[Sleep is] the golden chain that ties health and our bodies together. —Thomas Dekker (1572–1632)
Although the importance of sleep and well-being has been known for centuries, only recently has the medical profession begun to focus on sleep disorders and their effects. Although obstructive sleep apnea is probably the most well known, the disorders that can occur during sleep are diverse and often underdiagnosed or misdiagnosed [1]. Sleep disorders can be classified into one of four groups: insomnias, hypersomnias, parasomnias, and sleep–wake schedule disorders.
With increasing awareness that sleep disorders were posing a major public health problem, the United States Congress in 1988 created the National Commission on Sleep Disorders Research to assess the status of research and knowledge regarding sleep disorders, including available resources and facilities to address these problems. The Commission published its findings in 1993, highlighting the extensive prevalence and health and economic impacts of sleep disorders in our country [2]. According to the research, 40 million Americans were chronically ill with various sleep disorders, with an additional 20 to 30 million experiencing intermittent sleep-related problems. Sleep apnea alone was the cause of excessive daytime sleepiness in almost 20 million Americans, making it more prevalent than asthma in adults. The commission proposed that the direct cost of sleep disorders approached 16 billion dollars, with reduced workplace productivity costing an estimated 150 billion dollars. Other studies have confirmed similar numbers [3].
With sleep disorders apparently more prevalent among the elderly [4], the graying of the United States population suggests that an increasing public health burden will be encountered in the years to come. It is estimated that nearly 80 million Americans will have a sleep problem by the year 2010 and 100 million will by the year 2050. In a Gallup Poll performed in 1995 [5], 70% of American adults who claimed to have sleep problems stated that they never discussed these problems with a health care provider. This finding suggests that health care providers would have a high yield in the Review of Systems when asking their patients about sleep problems.
Besides excessive daytime sleepiness and loss of productivity, many studies have suggested that sleep disorders cause important cardiovascular morbidity and possibly mortality [6–8]. To better understand how sleep disorders may affect cardiovascular physiology, it is important to examine the normal physiology of sleep and arousal.
Based on electroencephalogram (EEG) and other physiologic measurements, sleep is divided into two distinct states: rapid eye movement (REM) sleep and non–rapid eye movement (NREM) sleep. NREM sleep is further classified into four stages: stage 1 (light sleep), stage 2 (consolidated sleep), and stage 3 and 4 (deep, or slow-wave sleep). During normal sleep, these stages tend to occur in succession; from wakefulness, into stage 1 sleep, followed by stage 2, 3, and 4, and then REM sleep.
The transition from wakefulness to NREM sleep is accompanied by marked alterations in respiratory and cardiovascular regulation. As one progresses into deeper sleep, parasympathetic nervous system tone increases while sympathetic nervous system activity, along with heart rate, blood pressure, and systemic vascular resistance decreases. As a result, workload on the heart is reduced and the cardiovascular system is in hemodynamic and autonomic quiescence [9,10]. During sleep, ventilation becomes tightly linked with metabolic rate, oxygen consumption (VO2), and carbon dioxide (CO2) production (VCO2), with behavioral influences being minimal to absent [11]. During NREM sleep, minute ventilation decreases mainly because of reductions in tidal volume, and thus, sleep is associated with a mild increase in end-tidal CO2 and decrease in oxygen saturation.
Arousals from sleep in normal subjects are associated with transient increases in blood pressure and heart rate produced by large bursts of sympathetic activity, which appear out of proportion to physiologic need and greater than those observed in periods of quiet wakefulness [12,13]. These elevations in sympathoexcitation persist, even after heart rate reverts back to baseline levels [14]. Arousal from sleep is associated with transient stimulation of ventilation above normal waking levels, which exceeds that expected for the elevated PaCO2 that was present during prior sleep [15].
