharmacon, Pittsburgh PA), PKCδ-siRNA (sense, 5′-GGCUGAGUUCUGGCUGGACTT-3′; Qiagen, Valencia, CA) or control-siRNA (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; Qiagen), with the Amaxa 4D-Nucleofector X-Unit (Lonza, Walkersville, MD), following the manufacturer's instructions. Cells were cultured for 24 hr in serum-containing media, followed by an additional 4 hr and 8 hr in serum-free medium. Percentages of apoptosis, active-caspase-3+ and cleaved PARP+ cells were assessed by co-staining with Annexin V-allophycocyanin/7-AAD, FITC-conjugated anti-cleaved PARP and phycoerythrin-conjugated anti-active-caspase-3 antibody, as previously described.15 Flow cytometry was performed using LSR II flow cytometer and FACSDiva software version 6.0.
LPS stimulation and ELISA analysis
Freshly isolated monocytes (1 × 106 cells/ml) were stimulated for 2, 4 and 8 hr with 10 ng/ml LPS (Escherichia coli 0127:B8, BD Biosciences). RNAi-transfected monocytes (1 × 106 cells/ml) were cultured for 24 hr and subsequently stimulated for 8 hr with 10 ng/ml LPS or PBS. The TNF-α was quantified by ELISA (R&D Systems, Minneapolis, MN), as previously described.31
Statistical analysis
All results are represented as mean ± standard error of mean (SEM). Statistical analyses were performed using the Student's t-test, a P-value < 0·05 was considered statistically significant.
Results
Distinct levels of spontaneous apoptosis in monocyte subpopulations
To study the mechanisms of CD16− and CD16+ monocyte lifespan, cells were isolated from healthy individuals and cultured for 4 and 8 hr. CD16+ monocytes displayed threefold increase in the percentage of apoptotic cells as shown by Annexin V/7-AAD staining compared with CD16− monocytes at 4 and 8 hr, respectively (Fig.1a). CD16+ cells had significantly higher levels of active-caspase-3+ cells at 4 and 8 hr compared with CD16− monocytes (Fig.1b). In addition, proteolytic processing of caspase-3 was observed in CD16+ cells at 4 hr and later at 8 hr in CD16− cells (Fig.1c). These results suggest a difference in lifespan between the CD16− and CD16+ monocyte populations.
Figure 1.
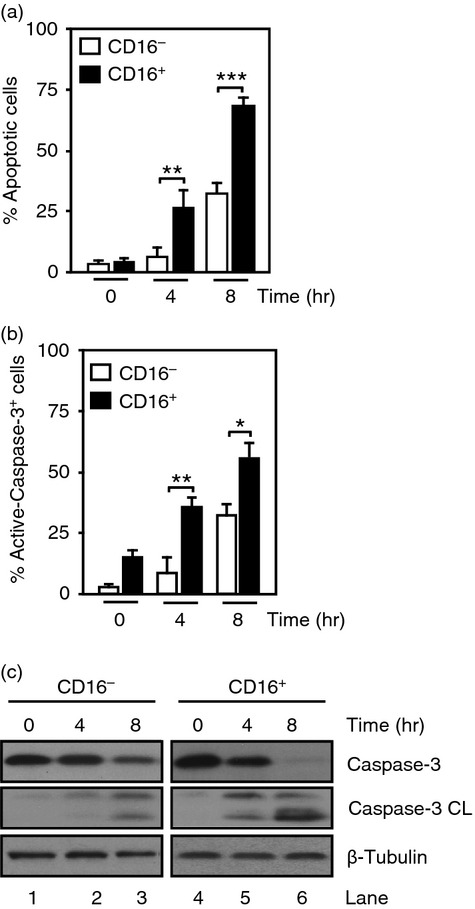
Spontaneous apoptosis in monocyte subsets. Purified human CD16− and CD16+ monocytes were cultured in serum-free media for 4 and 8 hr. (a) Percentage of apoptotic cells was assessed by Annexin V/7-AAD staining. (b) Percentage of cells stained with an anti-active-caspase-3-phycoerythrin antibody. Data represent mean ± SEM (n = 3, *P < 0·05, **P < 0·01, ***P < 0·001). (c) Immunoblots from the samples used in (a) probed with anti-inactive-caspase-3 (Casp-3) or anti-active-caspase-3 (Casp-3 CL) antibodies. β-Tubulin expression was used as loading control. Data are representative of three experiments.
