Abstract
CD91 is a scavenger receptor expressed by different immune cells and its ligands defensins have been demonstrated to contribute to immune responses against infections and tumours. We previously demonstrated that CD91 is expressed on human monocyte-derived dendritic cells (moDCs) and that human defensins stimulate in vitro the activation of these cells. In this study, we observed that CD91 is expressed at different levels on two distinct moDC subsets: CD91dim and CD91bright moDCs. Although CD91bright moDCs represented a small proportion of total moDCs, this subset showed higher levels of activation and maturation markers compared with CD91dim moDCs. The frequency of CD91bright moDCs increased by ∼ 50% after in vitro stimulation with recombinant human neutrophil peptide-1 (rHNP-1) and recombinant human β defensin-1 (rHBD-1), while lipopolysaccharide (LPS) stimulation decreased it by ∼ 35%. Both defensins up-regulated moDC expression of CD80, CD40, CD83 and HLA-DR, although to a lower extent compared with LPS. Notably, upon culture with rHNP-1 and rHBD-1, CD91bright moDCs maintained their higher activation/maturation status, whereas this was lost upon culture with LPS. Our findings suggest that defensins promote the differentiation into activated CD91bright DCs and may encourage the exploitation of the CD91/defensins axis as a novel therapeutic strategy to potentiate antimicrobial and anti-tumour immune response.
Keywords: CD91, defensins, dendritic cells, human β defensin-1, human neutrophil peptide-1
Introduction
CD91 is a member of the low-density lipoprotein receptor family that recognizes more than 40 structurally and functionally distinct ligands.1 Particularly, CD91 has been demonstrated to bind and internalize α2-macroglobulin,2 C1q3 and defensins.4 Reflecting this diversity of ligands, CD91 displays a wide tissue distribution and participates in a variety of physiological responses, including lipoprotein metabolism, proteinase homeostasis and cell migration. Subsequent studies defined CD91 as an immunologically relevant receptor for heat-shock proteins and molecular chaperones.5,6 It is expressed on different cell types, including dendritic cells, monocytes, macrophages, B and T cells as well as splenocytes and thymocytes.7 In addition, CD91 is expressed on monocyte-derived dendritic cells (moDCs) and it plays a role in the internalization of CD91-targeted antigens and their presentation to T cells.8 Moreover, the importance of this molecule in the immune response has been strengthened by the observation that CD91 represents an important route for stimulating CD8+ T cell responses by MHC class I-restricted presentation.9
The scavenger receptor CD91 is involved in a wide variety of diseases and infections. Kebba et al.,10 showed that a significantly higher surface expression of CD91 on monocytes of HIV-1-infected long-term non-progressors may contribute to host anti-HIV-1 defence and play a role in protection against HIV-1 infection. Further, the up-regulation of CD91 in patients affected by cancer may represent an additional strategy to improve DC activation and subsequent stimulation of a specific CD8+ T-cell response towards tumour cells dying an immunogenic cell death.11 Of note, CD91 ligands heat-shock proteins, α2-macroglobulin and defensins are currently under investigation in pre-clinical and clinical trials exploring new therapeutic strategies for the treatment of infectious diseases and cancers.12–14
In a previous study, we demonstrated that moDCs express CD91 and that moDC treatment with human defensins up-regulates the surface expression of CD91 as well as the activation/maturation markers on these cells.15 In this study, we describe two subsets of moDCs, characterized by variable levels of CD91 expression and different states of activation/maturation. We further investigate the effects of recombinant human α and β defensins, human neutrophil peptide-1 (HNP-1) and human β defensin-1 (rHBD-1), on these two subsets of moDCs.
