Abstract
Type 2 diabetes mellitus (DM) is associated with expanded frequencies of mycobacterial antigen-specific CD4+ T helper type 1 (Th1) and Th17 cells in individuals with active pulmonary tuberculosis (TB). No data are available on the role of CD8+ T and natural killer (NK) cells in TB with coincident DM. To identify the role of CD8+ T and NK cells in pulmonary TB with diabetes, we examined mycobacteria-specific immune responses in the whole blood of individuals with TB and DM (TB-DM) and compared them with those without DM (TB-NDM). We found that TB-DM is characterized by elevated frequencies of mycobacterial antigen-stimulated CD8+ T cells expressing type 1 [interferon-γ and interleukin-2 (IL-2)] and type 17 (IL-17F) cytokines. We also found that TB-DM is characterized by expanded frequencies of TB antigen-stimulated NK cells expressing type 1 (tumour necrosis factor-α) and type 17 (IL-17A and IL-17F) cytokines. In contrast, CD8+ T cells were associated with significantly diminished expression of the cytotoxic markers perforin, granzyme B and CD107a both at baseline and following antigen or anti-CD3 stimulation, while NK cells were associated with significantly decreased antigen-stimulated expression of CD107a only. This was not associated with alterations in CD8+ T-cell or NK cell numbers or subset distribution. Therefore, our data suggest that pulmonary TB complicated with type 2 DM is associated with an altered repertoire of cytokine-producing and cytotoxic molecule-expressing CD8+ T and NK cells, possibly contributing to increased pathology.
Keywords: CD8+ T cells, diabetes, natural killer cells, tuberculosis
Introduction
Type 2 diabetes mellitus (DM) is a major risk factor for tuberculosis (TB) with several lines of evidence demonstrating increased risk of TB in diabetic individuals. Moreover, the increasing prevalence of DM in certain parts of the world where TB infection is already rampant poses a major public health concern.1–3 In addition, there is also evidence that DM is associated with greater severity of TB disease affecting both disease presentation and response to treatment, including the radiological extent of disease, delay in the time to sputum conversion and increased risk of treatment failures and relapse.1,4 While the clinical and epidemiological implications of the TB–DM network are well understood, little is known about the immunological interaction between the two diseases.
It was assumed that as DM is known to be associated with impaired effector T-cell responses that TB susceptibility was the consequence of impaired T-cell responses in DM. Indeed, several early studies reported reduced pro-inflammatory cytokines in mouse models of DM after infection with Mycobacterium tuberculosis.5,6 However, more recent data clearly indicate that pro-inflammatory responses, inclusive of cytokine and T-cell responses, are elevated in TB-infected diabetic hosts – both in mice and in humans.7,8 In addition, TB with DM is characterized by an expansion of M. tuberculosis-specific CD4+ T helper type 1 (Th1) and Th17 cells and heightened plasma levels of type 1 and type 17 cytokines.9,10 CD8+ T and natural killer (NK) cells are also important producers of pro-inflammatory cytokines, including type 1 and type 17 cytokines in tuberculosis11 but their contribution to the cytokine environment in TB-DM co-morbidity is not known. Similarly, expression of the cytotoxic granule release mediators perforin, granzyme B and CD107a is an important component of the cytotoxic function of these cells.12
To study the influence of DM on CD8+ T and NK cell responses in active pulmonary TB, we examined baseline (ex vivo), antigen-specific and polyclonal induction of type 1 and type 17 cytokine-producing as well as cytotoxic marker expressing CD8+ T and NK cells in individuals with active TB with coincident DM (TB-DM) and compared them with those without DM (TB-NDM). We show that those with TB-DM have elevated frequencies of interferon-γ (IFN-γ), interleukin-2 (IL-2) and IL-17F secreting CD8+ T cells following mycobacterial antigen stimulation in comparison to TB-NDM individuals. We also show that those with TB-DM have elevated frequencies of tumour necrosis factor-α (TNF-α), IL-17A and IL-17F secreting NK cells following mycobacterial antigen stimulation. In contrast, the frequency of CD8+ T cells expressing perforin, granzyme B and CD107a and the frequency of NK cells expressing the degranulation molecule, CD107a alone, was considerably diminished in TB-DM. This was not associated with altered CD8+ T-cell or NK cell numbers or subset distribution. Hence, our data demonstrate that diabetes profoundly alters the CD8+ T and NK cell response to M. tuberculosis and possibly contributes to increased severity and/or immune-mediated pathology in TB.