Obstructive sleep apnea (OSA) syndrome is characterized by recurrent airflow cessation caused by the collapse of the upper airway despite continuing respiratory efforts. Because of its prevalence and association with neurocognitive and cardiovascular morbidity, it is the most studied chronic sleep disorder. Although controversial, the severity of OSA is measured by the apnea–hypopnea index (AHI), which is the average number of apneas plus hypopneas per hour of sleep. Apnea is defined as cessation of airflow that lasts at least 10 seconds. As there has been considerable debate on what constitutes a hypopnea, a consensus conference defined a hypopnea as one of the following three: substantial reduction in airflow (>50%), moderate reduction in airflow (<50%) associated with desaturation (>3%), or moderate reduction in airflow with electroencephalographic evidence of arousal [16]. During hypopneas and apneas, severe arterial hypoxemia and hypercapnia develop, until a brief arousal terminates the event and restores airway patency. In addition to the frequent arousals, exaggerated negative intrathoracic pressure against the occluded pharynx [17], hypoxemia, and hypercapnia [18] also contribute to the abnormal cardiovascular physiology seen with OSA. In patients who have OSA, the cardiovascular response to a postapneic arousal is markedly greater than to a spontaneous arousal [19].
The most convincing data regarding cardiovascular morbidity and sleep apnea is related to hypertension. Brooks et al [20] modeled OSA using a surgically implanted tracheostomy with an occlusion valve in dogs. Intermittent occlusion resulted in acute transient increases in nighttime blood pressure, and subsequently produced sustained daytime hypertension. Although recurrent arousals from sleep without airway occlusion produced acute surges in blood pressure, nonrespiratory arousals did not result in daytime hypertension. Several cross-sectional studies [21,22] showed a significant correlation between AHI and hypertension, even when adjusted for body mass index, age, and sex. Peppard et al [23] prospectively studied 709 participants who underwent polysomnography, and found a dose–response association between AHI at baseline and the presence of hypertension 4 years later, independent of known confounding factors. Treatment of OSA can include oral appliances and surgical treatment, but the most consistently effective therapy is continuous positive airway pressure (CPAP) to prevent pharyngeal collapse [24,25]. Mayer et al [26] showed that 6 months of treatment with CPAP in patients who had OSA and hypertension resulted in a mean decrease in systolic blood pressure from 147.1 to 126.4 mm Hg and diastolic blood pressure from 81.6 to 69.4 mm Hg. Minemura et al [27] demonstrated that patients who had OSA and were treated with CPAP had a significant reduction in urinary and morning plasma levels of norepinephrine.
Although OSA is clearly associated with other cardiovascular diseases, considerable controversy exists whether OSA is an independent risk factor. The Sleep Heart Health Study performed overnight, unattended, at-home polysomnography on a cohort of 6424 individuals and examined the association between sleep-disordered breathing and self-reported cardiovascular disease [28]. Sleep disordered breathing was found to be associated not only with hypertension and coronary artery disease but most strongly with congestive heart failure (CHF) and stroke. Further studies, however, are needed to prospectively define the attributable risk of OSA to these cardiovascular events.
Although the prevalence of obstructive sleep apnea has been found to be higher in patients who have CHF [29–31], the most common sleep-disordered breathing in patients who have CHF is central sleep apnea (CSA) [32]. CSA occurs when there is airflow cessation caused by lack of respiratory efforts, and can be classified as hypercapnia (decreased respiratory drive or ability to breathe) or hypocapnic. Hypocapnic CSA, which is the predominant form associated with CHF, is characterized by regular periodic waxing and waning of tidal volumes. This characteristic periodic breathing pattern [33] is also known as Cheyne-Stokes respiration (CSR). Hanly and Zuberi-Khokhar [34] showed that CHF patients who had CSR had significantly worse survival and heart transplant–free rates when compared with appropriately matched CHF patients who did not have CSR.
