Abstract
Diabetes is an oxidative stress disorder as a result of both hyperglycemia and increased levels of free fatty acids. Oxidative stress has been implicated in the pathogenesis of diabetes-related complications, and treatment with antioxidants seemed to be a promising therapeutic option. Although animal studies and preliminary human studies were initially encouraging, subsequent human studies have failed to show a clear benefit of antioxidants, whereas some studies have even suggested that they can be potentially harmful. Therefore, treatment with antioxidants cannot be currently recommended as a therapeutic option.
Introduction
One of the major complications of diabetes is the development of cardiovascular disease in both the macro -and microcirculations. Because diabetes-induced oxidative stress has been implicated in the pathogenesis of the cardiovascular complications of heart disease, antioxidants were thought to be a promising therapeutic option. A brief review of the current literature and the role of antioxidants in the treatment of diabetes is discussed.
Diabetes as an Oxidative Stress Disorder
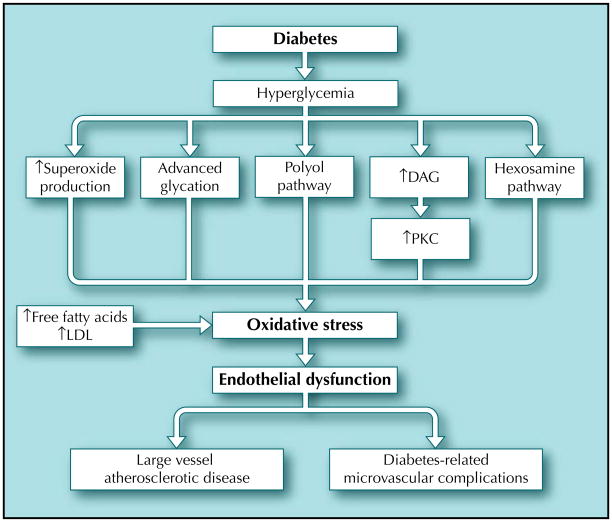
A substantial amount of evidence has demonstrated that diabetes is an oxidative stress disorder [1]. Oxidative stress describes the condition where the amount of reactive oxygen species (ROS) outstrips the amount of neutralizing agents or antioxidants. Hyperglycemia can induce oxidative stress through these four mechanisms: advanced glycation end product (AGE) formation, increased flux through the polyol pathway, increased activation of protein kinase C (PKC), and increased flux through the hexosamine pathway (Fig. 1). Creation of excess superoxide by the mitochondrial transport chain [2] during hyperglycemia may be the initiating factor of these four processes. The formation of superoxide and the subsequent increase in oxidative stress may lead to endothelial dysfunction and ultimately cardiovascular disease through several different mechanisms. Superoxide itself inactivates nitric oxide (NO) to form peroxynitrite [3]. The decreased bioavailability of NO impairs endothelial-dependent vasodilation and vascular smooth muscle function. By activating each of the four pathways, superoxide can further promote endothelial dysfunction by causing vasoconstriction, vascular smooth muscle cell growth, inflammation, further oxidative stress, and thrombosis ultimately leading to the development of cardiovascular disease.
Figure 1.
The pathogenesis of oxidative stress in diabetes. Hyperglycemia can induce oxidative stress through advanced glycation end product formation, increased flux thorough the polyol pathway, increased activation of protein kinase C (PKC), and increased flux through the hexosamine pathway. Hyperglycemia can also directly increase superoxide production. In addition to an increase in free fatty acids and low-density lipoprotein (LDL), these pathways promote oxidative stress, which in turn causes endothelial dysfunction. Endothelial dysfunction is likely the first step in the development of both the micro- and macrocirculatory complications of diabetes. DAG—diacylglycerol.
One way in which hyperglycemia causes oxidative stress is through the production of AGEs, which are non-enzymatically glycated proteins or lipids and are oxidized after exposure to aldose sugars [4]. AGEs can produce ROS, bind to receptors that promote oxidative stress, and trigger mechanisms that generate intracellular oxidants. In addition, AGEs have been found to alter cellular function, damage the extracellular matrix, cause vascular leak, decrease the bioavailability of endothelium-derived NO, and promote inflammation [5].
Another mechanism by which hyperglycemia promotes oxidative stress is by increasing polyol pathway flux [6]. Usually, aldose reductase has a low affinity for glucose, but in hyperglycemic states increased intracellular glucose increases aldose reductase activity in reducing glucose to sorbitol. The reduction of glucose to sorbitol, which consumes NADPH, decreases reduced glutathione and subsequently increases oxidative stress [2].