Materials and methods
Study population
We studied a group of 44 individuals with active pulmonary TB – 22 with diabetes and 22 without. Tuberculosis was diagnosed on the basis of sputum smear and culture positivity. This was the same group of patients for whom we had previously studied CD4+ T-cell responses.10 The TB was diagnosed on the basis of positive sputum smear using fluorescence microscopy and positive cultures on Lowenstein–Jensen medium. All individuals in the study were sputum smear and culture positive. Diabetes mellitus was diagnosed on the basis of glycated haemoglobin (HbA1c) levels and random blood glucose, according to the American Diabetes Association criteria (all DM individuals had HbA1c levels > 6·5% and random blood glucose > 200 mg/dl). All the individuals were HIV negative. The two groups did not differ significantly in terms of sputum smear grades or radiological extent of disease. All individuals were anti-tuberculous treatment naive. Anthropometric measurements, including height, weight and waist circumference, and biochemical parameters, including plasma glucose, lipid profile and HbA1c were obtained using standardized techniques as detailed elsewhere.13 The TB-DM individuals exhibited significantly increased levels of random glucose, total cholesterol, serum triglycerides and low-density cholesterol compared with TB-NDM individuals. There was no significant difference in age, sex or body mass index between the two groups. All individuals were examined as part of a natural history study approved by the Institutional Review Board of the National Institute of Research in Tuberculosis (NCT01154959), and informed written consent was obtained from all participants.
Ex vivo analysis
All antibodies used in the study were from BD Biosciences (San Jose, CA), BD Pharmingen (San Diego, CA), eBioscience (San Diego, CA) or R&D Systems (Minneapolis, MN). Absolute numbers of CD8+ T cells were enumerated in whole blood using BD Multiset 6-Color TBNK cocktail (BD Biosciences). Naive and memory T-cell phenotyping was performed using CD45RA and CCR7 staining on CD8+ T cells. The gating strategy for T-cell phenotyping is shown in the Supporting information, Fig. S1(b). Ex vivo intracellular staining for Ki-67 expression on CD8+ T and NK cells was performed.
Antigens
Tuberculosis antigens used were early secreted antigen-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) (both from Fitzgerald Industries Intl. Inc., Acton, MA). Final concentrations were 10 µg/ml for ESAT-6 and CFP-10 and 5 µg/ml for anti-CD3.
In vitro culture
Whole blood cell cultures were performed to determine the intracellular levels of cytokines. Briefly, whole blood was diluted 1 : 1 with RPMI-1640 medium, supplemented with penicillin/streptomycin (100 U/100 mg/ml), l-glutamine (2 mm), and HEPES (10 mm) (all from Invitrogen, Carlsbad, CA) and distributed in 12-well tissue culture plates (Costar®). The cultures were then stimulated with ESAT-6, CFP-10 or anti-CD3 or media alone in the presence of the co-stimulatory molecules, CD49d/CD28 at 37° for 6 hr. Brefeldin A (10 µg/ml) was added after 2 hr. After 6 hr, centrifugation, washing and red blood cell lysis were performed. The cells were fixed using cytofix/cytoperm buffer (BD Biosciences) and cryopreserved at – 80°.
Intracellular cytokine staining
The cells were thawed, washed and then stained with surface antibodies for 30–60 min. Surface antibodies used were CD3, CD4, CD8, CD16 and CD56. Gating strategies for CD8+ T cells (CD3+ CD8+ CD4−) and NK cells (CD56+ CD3−) are shown in Fig. S1(a). The cells were washed and permeabilized with BD Perm/Wash buffer (BD Biosciences) and stained with intracellular cytokines for an additional 30 min before washing and acquisition. Cytokine antibodies used were IFN-γ, TNF-α, IL-2, IL-17A, IL-17F and IL-22. Cytotoxic markers analysed were perforin, granzyme B and CD107a. Eight-colour flow cytometry was performed on a facscanto II flow cytometer with facsdiva software v.6 (Becton Dickinson, Franklin Lakes, NJ). The lymphocyte gating was set by forward and side scatter and 100 000 events were acquired. Data were collected and analysed using flow jo software (TreeStar Inc., Ashland, OR). All data are depicted as frequency of the various cell populations expressing cytokine(s). Baseline values following media stimulation are depicted as baseline frequency while frequencies following stimulation with antigens are depicted as net frequencies (with baseline values subtracted).
Statistical analysis
Data analyses were performed using graphpad prism (GraphPad Software, Inc., La Jolla, CA). Geometric means were used for measurements of central tendency. Statistically significant differences between two groups were analysed using the non-parametric Mann–Whitney U-test. Multiple comparisons were corrected using the Holm's correction.