Because improvement in cardiac output reduces periodic breathing, medical optimization of the underlying heart disease should occur before treatment is considered for the periodic breathing [35]. OSA and CSA often coexist and treatment of one may lead to the appearance or exacerbation of the other [36]. Controversy remains regarding the optimal treatment of CSR. Oxygen has been shown to significantly reduce the number of apneas [37], time of periodic breathing, and urinary norepinephrine levels [38]; however, the effect on sleep quality and patient's symptoms has been variable. There is concern regarding oxygen toxicity (eg, cardiotoxicity) [39] with prolonged treatment because of oxygen free radicals. CPAP therapy has been shown to reduce the amount of periodic breathing, apneas, arousals, and oxygen desaturations [40]. Sin et al [41] demonstrated that patients who had CHF and periodic breathing showed improvement in left ventricular ejection fraction and transplant-free survival when treated with CPAP, whereas CHF patients who did not have periodic breathing had no benefit. The roles of CO2 inhalation and acetazolamide in the treatment of CSA still need further study.
Function of sleep
Despite its obvious importance, the exact function of sleep is still unknown. That every living creature sleeps clearly points to sleep being a necessity of life. Total sleep deprivation in rats results in death within 11 to 32 days [42]. One theory, suggesting that sleep is important for energy conservation, is supported by findings that levels of ATP and glycogen in the brain decrease during wakefulness and are restored during sleep [43,44]. The restorative theory suggests that sleep is a time of growth and repair for the body and brain [45]. The findings that sleep increases after rigorous exercise and that growth hormone is mainly released during deep sleep [46] support this theory. Another postulated function of sleep is the organization and processing of large amounts of input gathered during the day. Other hypotheses include temperature regulation and immune defense mechanisms. The multiplicity of sleep theories indicates that sleep does not have a single function.
With sleep deprivation, higher-order cognitive tasks are affected early and disproportionately [47]. Tests requiring speed and accuracy reveal considerably slowed speed among participants before accuracy begins to fail. Glucose–positron emission tomography studies in sleep-deprived individuals show that after 24 hours of sustained wakefulness, the metabolic activity of the brain decreases significantly, especially in prefrontal and parietal associational areas, which are most important for judgment, impulse control, attention, and visual association [48]. In animal studies, sleep deprivation causes decreased weight despite increased caloric intake, possibly secondary to increased energy requirements [49]. In humans, sleep deprivation results in a decrease in core body temperature [50], immune system function as measured by white cell count and activity [51], and the release of growth hormone.
REM sleep represents a unique physiologic state. EEG findings resemble wakefulness with low-amplitude fast activity and eye movement activity, and there is evidence of increased energy expenditure by the brain. The skeletal muscles are atonic during REM sleep, probably as a protective mechanism to prevent the acting out of dreams that occur during this state. Despite extensive research, the exact function of REM sleep is far from being understood [52]. Experimental data suggest that REM is important in learning and memory consolidation processes. REM sleep deprivation disrupts learning of complex and new tasks; successful learning during wakefulness results in increased REM during the next sleep. However, certain people demonstrate marked decreases in REM sleep, yet show no clear impairment of neurocognitive functioning (eg, patients treated with selective serotonin reuptake inhibitors [SSRIs]).
Workup of excessive daytime sleepiness
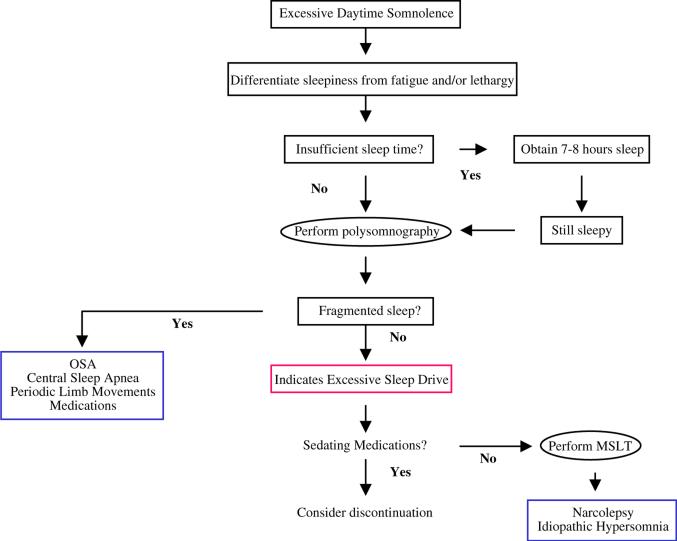
Most patients studied in sleep laboratories for clinical examination do so because of complaints related to excessive daytime sleepiness, nonrefreshing sleep, and chronic fatigue that cannot be ameliorated by sleep (Fig. 1). In 80% to 90% of these patients, laboratory examination uncovers breathing disorders during sleep [53].