Intrinsic and extrinsic activator caspases contribute to caspase-3-dependent apoptosis in CD16− and CD16+ monocyte subsets
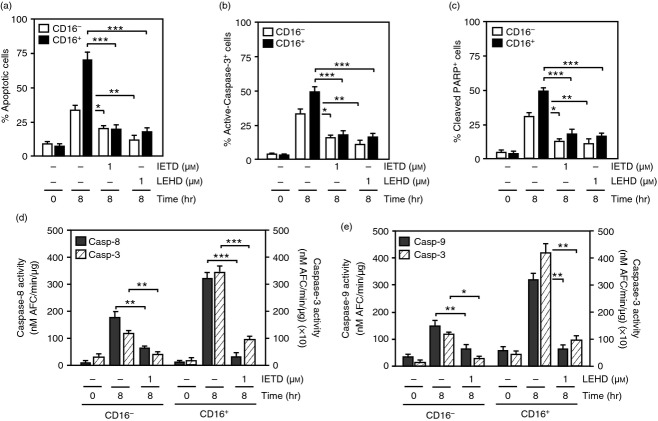
To evaluate the mechanisms regulating monocyte cell death, purified CD16− and CD16+ monocytes were cultured in the presence of the pharmacological caspase-3 inhibitor DEVD-fluoromethyl ketone (FMK) for 8 hr. Approximately 75% of the CD16+ and only 40% of the CD16− cells treated with the diluent control DMSO were apoptotic after 8 hr as indicated by the increased Annexin V/7-AAD staining (Fig.2a). Treatment with 1 or 25 μm DEVD-FMK reduced the percentage of apoptosis by about twofold in CD16− cells and by about fourfold in CD16+ monocytes (Fig.2a), reaching levels found in control cells. The presence of 1 or 25 μm DEVD-FMK significantly reduced the percentage of active-caspase-3+ cells in both CD16− and CD16+ monocytes (Fig.2b). Consistently, a reduction of caspase-3 activity, as determined by the cleavage of the DEVD-AFC substrate (Fig.2c) and a decrease in PARP cleavage were observed in both CD16− and CD16+ monocytes cultured with DEVD-FMK (Fig.2d). These results suggest that spontaneous apoptosis of both CD16− and CD16+ monocytes is regulated by caspase-3.
Figure 2.

The lifespan of CD16− and CD16+ monocytes is regulated by caspase-3. CD16− and CD16+ monocytes were cultured for the indicated times with 1 or 25 μm DEVD-FMK (DEVD) or diluent control DMSO (−). (a) Percentage of apoptotic cells was assessed by Annexin V/7-AAD staining. (b) Percentage of cells stained with an anti-active-caspase-3-phycoerythrin antibody. (c) The activity of caspase-3 was evaluated using the DEVD-AFC assay. (d) Percentage of cells stained with the anti-cleaved PARP-FITC antibody. Data represent mean ± SEM (n = 3, *P < 0·05, **P < 0·01, ***P < 0·001).
To determine the contribution of the activator caspase-8 and caspase-9 in spontaneous apoptosis, monocytes were incubated with 1 μm of the pharmacological caspase-8 inhibitor, IETD-FMK or the caspase-9 inhibitor LEHD-FMK for 8 hr. The percentage of apoptotic cells was reduced to ∼ 20% when either caspase-8 or caspase-9 was inhibited, comprising a threefold reduction in CD16+ cells and a twofold decrease in CD16− monocytes (Fig.3a). These effects were accompanied by a decrease of active-caspase-3+ and cleaved PARP+ cells in CD16− and CD16+ monocytes treated with either 1 μm IETD-FMK or LEHD-FMK (Fig.3b,c). CD16+ monocytes cultured for 8 hr with the diluent control DMSO showed about a twofold higher caspase-8 activity compared with CD16− cells (Fig.3d,e). Consistently, caspase-3 activity levels found at 8 hr were about threefold higher in CD16+ cells compared with CD16− monocytes (Fig.3d,e). The caspase-8 activity was inhibited in CD16− and CD16+ cells treated with 1 μm IETD-FMK reaching levels found in control cells (Fig.3d). In CD16+ monocytes, an approximately twofold higher caspase-9 activity was observed at 8 hr compared with CD16− cells (Fig.3e), which was inhibited to the basal levels found in control cells in the presence of 1 μm LEHD-FMK. These results showed that caspase-8, -9, and -3 activities are higher in CD16+ monocytes than CD16− cells. Collectively, these observations suggest a similar role of caspases in apoptosis with distinct kinetics, leading to a faster execution of apoptosis in CD16+ monocytes.
Figure 3.
Extrinsic and intrinsic activator caspases contribute to CD16+ and CD16− monocyte apoptosis. CD16− and CD16+ monocytes were cultured for 8 hr in the presence of 1 μm IETD-FMK (IETD), 1 μm LEHD-FMK (LEHD) or diluent control DMSO (−). (a) Percentage of apoptotic cells was determined by Annexin V/7-AAD staining. (b) Percentage of cells stained with an anti-active-caspase-3-phycoerythrin antibody. (c) Percentage of cells stained with an anti-cleaved PARP-FITC antibody. (d) Caspase-8 activity was assessed by cleavage of the IETD-AFC substrate. (e) Caspase-9 activity was determined by cleavage of the LEHD-AFC substrate. Data represent mean ± SEM (n = 3, *P < 0·05, **P < 0·01, ***P < 0·001).