Results
TB-DM is associated with increased frequencies of antigen-stimulated CD8+ T cells expressing IFN-γ, IL-2 and IL-17F
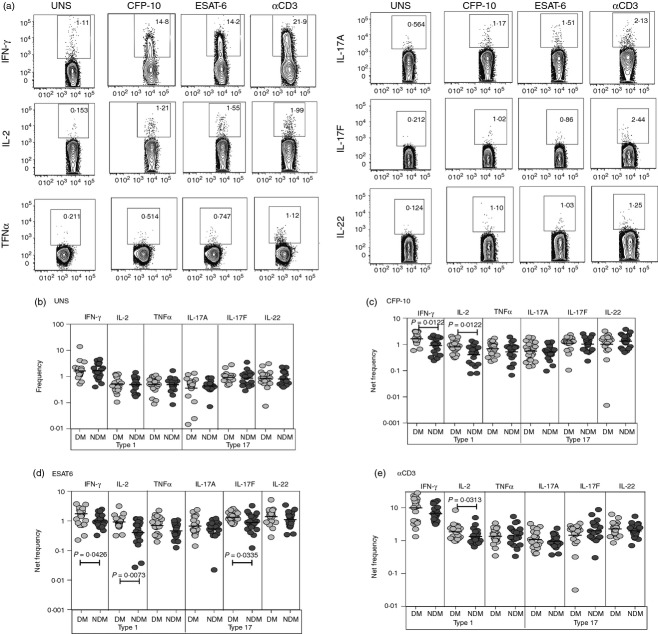
CD8+ T cells are thought to play an important role in the immune control of TB infection.14 To determine the influence of DM on CD8+ T cells in active TB, we used multi-parameter flow cytometry to define the frequencies CD8+ T cells expressing type 1 (IFN-γ, IL-2 or TNF-α) and type 17 (IL-17A, IL-17F or IL-22) cytokines at baseline and following stimulation with either mycobacterial antigens or anti-CD3 (Fig.1a). As shown in Fig.1(b), there were no differences in the frequencies of CD8+ T cells expressing type 1 or type 17 cytokines at baseline in TB-DM compared with TB-NDM. In contrast, in response to CFP-10 (Fig.1c) and ESAT-6 (Fig.1d), we observed significantly elevated frequencies of CD8+ T cells expressing either IFN-γ or IL-2 or IL-17F in TB-DM compared with TB-NDM individuals. Finally, there were no significant differences in the net frequencies of CD8+ T cells expressing pro-inflammatory cytokines between the two groups [with the exception of CD8+ T cells expressing IL-2 (Fig.1e)] following stimulation with anti-CD3 indicating that the increased frequency of pro-inflammatory cytokine-expressing CD8+ T cells induced in TB-DM individuals was predominantly antigen-specific.
Figure 1.
Elevated antigen-stimulated frequencies of type 1 and type 17 cytokine secreting CD8+ T cells in patients with tuberculosis and diabetes mellitus (TB-DM). (a) A representative whole-blood intracellular cytokine assay flow data from a TB-DM individual showing expression of interferon-γ (IFN-γ), interleukin-2 (IL-2), tumour necrosis factor-α (TNF-α), IL-17A, IL-17F and IL-22. The plots shown are gated on CD3+, CD8+ T cells. (b) The baseline frequency of CD8+ T cells expressing type 1 (IFN-γ, IL-2, TNF-α) or type 17 (IL-17A, IL-17F and IL-22) cytokines is shown as scatter plots with the line representing the geometric mean of the frequency of CD8+ T cells expressing the respective cytokine(s) in TB-DM (n = 22) and TB–no diabetes mellitus (TB-NDM; n = 22) individuals. (c, d) The net frequency of CD8+ T cells expressing type 1 (IFN-γ, IL-2, TNF-α) or type 17 (IL-17A, IL-17F and IL-22) cytokines in response to culture filtrate protein-10 (CFP-10) (c) and early secretory antigen 6 (ESAT-6) (d) are shown in TB-DM and TB-NDM individuals. (e) The net frequency of CD8+ T cells expressing the different cytokines in response to anti-CD3 stimulation is shown in TB-DM and TB-NDM individuals. Net frequencies were calculated by subtracting baseline frequencies from antigen-stimulated or anti-CD3-stimulated frequencies. P-values were calculated using the Mann–Whitney test.