Fig. 1.
Workup of hypersomnia. Important to initially differentiate sleepiness from fatigue and lethargy and determine sufficient sleep time. Polysomnography to assess for causes of fragmented sleep and mean sleep latency time (MSLT) to assess sleep drive can be important modalities in the workup. OSA, obstructive sleep apnea.
It is important to differentiate between complaints of sleepiness, fatigue, and lethargy, as the differential diagnosis and workup for these complaints are significantly different. Sleepiness is the increased likelihood of falling asleep, often during monotonous or sedentary activities, whereas fatigue is difficulty sustaining a high level of performance. There are three commonly available tools to aid in measuring the degree of sleepiness: the Epworth Sleepiness Scale (ESS), the Multiple Sleep Latency Test (MSLT), and the Maintenance Of Wakefulness Test (MWT). The ESS is a self-administered questionnaire that has patients rate on a scale of 0 to 3 their likelihood of falling asleep during eight common situations and provides a simple subjective measure of sleepiness (Box 1). The questionnaire reflects the level of the patient's daytime somnolence; the higher the score, the sleepier the patient. Patients who score up to six or seven have normal somnolence levels, whereas a score from 8 to 12 reflects mild sleepiness, a score of 13 to 17 indicates moderate sleepiness, and a score of 18 or above indicates severe sleepiness. The MSLT and MWT are objective tests of daytime sleepiness, testing length of time to achieving sleep onset and REM sleep during daytime naps. During the MSLT, the subject is instructed to try to sleep, whereas in the MWT the patient is instructed to try to stay awake. Thus, there are subjective and objective tests available to assess degrees of sleepiness, all measuring somewhat different phenomena.
There are three general causes of hypersomnia: insufficient sleep, fragmentation of sleep, and excessive sleep drive. Insufficient sleep can usually be determined from history, with particular emphasis regarding sleep schedule; sleep-onset latency; bedtime; shift working; number and duration of awakenings and naps; and information from the bed partner. Any patient who has complaints of daytime sleepiness should achieve at least 7 to 8 hours of sleep per night. If total sleep time appears to be sufficient, polysomnography (PSG) is indicated to determine if there is sleep fragmentation. MSLT can be performed to look for increased sleep drive if PSG does not show fragmentation.
PSG allows the continuous measurements of several neurophysiologic and cardiorespiratory parameters, which can be used to evaluate abnormalities during sleep or wakefulness. Electroencephalographic, electro-oculographic, and electromyographic channels provide information for the staging of successive epochs of wakefulness and NREM and REM sleep. Detection of airflow at the nose and mouth and analysis of breathing patterns recorded from sensors placed around the ribcage and abdomen provide information to assess physiologic events in relation to sleep structure. Airflow has been traditionally detected by oronasal thermistors. Many studies, however, suggest that measurement of nasal pressure may be more sensitive in detecting respiratory event during sleep [54], although the specificity of nasal pressure has been less well-characterized. Thus, some practitioners believe that subtle fluctuations in airflow may be subclinical and associated with no important sequelae. Other parameters often measured include snoring recording, ECG, and additional electromyogram (EMG) recordings, especially anterior tibialis, pulse oximetry, and body position.