Differential expression of PKC isoforms in monocyte populations
Protein kinase Cs play a central role in the homeostasis of survival and cell death.24 Previously, our findings showed that PKCδ is a central regulator of monocyte lifespan.16 To assess whether differences in PKC expression could account for the heterogeneous behaviour of CD16− and CD16+ cell death, we investigated the expression level of all PKC isoforms (Fig.4). No significant differences in the expression of classical or atypical PKC, including PKCα, PKCβI, PKCβII and PKCζ were observed in CD16− and CD16+ monocytes (Fig.4a,b). Neither PKCγ nor PKCι/λ was detected in either monocyte population, in agreement with previous findings.32 All novel PKC, including PKCη, PKCζ and PKCδ, were expressed at comparable levels in both subsets, with the exception of PKCε, which was about threefold higher in CD16+ compared with CD16− monocytes (Fig.4a,b). Next, the PKCδ kinase activity was assessed in both monocyte subsets at different times. A higher PKCδ kinase activity was observed in CD16+ cells compared with CD16− monocytes, starting at 30 min of culture and reaching about twofold higher activity at 2 hr, as represented by the increased phosphorylation of H2B (Fig.4c, lanes 5 and 10). Collectively, these results demonstrate that PKCδ reaches a higher kinase activity and has faster kinetics of activation during monocyte lifespan in CD16+ cells than CD16− monocytes.
Figure 4.
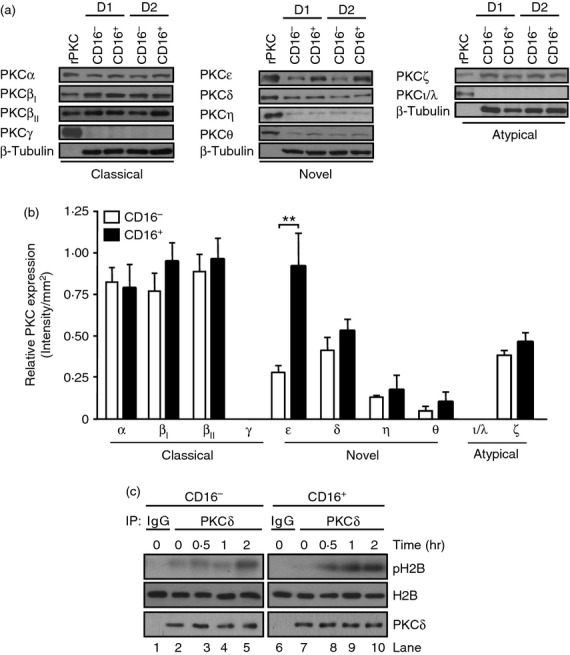
Expression of protein kinase C (PKC) isoforms and PKCδ activity in different monocyte subsets. (a) Immunoblot analyses using isoform specific anti-PKC antibodies in non-apoptotic CD16− and CD16+ monocytes from two independent donors (D1 and D2). Recombinant human PKC (rPKC) proteins were used as controls. The same membranes were re-probed with an anti-β-tubulin antibody. (b) Relative expression of PKC isoforms (Intensity/mm2) after normalization by the corresponding β-tubulin expression. Data represent mean ± SEM (n = 7, **P < 0·01). (c) CD16− and CD16+ monocytes were cultured in serum-free media for 0·5, 1 and 2 hr and lysates were immunoprecipitated (IP) with anti-PKCδ or isotype control (IgG) antibodies, followed by in vitro kinase assays in the presence of [γ32-P]-ATP and H2B as exogenous substrate. Phosphorylated H2B was visualized by autoradiography (pH2B) and the same membranes were re-probed with anti-PKCδ and anti-H2B antibodies. Data are representative of three experiments.
To determine the functional role of PKCδ and PKCε, the expression of either kinase was individually silenced by transfection with siRNA-PKCδ or siRNA-PKCε. PKCδ and PKCε were reduced by ∼ 75% and 90%, respectively, in CD16+ and CD16− monocytes, as determined by Western blot analyses (Fig.5a). CD16− and CD16+ monocytes transfected with siRNA-PKCδ, siRNA-PKCε or siRNA-Control were cultured for a further 4 and 8 hr in serum-free media to undergo spontaneous apoptosis. By 8 hr, only 25% of the siRNA-Control transfected CD16− cells were apoptotic, as determined by staining with Annexin V/7-AAD, whereas ∼ 75% of the CD16+ cells underwent cell death (Fig.5b, white bars). Silencing of PKCδ significantly reduced the percentage of apoptotic CD16− cells at 8 hr (Fig.5b, grey bars). A similar decrease of apoptosis was observed when PKCδ was silenced in CD16+ cells at 4 hr, reaching ∼ 25% at 8 hr (Fig.5b, grey bars). This effect was accompanied by a reduction in the percentage of active-caspase-3+ cells in CD16− and CD16+ monocytes when PKCδ was silenced (Fig.5c, grey bars). In contrast, silencing of PKCε increased the percentage of apoptotic cells by about twofold at 4 and 8 hr in CD16− monocytes compared with cells transfected with siRNA-Control, reaching ∼ 50% at 8 hr (Fig.5b, black bars). Inhibition of PKCε in CD16+ cells increased the percentage of apoptotic cells to ∼ 90% at 8 hr (Fig.5b, black bars). Similar results were observed when the percentage of active-caspase-3+ stained cells was evaluated (Fig.5c, black bars).