TB-DM is associated with increased frequencies of antigen-stimulated NK cells expressing TNF-α, IL-17A and IL-17F
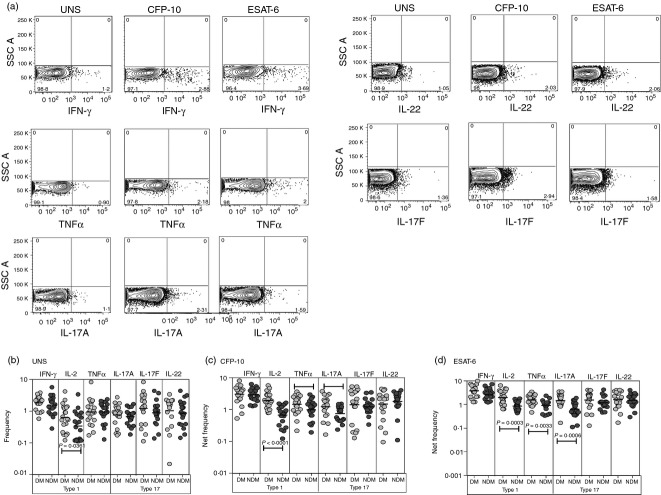
The role of NK cells in the immune response to TB is not well understood although NK cells are thought to contribute to protection.15 To determine the influence of DM on NK cells in active TB, we measured the frequencies of NK cells expressing type 1 (IFN-γ, IL-2 or TNF-α) and type 17 (IL-17A, IL-17F or IL-22) cytokines at baseline and following stimulation with mycobacterial antigens (Fig.2a). No significant differences in the frequency of NK cells expressing type 1 or type 17 cytokines were observed between TB-DM and TB-NDM individuals at baseline (Fig.2b). As shown in Fig.2(c,d), there were significantly elevated frequencies of NK cells expressing TNF-α and IL-17A following TB antigen stimulation in TB-DM compared with TB-NDM individuals. Hence, DM is associated with enhanced frequencies of NK cells expressing pro-inflammatory cytokines in active TB.
Figure 2.
Elevated antigen-stimulated frequencies of type 1 and type 17 cytokine-secreting natural killer (NK) cells in tuberculosis–diabetes mellitus (TB-DM). (a) A representative whole-blood intracellular cytokine assay flow data from a TB-DM individual showing expression of interferon-γ (IFN-γ), interleukin-2 (IL-2), tumour necrosis factor-α (TNF-α), IL-17A, IL-17F and IL-22. The plots shown are gated on CD56+, CD56+ CD3− cells. (b) The baseline frequency of CD8+ T cells expressing type 1 (IFN-γ, IL-2, TNF-α) or type 17 (IL-17A, IL-17F and IL-22) cytokines is shown as scatter plots with the line representing the geometric mean of the frequency of NK cells expressing the respective cytokine(s) in TB-DM (n = 22) and tuberculosis–no diabetes mellitus (TB-NDM) (n = 22) individuals. (c, d) The net frequency of NK cells expressing type 1 (IFN-γ, IL-2, TNF-α) or type 17 (IL-17A, IL-17F and IL-22) cytokines in response to culture filtrate protein 10 (CFP-10) (c) and early secretory antigen 6 (ESAT-6) (d) is shown in TB-DM and TB-NDM individuals. Net frequencies were calculated by subtracting baseline frequencies from antigen-stimulated frequencies. P-values were calculated using the Mann–Whitney test.
TB-DM is associated with decreased frequencies of antigen-stimulated CD8+ T cells expressing cytotoxic markers
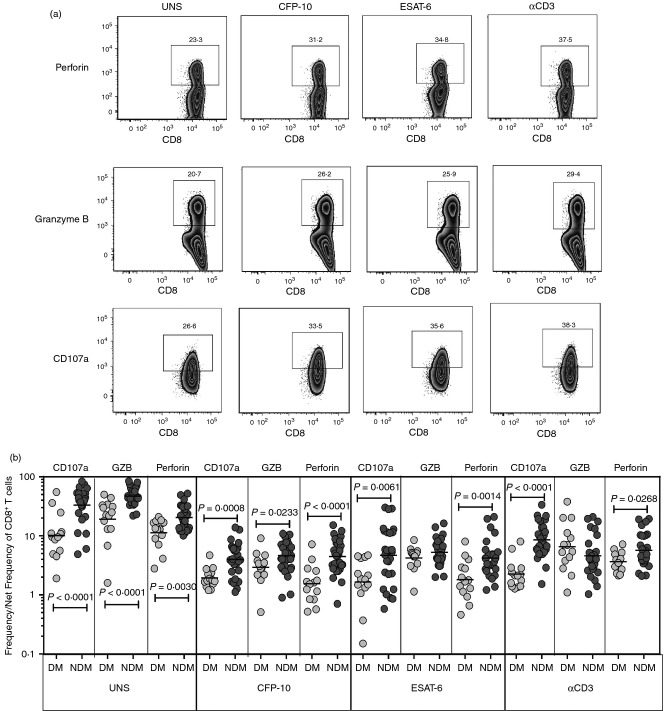
To determine the influence of DM on cytotoxic potential of CD8+ T cells in active TB, we used multi-parameter flow cytometry to define the frequencies CD8+ T cells expressing perforin, granzyme B and CD107a at baseline and following stimulation with either mycobacterial antigens or anti-CD3 (Fig.3a). As shown in Fig.3(b), we observed significantly decreased frequencies of CD8+ T cells expressing either perforin or granzyme B or CD107a in TB-DM compared with TB-NDM individuals at baseline and in response to CFP-10, ESAT-6 and anti-CD3. Hence, TB-DM is associated with an altered repertoire of cytotoxic molecule expressing CD8+ T cells.