Disorders of sleep fragmentation include sleep apnea, periodic limb movements (PLMs), and other causes, such as pain and medications. Periodic limb movement disorder (PLMD) is characterized by intermittent movements of the extremities during sleep, which may be associated with arousals from sleep. The most typical movements include extension of the hallux or dorsiflexion of the foot at the ankle. The diagnosis of PLMD requires a full night PSG study demonstrating characteristic limb movements in the anterior tibialis EMG. The severity of PLMD is determined by the number of PLMs and the proportion of movements that cause arousals. There is high night-to-night variability in PLMs; therefore, when PLMD is clinically strongly suspected and PSG does not reveal PLMs, a second diagnostic night may be indicated. Associated with PLMD, restless legs syndrome (RLS) is a disorder characterized by unpleasant sensations in the legs and an urge to move when at rest in an effort to relieve these feelings. RLS sensations are often described by people as aching, burning, or like insects crawling inside the legs. Although 80% of patients who have RLS have PLMD, RLS commonly presents with insomnia rather than sleep fragmentation. Medications that can cause sleep fragmentation include SSRIs.
Excessive sleep drive disorders include narcolepsy and idiopathic central nervous system hypersomnia, and can be caused by sedating medications. Narcolepsy is a relatively rare disorder characterized by excessive daytime sleepiness and cataplexy (ie, the sudden loss of muscle tone), and is usually triggered by a strong emotional stimulus. Two other classic symptoms include sleep paralysis (ie, transient but distressing inability to move at awakening or falling asleep) and hypnagogic hallucinations (ie, hallucina-tory experiences occurring at sleep onset). The sleepiness that accompanies narcolepsy manifests itself as irresistible episodes of drowsiness or sleep during monotonous activities and when actively engaged. Sleepiness in narcolepsy is transiently relieved by brief naps. The diagnosis of narcolepsy is confirmed by an MSLT demonstrating reduced sleep-onset latency in the presence of sleep-onset REM episodes.
Workup of Insomnia
Various surveys estimate that 20% to 35% of the general population has complaints of insomnia each year [55], with higher prevalence in the elderly, women, and hospitalized patients. One study estimated the total direct cost of treating American insomniacs to be around 14 millions dollars. The four most common complaints are difficulties falling asleep, frequent awakenings from sleep, difficulties falling back to sleep after nocturnal awakenings, and spontaneous early morning awakenings. These conditions are frequently associated with daytime symptoms, such as fatigue and impaired concentration.
Poor sleep hygiene is a frequent contributor to inadequate total sleep time among patients who have insomnia. Factors that may contribute to chronic insomnia include those that result in increased nocturnal alertness, such as excessive caffeine, frequent naps, and stressful work at night, and those that interfere with sleep continuity, such as falling asleep with the television or radio on, excessive time in bed, and other environmental factors. Even though poor sleep hygiene may not be the cause of insomnia, many patients will benefit from simple hygiene recommendations, such as those presented in Box 2.
Insomnia is usually not a disease but rather a symptom of another process. Insomnia has been classified into acute or chronic insomnia, with the arbitrary cutoff being 3 weeks. Most cases of acute insomnia do not progress to chronic insomnia and resolve spontaneously, or with reassurance or short-term hypnotic medications.
For some patients suffering from chronic insomnia, treatment of underlying medical disorders, such as chronic pain, pulmonary disease, or movement disorders, will help alleviate their insomnia. Many patients who have psychiatric disorders experience problems with sleep, which can be further confused by effects of their medications. In the remainder of patients, for whom no medical cause is found, many suffer from psychophysiologic insomnia. Typically, patients experience a stressful event that leads to insomnia. Although the insomnia would be transient in most individuals, the patient becomes concerned and anxious about the insomnia and these feelings thus become another source of stress. The result is a vicious cycle of anxiety leading to worsening insomnia, creating further anxiety. Oftentimes, a change in sleep environment or pharmacologic intervention to break the cycle is beneficial for these patients.
Although hypersomnia and insomnia are the most common sleep disorders seen in clinical practice, clinicians should be aware of other sleep disorders such as parasomnias and circadian rhythm disturbances. Parasomnias are undesirable physical and behavioral events that occur during sleep or are exacerbated by sleep. Primary parasomnias are disorders of the sleep state. They can be classified according to the state of sleep in which they tend to occur: arousal disorders, sleep–wake transition disorders, REM sleep disorders, and other parasomnias. Secondary parasomnias are disorders of other organ systems that manifest or worsen during sleep, such as nocturnal seizures and arrhythmias.