Materials and methods
In vitro culture of moDCs
Human peripheral blood mononuclear cells were isolated by Ficoll density gradient centrifugation from standard buffy coat preparations of healthy donors. Monocytes were obtained from adherent peripheral blood mononuclear cells (> 90% pure CD14+, as assessed by flow cytometry) and cultured in RPMI-1640 (Euroclone, Wetherby, UK) supplemented with 10% heat-inactivated fetal calf serum (Gibco, Invitrogen, Carlsbad, CA), in the presence of 800 U/ml recombinant human granulocyte–macrophage colony-stimulating factor (Strathmann Biotech, Hannover, Germany) and 10 ng/ml recombinant human interleukin-4 (R&D Systems, Minneapolis, MN) at 37° in 5% CO2 for 5 days. Immature moDCs were characterized by long dendrites and expression of high levels of CD1a (> 90%) and low levels of CD14 (< 5%). Cell viability was > 90% in all experiments, as assessed by trypan blue exclusion. These immature moDCs were stimulated for 18 hr by adding full-length 100 ng/ml rHNP-1 (30 amino acids), 500 ng/ml rHBD-1 (36 amino acids; both from AlphaDiagnostic, San Antonio, TX), 20 μg/ml heat-shock protein 90 (hsp 90; Enzo Life Sciences, Lausen, Switzerland), or 100 ng/ml lipolysaccharide (LPS; serotype 055:B5; Sigma Chemicals Co., St Louis, MO). The concentration and duration of culture stimulation were established based on preliminary experiments.15 Endotoxin contamination in culture media and defensins was excluded by Limulus amoebocyte lysate assay (BioWhittaker, Walkersville, MD) following the manufacturer's protocol.
Flow cytometric analysis
Phenotypic analysis was performed by five-colour flow cytometry. Briefly, 1 × 105 cells were incubated for 20 min at 4° with different monoclonal antibodies: FITC-conjugated anti-CD91 (Biomac, Leipzig, Germany), phycoerythrin (PE) -conjugated anti-CD80, allophycocyanin (APC) -conjugated anti-CD86, PE-conjugated anti-CD1a, APC-conjugated anti-CD83, APC-conjugated anti-CD14, PE-conjugated anti-CD40, PE-conjugated anti-CCR7, APC-Cy7-conjugated anti-HLA-DR (all from BD Biosciences, San Diego, CA). To optimize the staining, monoclonal antibodies were titrated in preliminary experiments. 7-Amino-actinomycin D (Sigma Aldrich, St Louis, MO) was used to exclude non-viable cells from the analysis. Fluorescence minus one samples were used as negative controls.
Cells were collected and analysed using a FACSCanto II (Becton Dickinson, San Jose, CA) flow cytometer, and data analysis was performed using FACSDiva (Becton Dickinson) and flowjo software (TreeStar Inc., Ashland, OR).
Statistical analysis
Statistical analyses were performed using graphpad prism 5.0a (GraphPad Software, San Diego, CA). All statistical analyses assumed a two-sided significance level of P < 0·05. The Student's paired t-test was used for comparisons between groups.
Results
Different levels of CD91 identify two moDC subpopulations
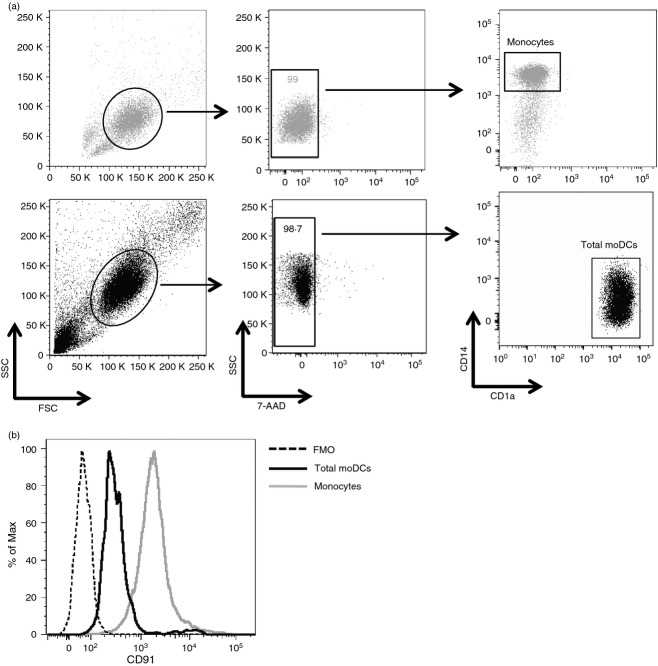
It has been shown that CD91 is expressed on moDCs.9,15–17 However, whether CD91 is expressed differently in moDC subpopulations is still unknown. To investigate the expression of CD91 receptor, we cultured human monocytes with granulocyte–macrophage colony-stimulating factor and interleukin-4 for 5 days to generate immature moDCs that were subsequently characterized by flow cytometry. As expected,15 we observed that during the differentiation of monocytes into moDCs characterized by down-regulation of CD14 and up-regulation of CD1a (Fig.1a), a partial down-regulation of CD91 occurred in parallel (Fig.1b). Moreover, our results clearly showed that CD91+ moDCs were segregated into two distinct populations: CD91 was highly expressed in a small proportion of total moDCs (CD91bright moDCs) (mean ± SE, 1·19 ± 0·28%), whereas the majority of cells expressed low levels of CD91 receptor (CD91dim moDCs) (Fig.2a).
