Abstract
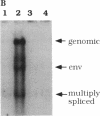
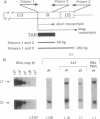
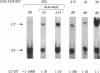
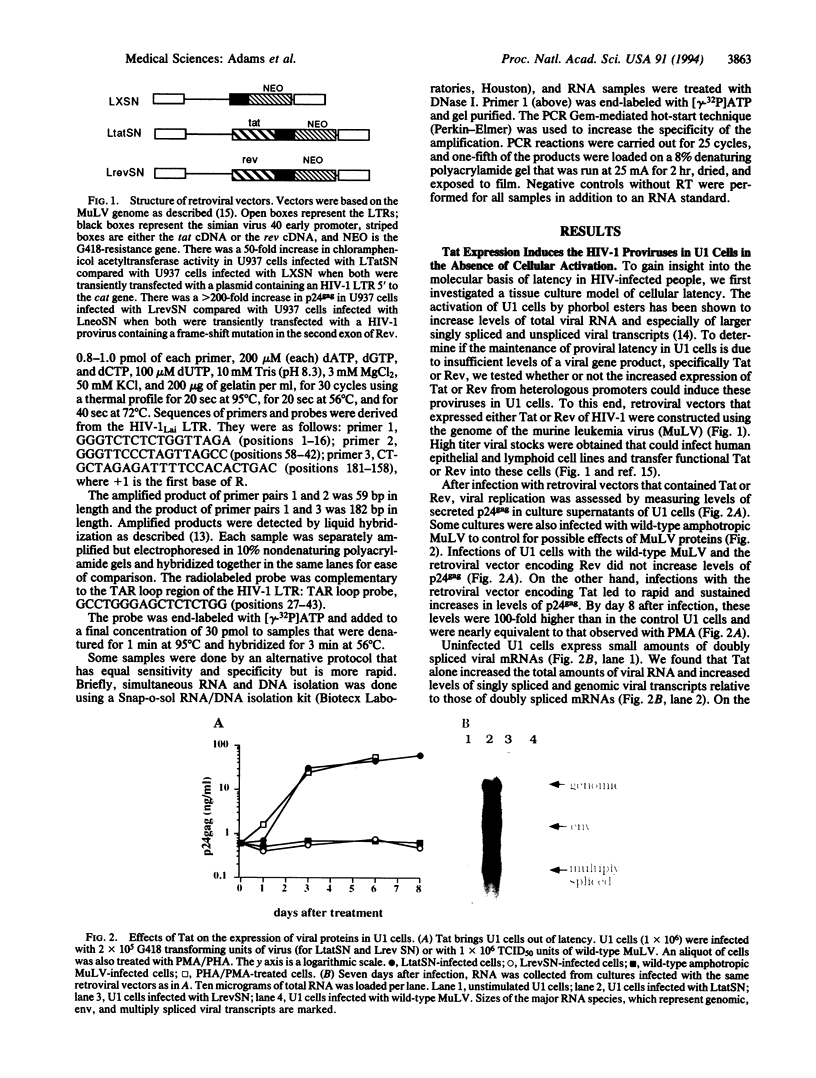
We have investigated the molecular basis of human immunodeficiency virus type 1 (HIV-1) latency in a tissue culture model and in HIV-infected people. We show that increased levels of Tat, but not Rev, can release the proviruses from latency in U1 cells. The absence of Tat in these cells is manifested by the accumulation of promoter-proximal viral transcripts, whereas the presence of Tat correlates with increased expression of viral proteins and an increase in promoter-distal transcripts. The presence of promoter-proximal transcripts also serves as a marker for latency in humans. We observed the exclusive presence of promoter-proximal viral transcripts in peripheral mononuclear cells from the majority (10/11) of asymptomatic HIV-infected individuals examined. Activation of these cells in vitro, and viremia in vivo, correlated with a switch from promoter-proximal transcription to promoter-distal transcription. These results suggest that the control between latency and replication of HIV in vivo is at the level of transcription elongation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnarelli P., Menzo S., Valenza A., Manzin A., Giacca M., Ancarani F., Scalise G., Varaldo P. E., Clementi M. Molecular profile of human immunodeficiency virus type 1 infection in symptomless patients and in patients with AIDS. J Virol. 1992 Dec;66(12):7328–7335. doi: 10.1128/jvi.66.12.7328-7335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik D. P., Folks T. M. Mechanisms of HIV-1 latency. AIDS. 1992 Jan;6(1):3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Stanwick T. L., Dempsey M. P., Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991 Oct 18;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993 Nov 12;262(5136):1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Baltimore D., Frankel A. D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4045–4049. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Schnittman S., Orenstein J., Poli G., Fauci A. S. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988 Feb 15;140(4):1117–1122. [PubMed] [Google Scholar]
- Garcia J. V., Miller A. D. Retrovirus vector-mediated transfer of functional HIV-1 regulatory genes. AIDS Res Hum Retroviruses. 1994 Jan;10(1):47–52. doi: 10.1089/aid.1994.10.47. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kimpton J., Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992 Apr;66(4):2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laspia M. F., Rice A. P., Mathews M. B. HIV-1 Tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989 Oct 20;59(2):283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Sunzeri F. J., Tobler L. H., Williams B. G., Busch M. P. Quantitative assessment of HIV-1 DNA load by coamplification of HIV-1 gag and HLA-DQ-alpha genes. AIDS. 1991 Jun;5(6):683–691. doi: 10.1097/00002030-199106000-00007. [DOI] [PubMed] [Google Scholar]