Disorders of arousals include sleep terrors, sleepwalking, confusional arousals, and sometimes sleep talking. These disorders usually occur after arousal from deep NREM sleep and are frequently worsened after sleep deprivation. They tend to occur during childhood and improve with age. Sleep terrors are characterized by sudden arousal from sleep with extreme panic and loud screams. Sleep terrors differ from nightmares in that patients who experience sleep terrors usually don't remember the event and can be disoriented and confused afterwards. Sleepwalking can be associated with other complex motor activity, such as driving a car and eating. Sleepwalking may be precipitated by several factors, including fever, stress, sleep deprivation, and medications (eg, lithium, phenothiazines). Treatment for sleepwalking is mainly aimed at ensuring safety and avoiding precipitating events.
Parasomnias associated with REM sleep include nightmares and REM sleep behavioral disorder (RBD). RBD is characterized by the acting out of dreams, caused by the loss of the normal muscle atonia of REM sleep. These movements can be injurious to the patient or bed partner. Because a substantial proportion of patients who have RBD have an underlying neurologic abnormality, such as subarachnoid hemorrhage, Parkinson's disease, and dementia [56], a neurologic workup including history, physical examination, EEG, and MRI is recommended. In addition, the patient should be informed of the potential risk of developing a neurologic disorder in the future. For treatment of RBD, clonazepam has been effective in most cases, but shorter-acting benzodiazepines are frequently used to minimize daytime hangover.
Bruxism, which does not fit into the above categories of parasomnias, is characterized by grinding of teeth during sleep and may occur in up to 6% to 12% of adults [57]. Bruxism can lead to sleep fragmentation, dental damage, and temporomandibular joint problems. Diagnosis can be made by PSG, and treatment is focused on relieving symptoms and preventing permanent sequelae.
Virtually all physiologic parameters, such as body temperature, blood pressure, cortisol and melatonin levels, exhibit regular variations over approximately 24 hours. These variations are known as circadian rhythms and are under control of an internal self-sustained oscillator located in the suprachiasmatic nucleus in the hypothalamus. These rhythms need to be entrained with the geophysical light/dark cycles. Circadian rhythm disturbances occur when they become dyssynchronous with environmental cues. Jet lag and night-shift work are examples of acute circadian rhythm disturbances. Chronic circadian rhythm disturbances include delayed sleep phase syndrome, in which patients habitually go to sleep late and awaken late, and advanced sleep phase syndrome, in which patients go to sleep early and awaken early. Treatment of these conditions may include the usage of bright light and melatonin, though the utility, timing, and dosages remain controversial.
As a result of the prevalence of sleep-disordered breathing and the limited number of sleep laboratories, there has been interest in at-home screening for sleep apnea. The simplest is overnight oximetry. However, oximetry has poor sensitivity in detecting subtle disordered breathing, and when positive, in-laboratory CPAP titration is generally required. Therefore, there is little clinical utility for overnight oximetry. Additional home diagnostic methods have been developed to measure other respiratory signals, such as airflow, effort, and patient position. The diagnostic accuracy of these systems is about 80%, with improved accuracy with more channels. It is still unclear what role these home diagnostic methods will provide, but it most likely will vary by country and region based on accessibility to sleep laboratories and reimbursement.
People spend approximately one third of their lives asleep, and yet the importance of sleep and its effects on health are only beginning to be understood. With the diversity and prevalence of sleep disorders, clinicians must always be alert to these problems in their patients.
Box 1. Epworth Sleepiness Scale.
Ask patient likelihood of falling asleep or dozing off in the following situations, in contrast to feeling just tired. Have the patient refer to their usually way of life in recent times.
-
0
= Would never doze
-
1
= Slight chance of dozing
-
2
= Moderate chance of dozing
-
3
= High chance of dozing
Situation Score
-
1)
Sitting and reading
-
2)
Watching television
-
3)
Sitting inactive in a public place (eg, church)
-
4)
As a passenger in a car for an hour without a break
-
5)
Lying down to rest in the afternoon when circumstances permit
-
6)
Sitting and talking to someone
-
7)
Sitting quietly after lunch without alcohol
-
8)
In a car, while stopped for a few minutes in the traffic
Box 2. Sleep hygiene recommendations.