Figure 5.
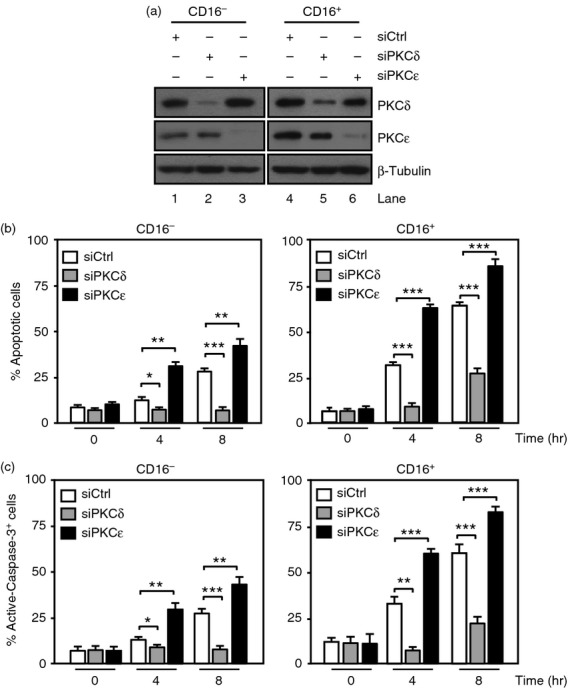
Distinct roles of protein kinase C δ (PKCδ) and PKCε in monocyte lifespan. CD16− and CD16+ monocytes were transfected with PKCε-small interfering RNA (siPKCε), PKCδ-siRNA (siPKCδ) or siRNA-Control (siCtrl) and lysates were used to evaluate the efficiency of silencing. (a) Immunoblots with anti-PKCε and anti-PKCδ antibodies. The same membrane was re-probed with an anti-β-tubulin antibody. The same cells used in (a) were cultured in serum-free media for a further 4 and 8 hr and used to evaluate the cellular lifespan. (b) Percentage of apoptotic cells was assessed by Annexin V/7-AAD staining. (c) Percentage of cells stained with anti-active-caspase-3-phycoerythrin antibodies. Data represent mean ± SEM (n = 3, *P < 0·05, **P < 0·01, ***P < 0·001).
These findings highlight differences in the contribution of PKCε and PKCδ to monocyte lifespan.
Increased expression of PKCε modulates TNF-α production in CD16+ monocytes
Previous findings demonstrating PKCε role in pro-inflammatory cytokine production26 and its elevated expression in CD16+ monocytes led us to hypothesize that high PKCε expression may contribute to the exacerbated immune response displayed by the CD16+ subset. To evaluate this possibility, CD16− and CD16+ monocytes were treated with 10 ng/ml LPS for 2, 4 and 8 hr. In agreement with previous reports,33 we found that stimulation with LPS resulted in a time-dependent increase of TNF-α in both monocyte subsets with an approximately fourfold higher TNF-α release in CD16+ cells at 8 hr compared with CD16− monocytes (Fig.6a). To investigate the role of PKC on TNF-α release, CD16− and CD16+ monocytes were transfected with siRNA-PKCε, siRNA-PKCδ, or siRNA-Control and subsequently treated with 10 ng/ml LPS for 8 hr. Silencing of PKCδ had no effect on LPS-induced TNF-α production in both monocyte subsets compared with levels found in siRNA-Control (Fig.6b). However, silencing of PKCε reduced LPS-induced TNF-α release by about twofold in CD16− and about threefold in CD16+ monocytes (Fig.6b). These results show that PKCδ is dispensable in the immunoregulation of TNF-α, while the increased expression of PKCε in CD16+ cells regulates the major production of TNF-α observed in the CD16+ population.
Figure 6.