Figure 3.
Diminished frequencies of cytotoxic marker expressing CD8+ T cells in patients with tuberculosis and diabetes mellitus (TB-DM). (a) A representative whole-blood intracellular cytotoxic marker flow data from a TB-DM individual showing CD8+ T-cell expression of perforin, granzyme B and CD107a. (b) The baseline or antigen or anti-CD3 stimulated net frequencies of CD8+ T cells expressing the cytotoxic markers perforin, granzyme B and CD107a are shown as scatter plots with the line representing the geometric mean of the frequency of CD8+ T cells expressing the respective marker in TB-DM (n = 22) and tuberculosis–no diabetes mellitus (TB-NDM) (n = 22) individuals. Net frequencies were calculated by subtracting baseline frequencies from antigen-stimulated or anti-CD3-stimulated frequencies. P-values were calculated using the Mann–Whitney test.
TB-DM is associated with altered frequencies of antigen-stimulated NK cells expressing CD107a
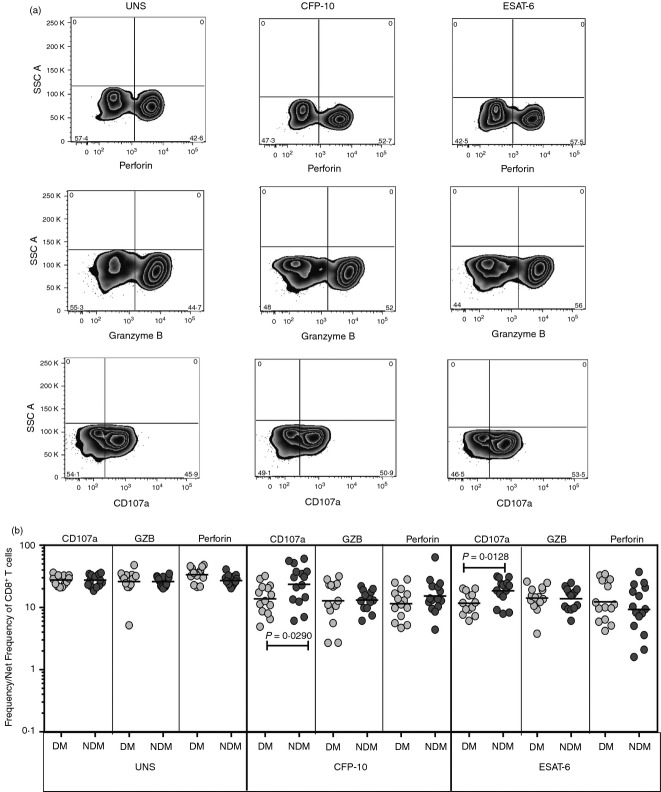
To determine the influence of DM on cytotoxic potential of NK cells in active TB, we used multi-parameter flow cytometry to define the frequencies of NK cells expressing perforin, granzyme B and CD107a at baseline and following stimulation with either mycobacterial antigen (Fig.4a). As shown in Fig.4(b), we observed no significant alterations in the frequencies of NK cells expressing either perforin or granzyme B in TB-DM compared with TB-NDM individuals at baseline and in response to CFP-10 and ESAT-6. On the other hand, the frequency of NK cells expressing CD107a was significantly lower in response to CFP-10 and ESAT-6 was significantly lower in TB-DM individuals. Hence, TB-DM is associated with an altered repertoire of CD107a-expressing NK cells.
Figure 4.