Time spent in bed should be limited to just sleeping. If awakening occurs, get out of bed and go back to bed only when ready to sleep again.
Do not try to force sleep.
Avoid physical activity before bed. Exercise should be done at least 2 hours before going to bed.
Avoid drinking coffee and alcohol or excessive amounts of beverages before going to bed.
Avoid eating a heavy meal before going to bed.
Avoid taking naps during the day.
Set a routine in bedtime and waking hours.
Make the sleep environment as comfortable as possible with respect to lighting, temperature, noise, and so forth.
References
- 1.Stores G. Misdiagnosing sleep disorders as primary psychiatric conditions. Adv Psychiatr Treat. 2003;9:69–77. [Google Scholar]
- 2.National Commission on Sleep Disorders Research . Executive summary and executive report. Vol. 1. National Institutes of Health; Bethesda (MD): Wake Up America: A National Sleep Alert. p. 1993. [Google Scholar]
- 3.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 4.Duran J, Esnaola S, Rubio R, et al. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3):685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 5.National Sleep Foundation . National sleep foundation sleep survey. National Sleep Foundation; Washington (DC): p. 1995. [Google Scholar]
- 6.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94(1):9–14. [PubMed] [Google Scholar]
- 7.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 8.Dyken ME, Somers V, Yamada T, et al. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 9.Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G. Autonomic modulation of the cardiovascular system during sleep. N Engl J Med. 1993;328:347–9. doi: 10.1056/NEJM199302043280511. [DOI] [PubMed] [Google Scholar]
- 11.White DP, Weil JV, Zwillich CW. Metabolic rate and breathing during sleep. J Appl Physiol. 1985;59:384–91. doi: 10.1152/jappl.1985.59.2.384. [DOI] [PubMed] [Google Scholar]
- 12.Morgan BJ, Crabtree DC, Puleo DS, et al. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996;80:1627–36. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- 13.Davies RJ, Belt PJ, Roberts SJ, et al. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74(3):1123–30. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- 14.Blasi A, Jo J, Valladares E, et al. Cardiovascular variability after arousal from sleep: time-varying spectral analysis. J Appl Physiol. 2003;95:1394–404. doi: 10.1152/japplphysiol.01095.2002. [DOI] [PubMed] [Google Scholar]
- 15.Horner RL, Rivera MP, Kozar LF, et al. The ventilatory response to arousal from sleep is not fully explained by differences in CO(2) levels between sleep and wakefulness. J Physiol. 2001;534(Pt 3):881–90. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.O'Donnell CP, Ayuse T, King ED, et al. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol. 1996;80:773–81. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, Mark AL, Zavala DC, et al. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67:2101–6. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 19.Ali NJ, Davies RJ, Fleetham JA, et al. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526–32. doi: 10.1378/chest.101.6.1526. [DOI] [PubMed] [Google Scholar]
- 20.Brooks D, Horner RL, Kozar LF, et al. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–9. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 22.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 24.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 25.Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 26.Mayer J, Becker H, Brandenburg U, et al. Blood pressure and sleep apnea: results of long-term nasal continuous positive airway pressure therapy. Cardiology. 1991;79:84–92. doi: 10.1159/000174864. [DOI] [PubMed] [Google Scholar]
- 27.Minemura H, Akashiba T, Yamamoto H, et al. Acute effects of nasal continuous positive airway pressure on 24-hour blood pressure and catecholamines in patients with obstructive sleep apnea. Intern Med. 1998;37:1009–13. doi: 10.2169/internalmedicine.37.1009. [DOI] [PubMed] [Google Scholar]
- 28.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 29.Tkacova R, Rankin F, Fitzgerald FS, et al. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98:2269–75. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 30.Sin D, Fitzgerald F, Parker JD, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 31.Parker JD, Brooks D, Kozar LF, et al. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. 1999;160:1888–96. doi: 10.1164/ajrccm.160.6.9807074. [DOI] [PubMed] [Google Scholar]