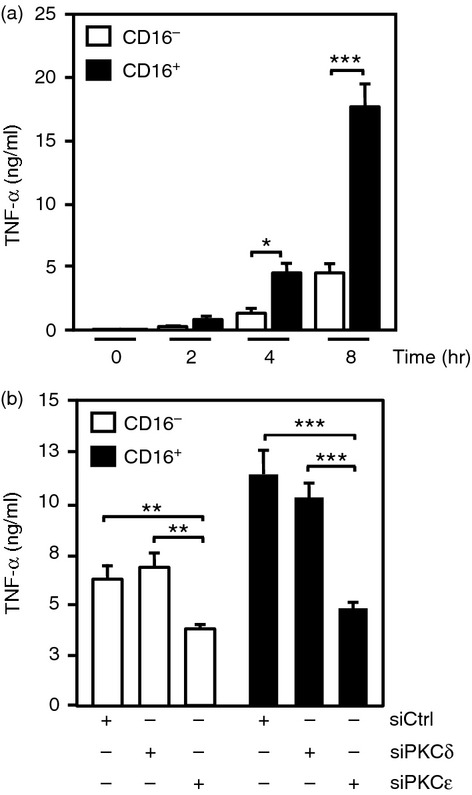
Protein kinase Cε (PKCε) regulates tumour necrosis factor-α (TNF-α) production in both monocyte subsets. (a) TNF-α production determined by ELISA in CD16− and CD16+ monocytes stimulated with 10 ng/ml LPS for 2, 4 and 8 hr. (b) Release of TNF-α in CD16− and CD16+ monocytes transfected with PKCε-small interfering RNA (siPKCε), PKCδ-siRNA (siPKCδ) or siRNA-Control (siCtrl) and subsequently stimulated with 10 ng/ml LPS for 8 hr. Data represent mean ± SEM (n = 3, *P < 0·05, **P < 0·01, ***P < 0·001).
Discussion
The emerging evidence that different monocyte populations7 have distinct contributions to the pathophysiology of inflammatory diseases has prompted great interest in understanding the molecular mechanisms involved in the regulation of monocyte numbers. Plasticity in the lifespan of monocytes is defined by a complex repertoire of regulators that respond to environmental cues including growth factors, and self and non-self stimuli. Hence, controlling monocyte numbers constitutes a dynamic process, with great clinical significance.13 Classical monocytes undergo spontaneous apoptosis20 through a mechanism that requires caspase-3 activation.4 Our findings showed that CD16+ monocytes underwent rapid spontaneous apoptosis (Fig.1) and that similar to CD16− cells,4 apoptosis of CD16+ monocytes depended on caspase-3 (Fig.2). We observed significantly higher activity of caspase-3 in CD16+ cells compared with CD16− monocytes (Fig.2). Recent gene expression analyses suggested that higher levels of caspase-3 transcript and lower anti-apoptotic gene expression might mediate the increased susceptibility to apoptosis observed in CD16+ cells.12 Yet, whether an increase of caspase-3 transcript resulted in higher apoptotic activity has not been demonstrated. Moreover, CD16+ monocytes execute oxidant-induced apoptosis faster than CD16− cells,12 an effect attributed to a higher expression of glutathione-metabolising genes in CD16− monocytes and higher reactive oxygen species production in CD16+ cells.12 Our results showed a faster and higher caspase-3 protease activity in CD16+ cells (Fig.2). Supporting the key contribution of caspase-3 in monocyte apoptosis, we found that treatment with DEVD-FMK induced a dose-dependent inhibition of caspase-3 activity (Fig.2c). This effect was accompanied by reductions of about threefold and twofold in the percentage of apoptosis in CD16+ and CD16− monocytes, respectively (Fig.2a). Yet, while caspase-3 activity decreased proportionally with increasing concentrations of DEVD-FMK, the number of apoptotic cells was reduced similarly when 1 or 25 μm DEVD-FMK was used. It is plausible that DEVD-FMK, a well-accepted inhibitor of caspase-3 and caspase-7,34 blocks caspase-3 activity, but other caspases, such as caspase-2 and caspase-6,35,36 capable of promoting cell death yet not susceptible to DEVD-FMK inhibition, may contribute to account for these small differences. Main mechanisms that regulate the apoptotic activity of caspase-3 include its proteolytic cleavage, mediated by activator caspases,4,34 and caspase-3 regulatory proteins.1,15,16 Proteomic analyses in different monocyte populations found no differences in the expression of activator caspases.12 Our studies revealed higher caspase-8 and caspase-9 activities in CD16+ cells compared with CD16− monocytes (Fig.3), which were accompanied by a faster activation of caspase-3, as shown by its proteolytic processing and the cleavage of the cellular substrate PARP (Fig.3). Pharmacological inhibition of either caspase-8 or caspase-9 blocked caspase-3-dependent apoptosis in both CD16− and CD16+ cells (Fig.3), suggesting the contribution of both extrinsic and intrinsic pathways on spontaneous monocyte apoptosis. These results support a common regulatory mechanism of spontaneous monocyte apoptosis for both subsets and reveal a faster activation of the cell death programme in CD16+ monocytes (Fig.7).
Figure 7.