Figure 1.
The expression of CD91 is partially down-regulated during differentiation of monocytes into monocyte-derived dendritic cells (moDCs). Monocytes obtained by plastic adhesion from freshly isolated peripheral blood mononuclear cells were stained for surface expression of CD14, CD1a and CD91 before and after their differentiation into moDCs obtained by 5 day-culture with recombinant human granulocyte–macrophage colony-stimulating factor and recombinant human interleukin-4. (a) Dot plots showing the gating strategy and the expression of CD14 and CD1a on monocytes (upper row) and moDCs (lower row). (b) Histogram showing the mean fluorescence intensity (MFI) of CD91 expression on monocytes (grey line), moDCs (black line), and fluorescence minus one (FMO) negative control (dotted line). One representative experiment is shown.
Figure 2.
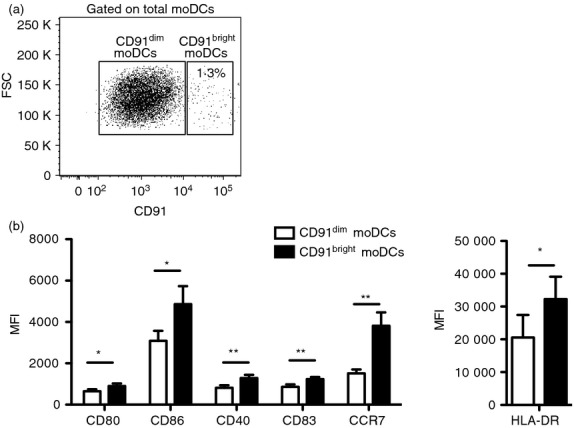
Different levels of CD91 identify two monocyte-derived dendritic cell (moDC) subpopulations. Immature moDCs were stained for surface expression of CD91 and activation/maturation markers. (a) Representative flow cytometric analysis showing the gating strategy used to define CD91dim and CD91bright moDCs. (b) Graphs show the mean fluorescence intensity (MFI) of CD80, CD86, CD40, CD83, CCR7 and HLA-DR molecules in CD91dim (white bars) and CD91bright (black bars) moDCs from 11 independent experiments. Data shown as mean ± SE. *P < 0·05, **P < 0·01.
CD91bright moDCs show an activated phenotype
The ability of DCs to activate immune responses depends on their maturation status and the expression of co-stimulatory molecules. To investigate whether CD91bright and CD91dim subsets differ in term of activation/maturation, we evaluated their surface expression of CD80, CD86, CD40, CD83, HLA-DR molecules. As shown in Fig.2(b), CD91bright moDCs expressed significantly higher levels of all the activation and maturation markers compared with CD91dim moDCs: CD80 [mean fluorescence intensity (MFI), mean ± SE, 905 ± 116 versus 647 ± 95, P < 0·05), CD86 (MFI, 4864 ± 861 versus 3093 ± 473, P = 0·05), CD40 (MFI 1287 ± 157 versus 813 ± 125, P < 0·01), CD83 (MFI, 1235 ± 99 versus 865 ± 120, P < 0·01) and HLA-DR (MFI, 32 273 ± 6815 versus 20 578 ± 6862, P < 0·05). Also shown in Fig.2(b), CD91bright moDCs expressed significantly higher levels of CCR7 than CD91dim moDCs (MFI 3814 ± 649 versus 1520 ± 185, P = 0·01).