- 32.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalence, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 33.Naughton M, Benard D, Tam A, et al. Role of hyperventilation in the pathogenesis of central sleep apnea in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–8. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 34.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 35.Dark DS, Pingleton SK, Kerby GR, et al. Breathing pattern abnormalities and arterial oxygen desaturation during sleep in the congestive heart failure syndrome. Improvement following medical therapy. Chest. 1987;91:833. doi: 10.1378/chest.91.6.833. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher EC. Recurrence of sleep apnea syndrome following tracheostomy. A shift from obstructive to central apnea. Chest. 1989;96:205–9. doi: 10.1378/chest.96.1.205. [DOI] [PubMed] [Google Scholar]
- 37.Franklin KA, Eriksson P, Sahlin C, et al. Reversal of central sleep apnoea with oxygen. Chest. 1997;111:163–9. doi: 10.1378/chest.111.1.163. [DOI] [PubMed] [Google Scholar]
- 38.Staniforth AD, Kinnear WJ, Starling R, et al. Effect of oxygen on sleep quality, cognitive function and sympathetic activity in patients with chronic heart failure and Cheyne-Stokes respiration. Eur Heart J. 1998;19:922–8. doi: 10.1053/euhj.1997.0861. [DOI] [PubMed] [Google Scholar]
- 39.Mak S, Azevedo E, Liu P, et al. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–73. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- 40.Naughton MT, Liu PP, Benard DC, et al. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:92–7. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- 41.Sin DD, Logan AG, Fitzgerald FS, et al. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102:61–6. doi: 10.1161/01.cir.102.1.61. [DOI] [PubMed] [Google Scholar]
- 42.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 43.Holden CP, Shepel PN, Mackiewicz M, et al. Brain glycogen levels are decreased with increased wakefulness and may represent a homeostatic drive for sleep. Soc Neurosci Abstr. 2000;26:1755. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karnovsky ML, Reich P, Anchors JM, et al. Changes in brain glycogen during slow-wave sleep in the rat. J Neurochem. 1983;41:1498–501. doi: 10.1111/j.1471-4159.1983.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 45.Oswald I. Sleep as a restorative process: human clues. Prog Brain Res. 1980;53:279–88. doi: 10.1016/s0079-6123(08)60069-2. [DOI] [PubMed] [Google Scholar]
- 46.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 47.Van Dongen HP, Maislin G, Mullington J, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 48.Petiau C, Harrison Y, Delfiore G, et al. Modification of fronto-temporal connectivity during a verb generation task after a 30-hour total sleep deprivation: A PET study [abstract]. J Sleep Res. 1998;7:208. [Google Scholar]
- 49.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1965;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 50.Landis CA, Savage MV, Lentz MJ, et al. Sleep deprivation alters body temperature dynamics to mild cooling and heating not sweating threshold in women. Sleep. 1998;21:101–9. doi: 10.1093/sleep/21.1.101. [DOI] [PubMed] [Google Scholar]
- 51.Irwin M, McClintick J, Costlow C, et al. Partial night sleep deprivation reduces natural killer cell and cellular immune responses in humans. FASEB J. 1996;10:643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 52.Siegel J. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–63. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.App WE, Boatwright GW, Ostrander SE, et al. Disorder of excessive daytime somnolence: a case series of 1000 patients. J Ky Med Assoc. 1990;88:393–6. [PubMed] [Google Scholar]
- 54.Sériès F, Marc I. Nasal pressure recording in the diagnosis of sleep apnoea hypopnoea syndrome. Thorax. 1999;54:506–10. doi: 10.1136/thx.54.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blais FC, Morin CM, Boisclair A, et al. Insomnia. Prevalence and treatment of patients in general practice. Can Fam Physician. 2001;47:759–67. [PMC free article] [PubMed] [Google Scholar]
- 56.Olson EJ, Boeve BF, Silber M. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 57.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]