Diminished frequencies of cytotoxic marker expressing natural killer (NK) cells in tuberculosis–diabetes mellitus (TB-DM). (a) A representative whole-blood intracellular cytotoxic marker flow data from a TB-DM individual showing NK cell expression of perforin, granzyme B and CD107a. (b) The baseline or antigen-stimulated net frequencies of NK cells expressing the cytotoxic markers perforin, granzyme B and CD107a are shown as scatter plots with the line representing the geometric mean of the frequency of NK cells expressing the respective marker in TB-DM (n = 22) and tuberculosis–no diabetes mellitus (TB-NDM) (n = 22) individuals. Net frequencies were calculated by subtracting baseline frequencies from antigen-stimulated frequencies. P-values were calculated using the Mann–Whitney test.
TB-DM is not associated with alterations in CD8+ T and NK cell numbers nor in the frequency of CD8+ T-cell memory subsets
We next examined the absolute numbers of CD8+ T cells and NK cells as well as the frequencies of CD8+ T-cell memory subsets at baseline (without stimulation) since alterations in any of the above-mentioned parameters could influence the frequency of type 1 and type 17 cytokine-secreting cells in TB-DM. The total white blood count was not significantly different between the two groups (geometric mean of 114·9 × 106/µl in TB-DM versus 101·1 × 106/µl in TB-NDM). Our data reveal that TB-DM is not associated with any significant differences in the absolute numbers of CD8+ T cells or NK cells in active TB (Fig.5a). Our data also reveal that TB-DM is not associated with significant alterations in the frequencies of central memory and effector memory CD8+ T cells in comparison to TB-NDM individuals but the former had significantly higher frequencies of naive CD8+ T cells compared with the latter (Fig.5b). Finally, our data using Ki-67 expression on stimulated CD8+ T and NK cells also did not reveal any significant differences between the groups (data not shown), suggesting that differential proliferation was also not responsible for the differential cytokine expression between the groups. Our data suggest that alterations in absolute cell numbers or memory subset frequencies are not associated with DM in TB.
Figure 5.
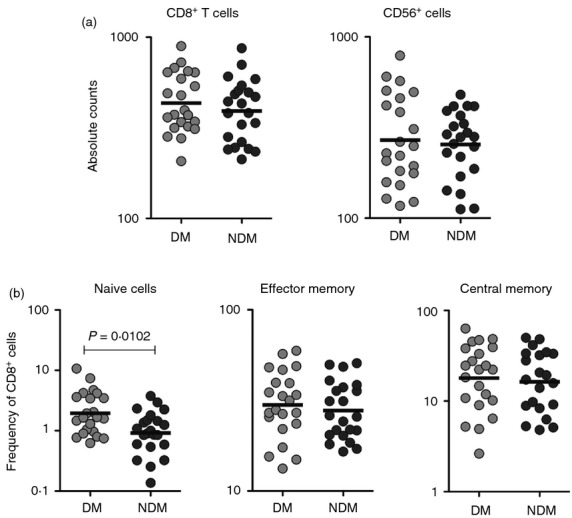
Tuberculosis–diabetes mellitus (TB-DM) is associated with increased frequencies of naive but not central or effector memory CD8+ T cells in TB-DM. (a) The absolute numbers of CD8+ T cells and natural killer (NK) cells in TB-DM and tuberculosis–no diabetes mellitus (TB-NDM) individuals. (b) Percentages of naive (defined as CD45RA+ CCR7+), central memory (defined as CD45RA– CCR7+), and effector memory (defined as CD45RA– CCR7–) CD8+ T cells in TB-DM and TB-NDM individuals. The results are shown as scatter plots with each circle representing a single individual. P-values were calculated using the Mann–Whitney test.