Hyperglycemia and elevated free fatty acids also increase PKC activity. By increasing de novo synthesis of diacylglycerol (DAG), by activating phospholipase C, and by inhibiting DAG kinase, hyperglycemia increases the amount of DAG. DAG activates PKC, which has a number of consequences [6]. Increased activity of PKC promotes oxidative stress through activation of mitochondrial NADPH oxidase. Increased PKC activity also has a number of other effects, including decreasing NO production, increasing endothelin-1 production, increasing vascular permeability, promoting vascular occlusion, and stimulating inflammation [1,7].
In addition to the above, hyperglycemia also shunts excess glucose through the hexosamine pathway [8]. By converting fructose 6-phosphate to glucosamine-6-phosphate, excess intracellular glucose promotes a series of reactions that increase oxidative stress by depleting NADPH, increases gene expression of transforming growth factor-β and plasminogen activator inhibitor-1, and inhibits endothelium NO synthase (NOS) activity [9].
Oxidative Stress and Endothelium Dysfunction
Endothelium dysfunction was first described in the early 1980s when Furchgott and Zawadzki first discovered that endothelial cells mediated acetylcholine-induced vasodilation. Since then, how the endothelium mediates vasodilation has been better delineated. Endothelium-derived NO acts as a vasodilator but has other functions that maintain the patency of blood vessels, such as inhibiting smooth muscle cell growth, preventing platelet aggregation, and inhibiting leukocyte adhesion. Stimulants, including thrombin, substance P, shear stress, acetylcholine, and blood flow, cause increased synthesis and endothelial production of NO. NO opposes the vasoconstrictive effects of catecholamines, serotonin, and the products of activated platelets [3]. When endothelium-derived NO is reduced, the protective functions of the endothelium are impaired. Endothelium dysfunction has since been shown to be an early marker of atherosclerotic disease and has been associated with a myriad of other disorders, including diabetes, impaired glucose tolerance, insulin resistance, aging, obesity, inflammation, transplantation, low birth weight, preeclampsia, obstructive sleep apnea, and smoking.
Although these diseases may show considerable clinical variability, oxidative stress is common to all of them and is thought to be a major mechanism mediating endothelium dysfunction. In general, high levels of ROS can be toxic to endothelial cells and smooth muscle cells by damaging DNA and inducing apoptosis. ROS also upregulate adhesion and chemotactic molecules, which allow monocytes to adhere to and migrate into the vascular wall. In addition, superoxide (O2•), the production of which is increased in hyperglycemia, reacts with NO to form the highly reactive molecule peroxynitrite (ONOO−·). Peroxynitrite mediates lipid peroxidation and protein nitration, both of which are known to be highly proatherogenic [10]. All of these factors lead to endothelium dysfunction and the subsequent development of cardiovascular disease.
Antioxidants
Because the production of ROS leads to endothelium dysfunction, a precursor to the development of cardiovascular disease, targeting ROS seems a logical approach to combat the vascular complications of diabetes. The human body has developed a variety of antioxidants to counteract the damaging effects of ROS. Enzymes such as superoxide dismutase convert the ROS superoxide to hydrogen peroxide, which is then transformed into water by catalase in lysosomes or by glutathione peroxidase in the mitochondria [11]. Diabetes alters the activity of these enzymes and reduces levels of glutathione (a direct free radical scavenger and cosubstrate for glutathione peroxidase), which may affect the body’s ability to defend against oxidative stress [12]. Nonenzymatic antioxidants include vitamins such as vitamins A, C, and E found in the everyday diet. These vitamins directly detoxify free radicals.
Other antioxidants that have been studied include glutathione, α-lipoic acid, cofactors (B1, B2, B6, and B12), carotenoids, trace elements (copper, zinc, and selenium), coenzyme Q, taurine, tetrahydrobiopterin (BH4), and acetylcysteine.
Animal Studies
The majority of animal studies using antioxidants to combat complications from oxidative stress have demonstrated favorable outcomes. Thus, vitamin E protected against endothelium dysfunction in cholesterol-fed rabbits and streptozotocin-diabetic rats [13,14]. However, not all studies yielded positive results, as some studies indicated that antioxidant vitamin supplementation may actually lead to endothelium dysfunction in both diabetic and normal animals [15,16]. Proposed mechanisms for this observation include pro-oxidant effects of vitamin E on vitamin C in the presence of NO and/or de novo synthesis of vasoconstrictive prostanoids [17]. Differing levels of vitamin E and C levels prior to repletion may also play a role.