rHNP-1 and rHBD-1 increase the proportion of the CD91bright moDC subset
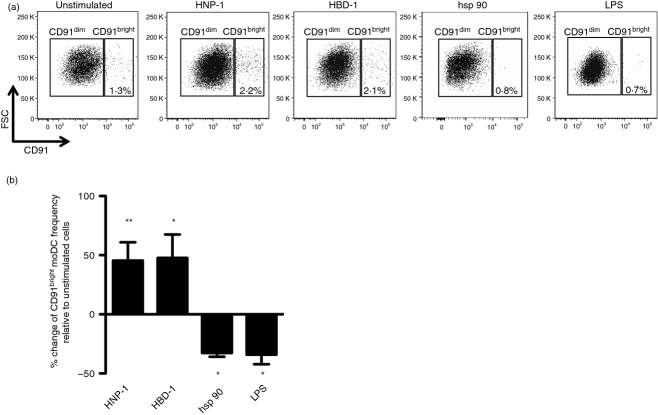
Based on our previous findings indicating that moDC treatment with human defensins up-regulates the surface expression of CD91, we next evaluated whether in vitro stimulation with rHNP-1 and rHBD-1 may indeed positively affect the frequency of CD91bright moDCs. Interestingly, we observed that the proportion of CD91bright moDCs increased after rHNP-1 stimulation by 45·4 ± 15·4% (mean ± SE, P < 0·01) and after rHBD-1 stimulation by 47·0 ± 17·4% (P < 0·05). On the contrary, the proportion of CD91bright moDCs decreased after treatment with the CD91 ligand hsp 90 by − 32·7 ± 6·2% (P < 0·05) and after LPS stimulation by − 34·0 ± 8·9% (P < 0·05) (Fig.3a,b).
Figure 3.
Recombinant human neutrophil peptide-1 (rHNP-1) and recombinant human β defensin-1 (rHBD-1) increase the proportion of the CD91bright monocyte-derived dendritic cell (moDC) subset. Immature moDCs were incubated with 100 ng/ml rHNP-1, 500 ng/ml rHBD-1, 20 μg/ml heat-shock protein 90 (hsp 90) or 100 ng/ml lipopolysaccharide (LPS). After 18 hr, the cells were analysed by flow cytometry to measure cell-surface CD91 expression. (a) Representative flow cytometric analysis showing the increased proportion of CD91bright moDC subset after rHNP-1 and rHBD-1 stimulation compared with the decreased proportion after hsp 90 and LPS challenge. (b) Change of CD91bright moDCs frequency after rHNP-1, rHBD-1, hsp 90 and LPS compared with unstimulated conditions. Data shown as mean ± SE from six independent experiments. *P < 0·05, **P < 0·01.