- Lu X., Welsh T. M., Peterlin B. M. The human immunodeficiency virus type 1 long terminal repeat specifies two different transcription complexes, only one of which is regulated by Tat. J Virol. 1993 Apr;67(4):1752–1760. doi: 10.1128/jvi.67.4.1752-1760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak R. A., Sharp P. A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991 Dec;10(13):4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N. L., Vahey M., Burke D. S., Redfield R. R. Viral DNA and mRNA expression correlate with the stage of human immunodeficiency virus (HIV) type 1 infection in humans: evidence for viral replication in all stages of HIV disease. J Virol. 1992 Jan;66(1):310–316. doi: 10.1128/jvi.66.1.310-316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Romeo J. M., Ulrich P. P., Busch M. P., Vyas G. N. Analysis of hepatitis C virus RNA prevalence and surrogate markers of infection among seropositive voluntary blood donors. Hepatology. 1993 Feb;17(2):188–195. [PubMed] [Google Scholar]
- Saksela K., Muchmore E., Girard M., Fultz P., Baltimore D. High viral load in lymph nodes and latent human immunodeficiency virus (HIV) in peripheral blood cells of HIV-1-infected chimpanzees. J Virol. 1993 Dec;67(12):7423–7427. doi: 10.1128/jvi.67.12.7423-7427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Greenhouse J., Justement J. S., Baseler M., Fauci A. S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M. J., Bain E. S., Luciw P. A., Peterlin B. M. Structure, sequence, and position of the stem-loop in tar determine transcriptional elongation by tat through the HIV-1 long terminal repeat. Genes Dev. 1989 Apr;3(4):547–558. doi: 10.1101/gad.3.4.547. [DOI] [PubMed] [Google Scholar]
- Seshamma T., Bagasra O., Trono D., Baltimore D., Pomerantz R. J. Blocked early-stage latency in the peripheral blood cells of certain individuals infected with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10663–10667. doi: 10.1073/pnas.89.22.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G., Hugerat Y. What determines whether chromosomes segregate reductionally or equationally in meiosis? Bioessays. 1993 Jan;15(1):1–8. doi: 10.1002/bies.950150102. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Gale M. J., Jr, Benveniste R. E., Clark E. A., Gallatin W. M. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J Immunol. 1990 May 15;144(10):3779–3783. [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]