BH4 is an essential cofactor for NOS [18]. BH4 is best known for stabilizing NOS dimers, which prevents uncoupling of NOS and subsequent superoxide formation. Because uncoupled NOS is a large source of ROS, BH4 deficiency is an important mechanism in promoting oxidative stress [19]. Oxidative stress can decrease the bioavailability of BH4, which furthers superoxide formation and decreases NO production. Studies in diabetic rats have shown that BH4 supplementation ameliorated endothelium dysfunction [20]. However, as the results in nondiabetic animal models have yielded contradictory results, it is not clear whether BH4 deficiency is an important mechanism in the diabetic model only [21].
Benfotiamine, a lipid-soluble thiamine (vitamin B1) derivative that achieves much higher blood and tissue levels than thiamine, has also been shown to have significant antioxidant effects. Thiamine and benfotiamine activate the enzyme transketolase that diverts glucose metabolites away from the mediators of oxidative stress, namely the polyol, hexosamine, DAG, and AGE pathways. In animal models, benfotiamine inhibited diabetic retinopathy and reversed the toxic effects of glucose on endothelial progenitor cell differentiation [22,23].
Lipid-lowering agents, such as 3-hydroxy-3-methlyglutaryl coenzyme A reductase inhibitors (statins), have also demonstrated potential antioxidant effects. However, because the main mode of action of these medications is not the reduction of oxidative stress, they will not be reviewed in this article.
Human Studies
Vitamins C and E
A substantial amount of human research has focused on the vitamin antioxidants such as vitamins B, C, and E. Vitamins C and E have been paid particular attention because of their known superoxide scavenger properties and they have been tested either alone or in combination.
Initial studies that acutely increased vitamin C levels showed an improvement of endothelial function, a main surrogate end point for cardiovascular disease, in multiple disease models of oxidative stress. Beckman et al. [24] found that hyperglycemia-induced endothelial dysfunction in nondiabetic subjects was reversed with vitamin C infusion. Intra-arterial infusions of vitamin C have been shown to improve endothelial function in both type 1 and type 2 diabetes [25,26]. Other studies reported similar results in subjects with essential hypertension; they showed that infusions of vitamin C improved endothelial function acutely, whereas other antioxidants such as N-acetylcysteine did not have the same effect [27].
Despite favorable studies showing acute improvements in endothelial function with vitamin C, long-term antioxidant therapy has not been as successful. A combination of vitamin C and vitamin E given to patients with both type 1 and 2 diabetes showed that endothelial function improved only in patients with type 1 diabetes [28]. In another study, high doses of oral vitamin C did not improve endothelial function in patients with type 2 diabetes [29]. Interestingly, in this study, although the diabetic patients with low vitamin C levels were able to replenish their vitamin C levels with the high-dose oral supplementation, they did not achieve as high levels as healthy nondiabetic subjects. Because high concentrations of vitamin C are needed to compete for superoxide, the failure to achieve these high levels was proposed as a reason of the failure of vitamin C in this group of patients [30].
High doses of vitamin E supplementation have not shown any clear beneficial effects in surrogate measurements of cardiovascular disease in diabetic patients. A study from our unit, which included patients with both type 1 and 2 diabetes treated with a high dose of vitamin E (1800 IU/d) for 12 months, found no improvement in endothelial-dependent or -independent vasodilation in both the skin microcirculation and the brachial artery, a macrocirculation test [31•]. Left ventricular function was also not affected by vitamin E supplementation. Additionally, endothelin (a potent vasoconstrictor) was increased in the treatment group after 6 months (but normalized by 12 months), whereas endothelial-independent vasodilation and the systolic blood pressure worsened slightly by the end of the 12-month treatment period. Of interest, C-reactive protein, a marker of inflammation, was decreased in the vitamin E–treated group. Thus, although vitamin E may be having a beneficial anti-inflammatory effect, it has no beneficial effect on cardiovascular function and, if anything, it may have a detrimental effect.