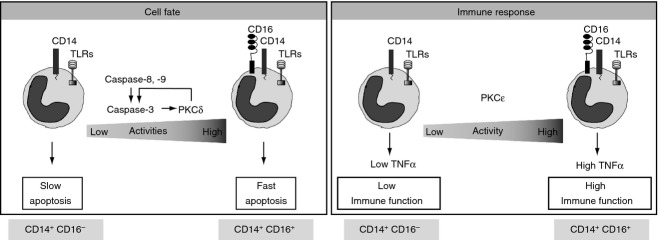
Working model of the contributions of protein kinase Cs and apoptotic factors in cell fate and immune response in different monocyte subpopulations.
Activation of caspases is tightly regulated and to a certain extent reversible.21 Dysregulation of monocyte numbers has been described under several inflammatory conditions, like atherosclerosis, arthritis and sepsis.13 Increased numbers of CD16+ monocytes have been reported in septic patients.37 Normalization of monocyte numbers correlates with resolution of inflammation and positive outcomes.38,39 Among the complex network of pro- and anti-apoptotic proteins, involved in monocyte function,13 PKC play a central role in monocyte biology.40 Our previous findings demonstrated that PKCδ is essential for the activation of caspase-3 in classical monocyte apoptosis.16 Here, we evaluated the expression of all PKC in both monocyte subsets (Fig.4). PKCδ expression was similar in CD16+ and CD16− monocytes (Fig.4a,b). However, the kinase activity of PKCδ was significantly higher and showed faster kinetics in CD16+ cells (Fig.4c). The regulation of PKCδ is multifactorial and remains poorly understood. In addition to the role of co-factors, such as PS and DAG, the former especially well studied in the regulation of classical PKC, some additional DAG-independent mechanisms have been described for PKCδ.24,41,42 Among them, phosphorylation, association to regulatory proteins, different cellular pools with distinct activation requirements, and a positive feedback loop that requires the proteolytic cleavage of PKCδ by caspase-3 have been previously reported.16,43–47 Recent studies showed that CD16+ cells express higher levels of genes encoding DAG-catalysing enzymes, including phospholipases γ2 and β1,48 but whether these changes in gene expression result in increased enzymatic activity has not been established. Our findings showed a faster proteolytic activation kinetics of caspase-3 in CD16+ cells (Fig.1) with a concurrent faster kinetics of PKCδ (Fig.4), supporting a positive feedback loop between PKCδ and caspase-3 that contributes to the differences in lifespan found in CD16+ monocytes (Fig.7).
Silencing experiments demonstrated that PKCδ has a pro-apoptotic function in both CD16− and CD16+ monocytes (Fig.5). Differences in the expression of certain PKC isoforms have been observed in classical monocytes.32 We found no differences in the expression of PKC between CD16− and CD16+ cells except for PKCε (Fig.4). PKCε regulates the survival of various cancer cells49,50 and immune response in macrophages26 but its role in the monocyte lifespan remains poorly understood. Supporting its role in survival, we found that PKCε-silencing increased spontaneous apoptosis in both monocyte subsets (Fig.5). However, higher expression of PKCε in CD16+ cells, a population more susceptible to undergo apoptosis, was intriguing and suggested an additional role of PKCε. Previous studies showed that macrophages from PKCε−/− mice have reduced NF-κB activation and decreased TNF-α expression in response to LPS,26 suggesting that PKCε plays a role in the immune response. PKCε is recruited to the Toll-like receptor 4 receptor in a MyD88-dependent manner upon LPS stimulation, increasing inflammatory cytokine production.51 Consistent with the role of PKCε in pro-inflammatory cytokine production we observed higher TNF-α release in LPS-stimulated CD16+ cells compared with CD16− monocytes (Fig.6), in agreement with previous studies.33 We further found that PKCδ-silencing had no effect on TNF-α production. In contrast, silencing of PKCε reduced LPS-induced TNF-α release in both monocyte populations with a more profound effect in the CD16+ subset (Fig.6).