rHNP-1 and rHBD-1 increase the activation of CD91bright moDC subset
Next, we performed experiments to test the ability of CD91bright moDCs to respond to defensins. First, we confirmed previously published data that both defensins promoted the activation and maturation of total moDCs: rHNP-1 versus unstimulated CD80 (MFI, mean ± SE, 739 ± 37 versus 611 ± 39, P < 0·05), CD86 (3482 ± 1162 versus 2984 ± 557, P = ns), CD40 (1432 ± 101 versus 1018 ± 174, P < 0·05), CD83 (1288 ± 252 versus 738 ± 165, P < 0·05) and HLA-DR (24 026 ± 3209 versus 14 490 ± 3905, P < 0·05); rHBD-1 versus unstimulated CD80 (830 ± 64 versus 611 ± 39, P < 0·05), CD86 (5327 ± 1589 versus 2984 ± 557, P = ns), CD40 (1549 ± 145 versus 1018 ± 174, P < 0·05), CD83 (1229 ± 253 versus 738 ± 165, P = ns) and HLA-DR (26 580 ± 4690 versus 14 490 ± 3905, P < 0·05) (Fig.4). We further analysed the in vitro effects of rHNP-1, rHBD-1 and LPS stimulation on CD91bright compared with CD91dim moDCs. As also shown in Fig.4, rHNP-1 induced significantly higher levels of all the activation markers on CD91bright moDCs compared with CD91dim moDCs (MFI on CD91bright versus CD91dim cells, CD80: 864 ± 51 versus 721 ± 45, P < 0·05; CD86: 4373 ± 1260 versus 3463 ± 1154, P < 0·05; CD40: 2164 ± 289 versus 1367 ± 132, P < 0·05; HLA-DR: 40 483 ± 5467 versus 23 769 ± 7002, P < 0·05). Similar results were observed when moDCs were stimulated with rHBD-1 (CD80: 1216 ± 264 versus 1012 ± 190, P < 0·05; CD40: 1956 ± 138 versus 1323 ± 196, P < 0·05; CD83: 1775 ± 279 versus 1224 ± 253, P < 0·05; HLA-DR: 41 818 ± 5536 versus 26 457 ± 3637, P < 0·05). Conversely, when moDCs were stimulated with LPS, no significant differences in the expression of any activation marker were observed between CD91bright and CD91dim cells. Overall, these results indicate that CD91bright moDCs that had undergone stimulation with rHNP-1 and rHBD-1 maintained their higher state of activation/maturation compared with CD91dim moDCs while this property was lost upon LPS stimulation.
Figure 4.
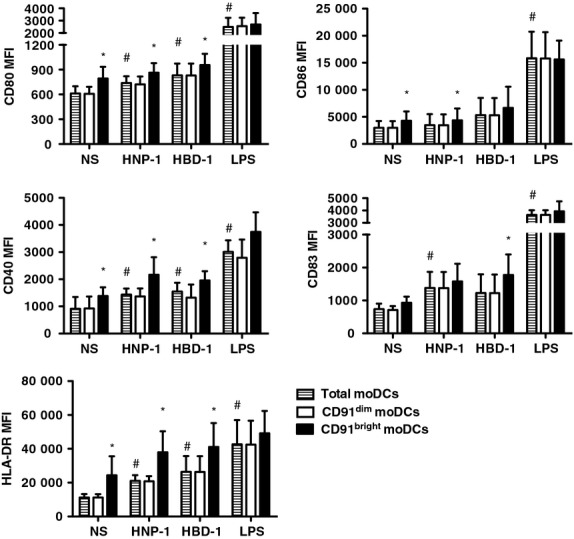
Human neutrophil peptide-1 (HNP-1) and human β defensin-1 (HBD-1) increase the activation of CD91bright monocyte-derived dendritic cell (moDC) subset. Immature moDCs were stimulated with 100 ng/ml recombinant HNP-1, 500 ng/ml recombinant HBD-1, or 100 ng/ml lipopolysaccharide (LPS). After 18 hr, the cells were analysed by flow cytometry to measure cell-surface activation and maturation markers expression on total moDCs (shaded bars), CD91dim moDCs (white bars) and CD91bright moDCs (black bars). Graphs show the mean fluorescence intensity (MFI) of CD80, CD86, CD40, CD83 and HLA-DR molecules. Data shown as mean ± SE from six independent experiments and analysed by the paired t-test. #P < 0·05 stimulated total moDCs compared to unstimulated (NS) cells; *P < 0·05: CD91dim moDCs compared with CD91bright moDCs.