Discussion
Type 2 diabetes is a major risk factor for active pulmonary TB as well as a predictor of poor treatment outcomes and reduced survival among those with TB disease.1 A detailed meta-analysis of 13 observational studies on the risk for TB disease in diabetes determined that diabetic patients were 3·1 times more likely to develop TB than non-diabetic individuals.3 In addition, data emerging from India indicate that while the incidence of TB remains high, the prevalence of DM is rising alarmingly. In fact, two recent studies from Chennai and Kerala have demonstrated that the prevalence of type 2 DM in TB patients attending outpatient clinics are approximately 25% with an additional 25% of these patients being pre-diabetic.16,17 The immunological basis for this susceptibility to TB among those with DM is poorly understood. The initial explanation for the increased susceptibility to TB related to the suggestion that an impaired immune response in diabetic patients could potentially facilitate either primary infection with TB or reactivation of latent TB.18 Indeed, a few studies examining the innate and adaptive immune response to microbial antigens in diabetic patients had suggested that these responses are compromised, particularly in patients with chronic hyperglycaemia.19–21 Whether this applies to TB infection remains unclear. However, more recent data from animal models suggest that diabetic mice infected with TB actually manifest an exuberant (not impaired) Th1 response that leads to immune-mediated pathology.22 Interestingly, these data mirror the finding that human TB patients with DM over-express cytokines that are normally protective in TB.23 In addition, we have previously demonstrated that TB-DM is associated with the presence of expanded frequencies of antigen-specific CD4+ Th1 and Th17 cells as well as a pro-inflammatory cytokine milieu.9,10
CD8+ T cells are known to play a protective role in the immune response to murine TB and M. tuberculosis-specific CD8+ T cells have also been found in humans.14 These cells have the capacity to activate macrophage defence mechanisms by secreting IFN-γ and TNF-α and also help in eliminating the bacteria by the granule exocytosis pathway.24 Similarly, NK cell production of IFN-γ, IL-17 and IL-22 is thought to play an important role in host defence against mycobacterial infection.15 As very little is known about the regulation of CD8+ T cells and NK cells in TB with DM, we sought to determine the role of these lymphocytes in TB-DM. Our findings reveal that at baseline, very few differences exist in the frequencies of type 1 or type 17 secreting T or NK cells in TB-DM compared with TB-NDM, indicating that DM per se does not significantly alter the homeostatic regulation of CD8+ T and NK cells. Similarly, intrinsic potential of CD8+ T cells to secrete type 1 or type 17 cytokines in active TB is also not influenced by DM because stimulation with a polyclonal stimulus (anti-CD3) did not reveal any major differences in the frequency of pro-inflammatory cytokine secreting CD8+ T cells. In contrast, mycobacterial antigen stimulation appears to play a major role in driving the expansion of type 1 or type 17 cytokine expressing CD8+ T or NK cells in diabetic individuals. Both the immunodominant antigens of M. tuberculosis, CFP-10 and ESAT-6, induce specific expansion of CD8+ T and NK cell secreting type 1 or type 17 cytokines. Interestingly, the diversity of the expanded repertoire of CD8+ T and NK cells was not as extensive as the repertoire of antigen-driven CD4+ Th1 and Th17 cells, observed in our previous study.10 This is likely due to the fact that we used recombinant antigens as our stimulus and not peptides, which may be better for CD8+ T-cell stimulation in short term assays. Nevertheless, our study confirms an important association of CD8+ T and NK cells with the pathogenesis of TB disease in DM and suggest that elevated frequencies of these could potentially lead to enhanced severity of disease or a higher bacillary load. As unrestrained expansion of type 1 cytokine-secreting CD8+ T and NK cells is also known to contribute to pathology in several infectious and autoimmune diseases,25 our findings also suggest that this expanded population could possibly contribute to lung pathology in diabetic individuals. However, we do not observe any direct correlation between the frequencies of CD8+ T and NK cells and the glycaemic control in TB-DM individuals.
Type 17 cytokines, most notably IL-17A and IL-17F have been shown to play a central role in mediating immunity to both extracellular and intracellular bacteria, including M. tuberculosis.26,27 However, due to the potential for IL-17 to mediate immune pathology as seen in autoimmune diseases and infection models,28 it is also postulated that IL-17 may have detrimental effects in chronic bacterial infections such as TB. Our data suggest that DM influences the expression pattern of TB antigen-specific CD8+ T and NK cells producing IL-17A and IL-17F and therefore, could be potentially associated with immune-mediated pathology in individuals with TB-DM co-morbidity. Interestingly, while we observed a differential expression of CD8+ T and NK cells secreting IL-17, no similar difference was observed in the expression pattern of IL-22-expressing cytotoxic cells. We also observed minor differences in the cytokine response to ESAT-6 versus CFP-10, which could reflect minor variations in the antigen-induced T-cell repertoire. Therefore, an exaggerated Type 17 response, similar to the Type 1 response, occurs in active TB individuals with diabetes and possibly contributes to immune-mediated pathology as well. Our study also confirms previous reports that T cell profiles in patients with insulin resistance are altered, which could indicate that T-cell factors are linked to disturbed insulin sensitivity.29 In patients with type 2 DM, circulating T cells produce increased levels of IL-17 and IFN-γ and promote inflammation and several cytokines produced by Th1 or Th17 cells have been linked to insulin resistance.30 Therefore, our data on the lack of differences at baseline coupled with the altered frequency of TB antigen-specific CD8+ T and NK cells upon antigen stimulation strongly support an important role for DM in contributing to the modulation of TB antigen-specific immune responses in pulmonary TB. Our exploration of NK cell responses in this study is preliminary and more in depth studies using additional markers and additional stimulations are necessary to elucidate the regulation of these cells in TB-DM. Also, the antigen mediated induction of NK cell responses could reflect pathogen-associated activation of NK cell activating receptors, such as NKp30, NKp44 and NKp46.