Recent findings showed that reactive oxygen species generation upon TNF-α treatment could induce cell death in various cell types, including primary monocytes.12,52 However, this mechanism can be inhibited by the concomitant activation of NF-κB.53 Preceding data further showed that CD16+ monocytes were more susceptible to oxidant-induced apoptosis due to elevated levels of intracellular reactive oxygen species.12 However, within a pro-inflammatory microenvironment, monocytes escape their apoptotic fate through the activation of survival factors like AKT and NF-κB18,54 and respond to the bacteria-associated insults by initiating an immune response. These findings would suggest that reactive oxygen species-induced apoptosis is blocked in an inflammatory microenvironment to favour the generation of pro-inflammatory cytokines, like TNF-α, upon immune response initiation. In fact, our findings identified CD16+ monocytes as the major producers of TNF-α and further identified PKCε as an important regulator of this cytokine (Fig.6). Moreover, additional findings indicate that CD16+ monocytes are more efficient in responding against various microorganisms due to their faster migration into the inflammatory site, higher phagocytic capability and a greater production of β2-defensin and the key pro-inflammatory cytokines, TNF-α and interleukin-6.55–57
In summary, our findings revealed unique aspects of the molecular mechanisms regulating apoptosis and the immunological response of CD16− and CD16+ monocyte subpopulations. Although the mechanisms regulating lifespan and immune response were common to both monocyte subsets, a striking difference found here was the amplitude of such responses. Our findings showed that the susceptibility of CD16+ monocytes to undergo apoptosis is due to the increased activity of both executioner and activator caspases (Fig.7). We previously demonstrated that PKCδ is a positive regulator of caspase-3 in classical monocytes.16 Here, we showed an increased activity of PKCδ in CD16+ cells compared with CD16− monocytes, but no differences in the expression of PKCδ were noted. Hence, our findings suggest that rather than gene expression, other mechanisms such as the caspase-3-PKCδ-positive feedback loop may contribute to the distinct regulation of PKCδ in monocyte subsets. Results of the silencing experiments indicated that PKCδ, but not PKCε, acts as a positive regulator of CD16+ and CD16− apoptosis (Fig.5), whereas PKCε is dispensable to regulate cell fate, yet central in the regulation of the immune response. Our results suggest distinct contributions of PKC in controlling cell fate and immune response of monocyte subsets (Fig.7).
These findings provide evidence of the heterogeneous nature of monocytes beyond their cell surface receptor expression revealing unique aspects of the protein network that regulates the number of monocytes and their immune response.
Author contributions
This work was supported by grant RO1HL075040-01 to AID. A.I.D., Y.M. and O.V. designed the research; Y.M., E.G.M, and O.V. performed the research; and O.V., Y.M., A.P., and A.I.D., analysed data and wrote the paper.
Disclosure
The authors have no financial or commercial conflicts of interest.
References
- Gonzalez-Mejia ME, Doseff AI. Regulation of monocytes and macrophages cell fate. Front Biosci. 2009;14:2413–31. doi: 10.2741/3387. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–62. [PubMed] [Google Scholar]
- van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–35. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuluundorj D, Harding SA, Abernethy D, La Flamme AC. Expansion and preferential activation of the CD14/CD16 monocyte subset during multiple sclerosis. Immunol Cell Biol. 2014;92:1–9. doi: 10.1038/icb.2014.15. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–7. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+ CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–90. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayyani F, Belge KU, Frankenberger M, Mack M, Berki T, Ziegler-Heitbrock L. Mechanism of glucocorticoid-induced depletion of human CD14+ CD16+ monocytes. J Leukoc Biol. 2003;74:33–9. doi: 10.1189/jlb.1202612. [DOI] [PubMed] [Google Scholar]
- Zhao C, Tan YC, Wong WC, et al. The CD14+ CD16+ monocyte subset is more susceptible to spontaneous and oxidant-induced apoptosis than the CD14+ CD16− subset. Cell Death Dis. 2010;1:e95. doi: 10.1038/cddis.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2:204–15. doi: 10.1159/000296507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordet O, Rebe C, Plenchette S, et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–53. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- Voss OH, Batra S, Kolattukudy SJ, Gonzalez-Mejia ME, Smith JB, Doseff AI. Binding of caspase-3 pro-domain to Heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J Biol Chem. 2007;282:25088–99. doi: 10.1074/jbc.M701740200. [DOI] [PubMed] [Google Scholar]
- Voss OH, Kim S, Wewers MD, Doseff AI. Regulation of monocyte apoptosis by the PKCδ-dependent phosphorylation of caspase-3. J Biol Chem. 2005;280:17371–9. doi: 10.1074/jbc.M412449200. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Goyal A, Wang Y, Graham MM, Doseff AI, Bhatt NY, Marsh CB. Monocyte survival factors induce Akt activation and suppress caspase-3. Am J Respir Cell Mol Biol. 2002;26:224–30. doi: 10.1165/ajrcmb.26.2.4640. [DOI] [PubMed] [Google Scholar]
- Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/ PKCβ. J Biol Chem. 1999;274:26393–8. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- Mangan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–12. [PubMed] [Google Scholar]
- Doseff AI, Baker JH, Jr, Bourgeois TA, Wewers MD. Interleukin-4-induced apoptosis entails caspase activation and suppression of extracellular signal-regulated kinase phosphorylation. Am J Respir Cell Mol Biol. 2003;29:367–74. doi: 10.1165/rcmb.2002-0158OC. [DOI] [PubMed] [Google Scholar]