A number of large clinical trials that have employed clinical end points have also been conducted. One of the initial studies, the CHAOS (Cambridge Heart Antioxidant Study), employed vitamin E (400–800 IU) and reported a significant risk reduction from nonfatal myocardial infarction after an 18-month follow-up period [32]. This reduction was accompanied by a nonsignificant excess of cardiovascular deaths in the same group. Subsequent large-scale trials failed to yield similar beneficial outcomes. A large Italian study, the GISSI-Prevenzione (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico) trial, employed vitamin E (300 mg/d) and n-3 polyunsaturated fatty acids (PUFA) or placebo for a median of 3.5 years [33]. Vitamin E had no effect in preventing cardiovascular outcomes, in contrast to n-3 PUFA, which had a clinically important and statistically significant benefit.
The largest North American trial to date has been the HOPE (Heart Outcomes Prevention Evaluation study), which was published in 2000 [34]. It enrolled over 9500 subjects, aged 55 years old and above, 3654 of whom had diabetes, and employed 400 IU of vitamin E daily for a mean follow-up of 4.5 years. Vitamin E had no effect on cardiovascular outcomes in all subgroups, including those with diabetes. The HOPE trial was subsequently extended to the HOPE-TOO (HOPE-The Ongoing Outcomes) trial, which included a large number of the participants of the original study (7030 patients, out of whom 2680 had diabetes) [35]. Subjects were followed up an average of 7 years in this study and were randomized to placebo or 400 IU of vitamin E daily. There was no difference in cardiovascular outcomes (including myocardial infarction, stroke, and death from cardiovascular causes) between the treatment and placebo groups. Surprisingly, subjects treated with vitamin E had higher rates of heart failure and heart failure–related hospital admissions. This finding was consistent among all subgroups, including diabetes, and was persistent through both HOPE and HOPE-TOO. Although the mechanisms related to the vitamin E–associated excess rate of heart failure were not clear, the HOPE-TOO investigators hypothesized that one possibility could be that vitamin E may be pro-oxidative in certain circumstances and subsequently depress myocardial function.
Although initial meta-analyses did not show an effect of vitamin E on survival, one common shortcoming in these studies was that the effect of dose was not analyzed [36,37]. The dose was taken into account in a recent meta-analysis, which included 19 clinical trials and examined the relationship between vitamin E supplementation and total mortality. The results showed that in nine of 11 trials testing high-dose vitamin E (≥400 IU/d), the all-cause mortality risk increased, prompting the conclusion that high doses of vitamin E ≥ 400 IU/d should be avoided [38••].
In summary, there is currently no compelling evidence to support the use of vitamins C and E for preventing cardiovascular disease in diabetes. Furthermore, because the data indicate that high doses of vitamin E may be associated with serious side effects, it is reasonable to suggest that such high doses should be avoided.
Tetrahydrobiopterin (BH4)
The bioavailability of BH4 decreases during oxidative stress conditions (ie, hyperglycemia), which in turn reduces endothelial NO production and subsequently causes endothelial dysfunction. Repletion of BH4 is believed to improve production of NO because it is an essential cofactor for NOS. In healthy adults, oral glucose-induced endothelial dysfunction was reversed acutely with BH4 [39]. Furthermore, in subjects with type 2 diabetes, infusion of BH4 was shown to improve endothelial function acutely [40]. Currently, there are no clinical trials that have examined the effect of BH4 in cardiovascular risk in diabetes and further research in this field is required before any recommendations can be made.
Benfotiamine
Although large-scale clinical trials using benfotiamine are lacking, small studies have shown promising results. Thus, a recent small clinical trial examined the effect of a 3-day therapy with benfotiamine (1050 mg/d) on the macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in AGEs in individuals with type 2 diabetes [41]. Benfotiamine prevented the endothelial dysfunction that was caused by the ingestion of a meal with high AGE content in both the micro- and macrocirculation and prevented an increase in biochemical markers of endothelial dysfunction and oxidative stress.
In addition, benfotiamine has been employed for the management of diabetes-related microvascular complications. A small trial that randomized 40 patients with diabetes and polyneuropathy to benfotiamine (50 mg four times daily) or placebo and followed them for a 3-week period reported a decrease in neuropathic pain without significant adverse effects [42].
In summary, these studies indicate that benfotiamine may have a beneficial effect and reduce cardiovascular risk in diabetes. However, its use cannot be recommended on the basis of the existing data, and large clinical trials with clinical end points will be required before any recommendations can be made.
Antioxidants and diet
In 1996, a study of 34,486 postmenopausal women found that although vitamin E supplementation did not affect risk of death from cardiovascular disease, increased intake of vitamin E through diet was associated with decreased risk of death from coronary artery disease [43]. This study exemplifies the paradox noted in several large-scale clinical and epidemiologic studies, that diet but not vitamin supplementation seems to improve cardiovascular outcomes. Because diet is beyond the scope of the current review, the efficacy of diet antioxidants is briefly reviewed.