One potential mechanism for the increased expansion of CD8+ T and NK cells expressing pro-inflammatory cytokines could be increased CD8+ T and NK cell total counts or altered subset distribution or differential proliferation. However, our data suggest that it is unlikely that alterations in T or NK cell numbers or subset distribution or intrinsic proliferation status was the primary cause for the enhanced expansion of cytokine-producing T cells. The expression of cytotoxic markers including perforin and granzyme B and the degranulation marker CD107a is an indirect estimation of the cytotoxic potential of CD8+ T and NK cells.12 Our data reveal that in contrast to cytokine-secreting function, cytotoxic potential of CD8+ T cells and to a lesser extent NK cells is significantly compromised in type 2 DM. Interestingly, this decreased frequency of CD8+ T cells expressing perforin is also observed with anti-CD3 indicating a cell intrinsic effect of hyperglycaemia on cytotoxic marker expression in these cells. Whether this accounts for the increased susceptibility of type 2 diabetics to intracellular infections cannot be ascertained from our study. Nevertheless, they do reveal an important association of altered cytotoxic T and NK cell potential with the pathogenesis of TB-DM co-morbidity.
Our study highlights the contribution of poorly controlled type 2 DM to the pathogenesis of TB disease by its role in modulating the phenotype and function of CD8+ T and NK cells. Since CD8+ T and NK cell are potent cytokine-secreting cells, their contribution to the pro-inflammatory milieu of diabetic individuals with TB would be expected to substantially influence the TB-DM co-morbidity. Future studies examining immune responses in diabetic patients with latent TB should provide additional insights into the pathogenesis of TB disease by dissecting the complex immunological interaction between diabetes and TB.
Acknowledgments
We thank the staff of the Department of Clinical Research and the Department of Social Work, NIRT especially Ms Kalaiselvi and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study, and R. Anuradha, V. Gopinath and Jovvian George of the NIH–ICER for technical assistance. This study was funded by the Division of Intramural Research, NIAID, NIH.
Glossary
Abbreviations:
- CFP-10
culture filtrate protein-10
- DM
diabetes mellitus
- ESAT-6
early secreted antigen-10
- HbA1c
haemoglobin A1c
- Mtb
Mycobacterium tuberculosis
- NDM
non-diabetes mellitus
- PPD
purified protein derivative
- TB
tuberculosis
Author contributions
TBN and SB designed the experiment:; NPK performed the experiments; NPK and SB analysed the data; RS, DN and VVB provided patient samples and TBN and SB wrote the paper.
Disclosures
The authors have no potential conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Gating strategy for CD8+ T and natural killer (NK) cells and representative cytokine plots.
References
- Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries AD, Lin Y, Satyanarayana S, et al. The looming epidemic of diabetes-associated tuberculosis: learning lessons from HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2011;15:1436–44. doi: 10.5588/ijtld.11.0503. , i. [DOI] [PubMed] [Google Scholar]
- Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attiyah RJ, Mustafa AS. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovis bacilli Calmette–Guérin (BCG)-vaccinated healthy subjects. Clin Exp Immunol. 2009;158:64–73. doi: 10.1111/j.1365-2249.2009.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalenhoef JE, Alisjahbana B, Nelwan EJ, et al. The role of interferon-γ in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2008;27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44:617–26. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo BI, Schlesinger LS. Host–pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis. 2013;93(Suppl):S10–4. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10:441–9. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Banurekha VV, et al. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208:739–48. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Redford PS, McNab FW, et al. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–62. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- Deepa M, Pradeepa R, Rema M, et al. The Chennai Urban Rural Epidemiology Study (CURES)–study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- Vankayalapati R, Barnes PF. Innate and adaptive immune responses to human Mycobacterium tuberculosis infection. Tuberculosis. 2009;89(Suppl 1):S77–80. doi: 10.1016/S1472-9792(09)70018-6. [DOI] [PubMed] [Google Scholar]
- Balakrishnan S, Vijayan S, Nair S, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V, Kumpatla S, Aravindalochanan V, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, et al. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–50. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–82. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12:597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–62. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–6. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–16. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–72. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy for CD8+ T and natural killer (NK) cells and representative cytokine plots.