- Bai X, Margariti A, Hu Y, et al. PKCd deficiency accelerates neointimal lesions of mouse injured artery involving delayed reendothelialization and vasohibin-1 accumulation. Arterioscler Thromb Vasc Biol. 2010;30:2467–74. doi: 10.1161/ATVBAHA.110.215723. [DOI] [PubMed] [Google Scholar]
- Morgan S, Yamanouchi D, Harberg C, et al. Elevated PKCδ contributes to aneurysm pathogenesis through stimulation of apoptosis and inflammatory signaling. Arterioscler Thromb Vasc Biol. 2012;32:2493–502. doi: 10.1161/ATVBAHA.112.255661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF. Structural basis of PKC isoform function. Physiol Rev. 2008;88:1341–78. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale KA, Cathcart MK. PKCβ is required for human monocyte chemotaxis to MCP-1. J Biol Chem. 2003;278:25317–22. doi: 10.1074/jbc.M304182200. [DOI] [PubMed] [Google Scholar]
- Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Bosca L. PKCe is required for macrophage activation and defense against bacterial infection. J Exp Med. 2001;194:1231–42. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Lee HM, Leu SJ, Tsai YH. The essentiality of PKCα and PKCβΙ translocation for CD14+ monocyte differentiation towards macrophages and dendritic cells, respectively. J Cell Biochem. 2007;102:429–41. doi: 10.1002/jcb.21305. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mo X, Piper MG, Wang H, Parinandi NL, Guttridge D, Marsh CB. M-CSF induces monocyte survival by activating NF-κB p65 phosphorylation at Ser276 via PKC. PLoS ONE. 2011;6:e28081. doi: 10.1371/journal.pone.0028081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mejia ME, Voss OH, Murnan EJ, Doseff AI. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of Heat shock protein 27. Cell Death Dis. 2010;1:e64. doi: 10.1038/cddis.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Phenix BN, Lum JJ, et al. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome C, caspase cleavage and nuclear fragmentation. Cell Death Differ. 2002;9:1172–84. doi: 10.1038/sj.cdd.4401094. [DOI] [PubMed] [Google Scholar]
- Nicholas C, Batra S, Vargo MA, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and pro-inflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J Immunol. 2007;179:7121–7. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- Monick MM, Carter AB, Gudmundsson G, Geist LJ, Hunninghake GW. Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am J Physiol. 1998;275:L389–97. doi: 10.1152/ajplung.1998.275.2.L389. [DOI] [PubMed] [Google Scholar]
- Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+ CD16+ DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–6. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci U S A. 2008;105:12815–9. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzeczynska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+ CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55:629–38. doi: 10.1046/j.1365-3083.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- Nishibori M, Takahashi HK, Katayama H, et al. Specific removal of monocytes from peripheral blood of septic patients by polymyxin b-immobilized filter column. Acta Med Okayama. 2009;63:65–9. doi: 10.18926/AMO/31855. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Ono S, Hiraki S, et al. Hemoperfusion with polymyxin b-immobilized fibers reduced the number of CD14+ CD16+ monocytes in patients with septic shock. J Endotoxin Res. 2004;10:229–37. doi: 10.1179/096805104225005814. [DOI] [PubMed] [Google Scholar]
- Malavez Y, Gonzalez-Mejia ME, Doseff AI. PRKCδ (protein kinase C, delta) Atlas Genet Cytogenet Oncol Haematol. 2009;13:55–80. [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of PKC by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–7. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Yamauchi E, Taniguchi H, et al. Phosphorylation sites of PKCδ in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc Natl Acad Sci U S A. 2001;98:6587–92. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass M, Kronfeld I, Kazimirsky G, Blumberg PM, Brodie C. Tyrosine phosphorylation of PKCd is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–95. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. PKC isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–38. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCδ is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–60. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of PKCδ in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–20. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- Leverrier S, Vallentin A, Joubert D. Positive feedback of PKC proteolytic activation during apoptosis. Biochem J. 2002;368:905–13. doi: 10.1042/BJ20021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- Ding L, Wang H, Lang W, Xiao L. PKCε promotes survival of lung cancer cells by suppressing apoptosis through dysregulation of the mitochondrial caspase pathway. J Biol Chem. 2002;277:35305–13. doi: 10.1074/jbc.M201460200. [DOI] [PubMed] [Google Scholar]
- Okhrimenko H, Lu W, Xiang C, Hamburger N, Kazimirsky G, Brodie C. PKCε regulates the apoptosis and survival of glioma cells. Cancer Res. 2005;65:7301–9. doi: 10.1158/0008-5472.CAN-05-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The scaffold Myd88 acts to couple PKCε to TLR. J Biol Chem. 2008;283:18591–600. doi: 10.1074/jbc.M710330200. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee SB, Park JK, Yoo YD. TNF-α-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L) Cell Death Differ. 2010;17:1420–34. doi: 10.1038/cdd.2010.19. [DOI] [PubMed] [Google Scholar]
- Sakon S, Xue X, Takekawa M, et al. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-κB response in macrophages. BMC Immunol. 2007;8:1–17. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Ruiz SR, Torres-Aguilar H, Gonzalez-Dominguez E, et al. Human CD16+ and CD16− monocyte subsets display unique effector properties in inflammatory conditions in vivo. J Leukoc Biol. 2011;90:1119–31. doi: 10.1189/jlb.0111022. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–70. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]