Mediterranean diet
Although it may be challenging to fully define the Mediterranean diet, because it is consumed in more than 15 countries that border the Mediterranean Sea, extensive work over the past few decades has shown that this type of diet has impressive effects in reducing cardiovascular risk [44]. Olive oil, a main component of the diet, has considerable antioxidant properties and is considered to be among the primary factors that contribute to these beneficial effects [45]. In a recent study that involved subjects with the metabolic syndrome, the diet had anti-inflammatory and antithrombotic properties and improved the endothelial function and insulin sensitivity [46]. Therefore, the current consensus is that a diet that encompasses the main components of the Mediterranean diet can greatly reduce cardiovascular risk in diabetic patients.
Green tea and coffee
Separate food ingredients and beverages, which may have antioxidant effects, have also been studied across different populations to examine whether they affect health and cardiovascular disease in particular. Coffee, a common beverage in Western countries, may have antioxidant effects through minerals (ie, as magnesium), phytochemicals (in caffeine), and antioxidants. Several studies have shown that coffee decreases the risk of type 2 diabetes, although there have been reports that caffeine itself may impair glucose metabolism in patients with type 2 diabetes [47,48]. It is not clear, however, how coffee decreases the risk of type 2 diabetes, especially because caffeine (and its phytochemicals) does not seem to play a large role.
Green tea, another widely consumed beverage, also seems to have protective effects because its polyphenols have antioxidant properties. A recent large study that followed Japanese subjects for 11 years found that green tea consumption was associated with decrease in all-cause mortality and mortality from cardiovascular disease [49]. In another recently published study, consumption of green tea, coffee, and total caffeine among Japanese subjects was associated with a decreased risk for type 2 diabetes in a 5-year follow-up period [50].
Conclusions
Although there was initially much enthusiasm for antioxidant therapy in diabetes, especially in the form of supplemental vitamins, clinical trials have not shown decreased risk of cardiovascular outcomes. Furthermore, some studies have suggested detrimental effects of vitamins, especially vitamin E. Therefore, supplementation with vitamins E and C cannot be currently recommended. The same can be said for other antioxidants, such as BH4 and benfotiamine, because there are no adequate data from large clinical trials despite initial encouraging preliminary reports. On the other hand, diet rich in antioxidants, especially Mediterranean diet, can provide considerable reduction of cardiovascular risk and may be of particular benefit to subjects with the metabolic syndrome and/or diabetes.
Acknowledgments
This work was partially supported by National Institutes of Health Grants RO1-HL73146 (to AM) and R01-HL075678 and R01-NS046710 (to AV).
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 3.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 4.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats. Evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994;93:311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolm-Litty V, Sauer U, Nerlich A, et al. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacher P, Szabo C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 13.Stewart-Lee AL, Forster LA, Nourooz-Zadeh J, et al. Vitamin E protects against impairment of endothelium-mediated relaxations in cholesterol-fed rabbits. Arterioscler Thromb. 1994;14:494–499. doi: 10.1161/01.atv.14.3.494. [DOI] [PubMed] [Google Scholar]
- 14.Cinar MG, Ulker S, Alper G, Evinc A. Effect of dietary vitamin E supplementation on vascular reactivity of thoracic aorta in streptozotocin-diabetic rats. Pharmacology. 2001;62:56–64. doi: 10.1159/000056072. [DOI] [PubMed] [Google Scholar]
- 15.Palmer AM, Thomas CR, Gopaul N, et al. Dietary antioxidant supplementation reduces lipid peroxidation but impairs vascular function in small mesenteric arteries of the streptozotocin-diabetic rat. Diabetologia. 1998;41:148–156. doi: 10.1007/s001250050883. [DOI] [PubMed] [Google Scholar]
- 16.Alper G, Olukman M, Irer S, et al. Effect of vitamin E and C supplementation combined with oral antidiabetic therapy on the endothelial dysfunction in the neonatally streptozotocin injected diabetic rat. Diabetes Metab Res Rev. 2006;22:190–197. doi: 10.1002/dmrr.586. [DOI] [PubMed] [Google Scholar]
- 17.Gorbunov NV, Osipov AN, Sweetland MA, et al. NO-redox paradox: direct oxidation of alpha-tocopherol and alpha-tocopherol-mediated oxidation of ascorbate. Biochem Biophys Res Commun. 1996;219:835–841. doi: 10.1006/bbrc.1996.0319. [DOI] [PubMed] [Google Scholar]