Discussion
Dendritic cells are potent antigen-presenting cells that sense environmental stimuli through a wide repertoire of receptors expressed on their surface.18 We and others previously described that moDCs express CD91, a polyfunctional receptor that binds several molecules including defensins.4,9,15–17 In this study we confirmed our previous observations and, by using multi-parametric flow cytometry analysis, we showed for the first time to our knowledge the presence of two distinct subsets of moDCs based on their CD91 expression: CD91bright and CD91dim moDCs. These subsets were characterized by a different phenotype. In fact, although CD91bright cells represented a small proportion of moDCs, this subset showed greater expression of activation/maturation markers compared with CD91dim moDCs, suggesting that these two subsets could have different roles in the immune response. This hypothesis may be further supported by a previous study that correlated the full maturation status of plasmacytoid DCs with a greater increase of CD91 expression and increased CD91 ligand-binding capacity.19 Interestingly, in our study in vitro stimulation with defensins increased the proportion of CD91bright moDCs and preserved their higher activation/maturation status compared with CD91dim moDCs, suggesting that CD91bright moDCs may be more sensitive to CD91 stimulation. Notably, defensins appeared unique in their ability to up-regulate their own receptor, as CD91 up-regulation was not shared by hsp 90, another CD91 ligand highly expressed in eukaryotic cells. Indeed, in accordance with one of two previous studies addressing the effects of heat-shock proteins on the expression of CD91 on different antigen-presenting cells,19,20 we observed that hsp 90 decreased the frequency of CD91bright moDCs. Not even the ability of defensins to up-regulate CD91 was shared by LPS, a common pro-inflammatory molecule that signals through toll-like receptor 4. Indeed, LPS decreased the frequency of CD91bright moDCs. This finding is in accordance with our previous study,15 reporting LPS-induced down-regulation of CD91 expression on moDCs, possibly supported by shedding of CD91 from cell surface.21
Although there is evidence that CD91-mediated immune activation can represent a powerful mechanism of immune regulation, the pathway(s) regulating expression of CD91 have not been fully defined so far. CD91 is up-regulated on monocytes of people who are HIV-positive long-term non-progressors, it may enhance cross-presentation of HIV antigens and may in turn enhance the stimulation of activated anti-HIV cytotoxic T lymphocytes.22 Moreover, the pivotal role of CD91 regulation on DCs has been demonstrated in the context of primary effusion lymphoma: DC activation was completely inhibited when these cells were pre-treated with a neutralizing antibody directed against CD91,11 suggesting that the up-regulation of CD91 in cancer patients may represent an additional strategy to improve DC activation and subsequent stimulation of a specific CD8+ T-cell response. Notably, we observed that CD91bright cells expressed significantly higher levels of CCR7 than CD91dim cells, suggesting that the CD91bright moDC subset might preferentially migrate to secondary lymph nodes, where they may elicit adaptive immune responses.
Our results are intriguing because of the plasticity of dendritic cells in the setting of different immune responses. Hence, we could hypothesize a differential role of CD91bright and CD91dim moDC subsets in activating the immune response, possibly involving a differential production of pro-inflammatory and regulatory cytokines. These two cell subsets may also differentially privilege MHC-I rather than MHC-II presentation. Because of the low frequency of CD91bright moDCs in our cultures, we could not separate these populations to compare their antigen-presenting ability. Experiments of cell depletion were not undertaken, as they may provide only approximate and indirect functional data. Additional studies will be required in the future to further characterize the role of CD91bright and CD91dim DCs in the immune response.
Taken together, our results suggest that moDCs preferentially respond to defensins by increasing the frequency of CD91bright moDCs and promoting a higher state of activation/maturation of these cells compared with CD91dim moDCs. The identification of a subset of moDCs expressing high levels of CD91 and molecules involved in DC–T-cell interaction suggests that these CD91bright cells may be particularly devoted to the activation of adaptive immune responses against antigens that may be delivered to DCs through CD91 ligands, in particular defensins. These results may provide new insights into the immune potentiating effects of defensins that are currently under intense investigation as possible new therapeutic agents aimed to potentiate antimicrobial and anti-tumour immunity.
Acknowledgments
This study was supported by grants from Università degli Studi di Milano (2009-ATE-0573) to SDB. MC was supported by a fellowship of the Doctorate School of Molecular Medicine, University of Milan, Italy. FC is a recipient of a fellowship awarded by the Fondazione Italiana per la Ricerca sul Cancro.
Glossary
Abbreviations:
- hsp 90
heat-shock protein 90
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- moDCs
monocyte-derived dendritic cells
- rHBD-1
recombinant human β defensin-1
- rHNP-1
recombinant human neutrophil peptide-1
Disclosure
The authors declare no conflicting financial interests.
References
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar T, Akkawi S, Bar-Shavit R, Haj-Yehia A, Bdeir K, Al-Mehdi AB, Tarshish M, Higazi AA. Human α-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/α2-macroglobulin receptor. Blood. 2002;100:4026–32. doi: 10.1182/blood-2002-04-1080. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–84. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- Robert J, Ramanayake T, Maniero GD, Morales H, Chida AS. Phylogenetic conservation of glycoprotein 96 ability to interact with CD91 and facilitate antigen cross-presentation. J Immunol. 2008;180:3176–82. doi: 10.4049/jimmunol.180.5.3176. [DOI] [PubMed] [Google Scholar]
- Maniecki MB, Moller HJ, Moestrup SK, Moller BK. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology. 2006;211:407–17. doi: 10.1016/j.imbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Stebbing J, Gazzard B, Portsmouth S, et al. Disease-associated dendritic cells respond to disease-specific antigens through the common heat shock protein receptor. Blood. 2003;102:1806–14. doi: 10.1182/blood-2003-03-0891. [DOI] [PubMed] [Google Scholar]
- Kebba A, Stebbing J, Rowland S, et al. Expression of the common heat-shock protein receptor CD91 is increased on monocytes of exposed yet HIV-1-seronegative subjects. J Leukoc Biol. 2005;78:37–42. doi: 10.1189/jlb.0105049. [DOI] [PubMed] [Google Scholar]
- Cirone M, Di Renzo L, Lotti LV, et al. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS ONE. 2012;7:e31732. doi: 10.1371/journal.pone.0031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawaria S, Kropp LE, Binder RJ. Immunotherapy of tumors with α2-macroglobulin-antigen complexes pre-formed in vivo. PLoS ONE. 2012;7:e50365. doi: 10.1371/journal.pone.0050365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty S, Colaco CA, Blandford LE, Bailey CR, Baschieri S, Todrik S. Heat-shock proteins as dendritic cell-targeting vaccines – getting warmer. Immunology. 2013;139:407–15. doi: 10.1111/imm.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei HF, Jin XB, Zhu JY, Zeng AH, Wu Q, Lu XM, Li XB, Shen J. β-defensin 2 as an adjuvant promotes anti-melanoma immune responses and inhibits the growth of implanted murine melanoma in vivo. PLoS ONE. 2012;7:e31328. doi: 10.1371/journal.pone.0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presicce P, Giannelli S, Taddeo A, Villa ML, Della Bella S. Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J Leukoc Biol. 2009;86:941–8. doi: 10.1189/jlb.0708412. [DOI] [PubMed] [Google Scholar]
- Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–91. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Navarrete AM, Andre S, et al. Factor VIII bypasses CD91/LRP for endocytosis by dendritic cells leading to T-cell activation. Haematologica. 2008;93:83–9. doi: 10.3324/haematol.11535. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- De Filippo A, Binder RJ, Camisaschi C, et al. Human plasmacytoid dendritic cells interact with gp96 via CD91 and regulate inflammatory responses. J Immunol. 2008;181:6525–35. doi: 10.4049/jimmunol.181.9.6525. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–5. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gorovoy M, Gaultier A, Campana WM, Firestein GS, Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol. 2010;88:769–78. doi: 10.1189/jlb.0410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J, Gazzard B, Kim L, et al. The heat-shock protein receptor CD91 is up-regulated in monocytes of HIV-1-infected “true” long-term nonprogressors. Blood. 2003;101:4000–4. doi: 10.1182/blood-2002-11-3353. [DOI] [PubMed] [Google Scholar]