- 18.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 19.Akamine EH, Kawamoto EM, Scavone C, et al. Correction of endothelial dysfunction in diabetic female rats by tetrahydrobiopterin and chronic insulin. J Vasc Res. 2006;43:309–320. doi: 10.1159/000093196. [DOI] [PubMed] [Google Scholar]
- 20.Meininger CJ, Marinos RS, Hatakeyama K, et al. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.d’Uscio LV, Katusic ZS. Increased vascular biosynthesis of tetrahydrobiopterin in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2006;290:H2466–H2471. doi: 10.1152/ajpheart.00366.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti V, Menghini R, Rizza S, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231–2237. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103:1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- 25.Timimi FK, Ting HH, Haley EA, et al. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1998;31:552–557. doi: 10.1016/s0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- 26.Ting HH, Timimi FK, Boles KS, et al. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider MP, Delles C, Schmidt BM, et al. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am J Hypertens. 2005;18:1111–1117. doi: 10.1016/j.amjhyper.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Beckman JA, Goldfine AB, Gordon MB, et al. Oral antioxidant therapy improves endothelial function in type 1 but not type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H2392–H2398. doi: 10.1152/ajpheart.00403.2003. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Karne RJ, Hall G, et al. High-dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006;290:H137–H145. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- 30.Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- 31•.Economides PA, Khaodhiar L, Caselli A, et al. The effect of vitamin E on endothelial function of micro- and macro-circulation and left ventricular function in type 1 and type 2 diabetic patients. Diabetes. 2005;54:204–211. doi: 10.2337/diabetes.54.1.204. This paper shows that vitamin E has no benefits in cardiovascular disease risk in diabetes and identifies possible mechanisms for these results. [DOI] [PubMed] [Google Scholar]
- 32.Stephens NG, Parsons A, Schoffeld PM, et al. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 33.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. no authors listed. [PubMed] [Google Scholar]
- 34.Yusuf S, Dagenais G, Pogue J, et al. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 35.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 36.Eidelman RS, Hollar D, Hebert PR, et al. Randomized trials of vitamin E in the treatment and prevention of cardiovascular disease. Arch Intern Med. 2004;164:1552–1556. doi: 10.1001/archinte.164.14.1552. [DOI] [PubMed] [Google Scholar]
- 37.Shekelle PG, Morton SC, Jungvig LK, et al. Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med. 2004;19:380–389. doi: 10.1111/j.1525-1497.2004.30090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. This paper shows that long-term vitamin supplementation may not have any beneficial effect and may also be harmful. [DOI] [PubMed] [Google Scholar]
- 39.Ihlemann N, Rask-Madsen C, Perner A, et al. Tetrahy-drobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol. 2003;285:H875–H882. doi: 10.1152/ajpheart.00008.2003. [DOI] [PubMed] [Google Scholar]
- 40.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydro-biopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 41.Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29:2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 42.Haupt E, Ledermann H, Kopcke W. Benfotiamine in the treatment of diabetic polyneuropathy—a three-week randomized, controlled pilot study (BEDIP study) Int J Clin Pharmacol Ther. 2005;43:71–77. doi: 10.5414/cpp43071. [DOI] [PubMed] [Google Scholar]
- 43.Kushi LH, Folsom AR, Prineas RJ, et al. Dietary antioxidant vitamins and death from coronary heart disease in post-menopausal women. N Engl J Med. 1996;334:1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 44.Kris-Etherton P, Eckel RH, Howard BV, et al. Lyon Diet Heart Study: benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association step I dietary pattern on cardiovascular disease. Circulation. 2001;103:1823–1825. doi: 10.1161/01.cir.103.13.1823. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Jimenez F, Alvarez de Cienfuegos G, Badimon L, et al. International conference on the healthy effect of virgin olive oil. Eur J Clin Invest. 2005;35:421–424. doi: 10.1111/j.1365-2362.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 46.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 47.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 48.Pereira MA, Parker ED, Folsom AR. Coffee consumption and risk of type 2 diabetes mellitus: an 11-year prospective study of 28 812 postmenopausal women. Arch Intern Med. 2006;166:1311–1316. doi: 10.1001/archinte.166.12.1311. [DOI] [PubMed] [Google Scholar]
- 49.Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 50.Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554–562. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]