Abstract
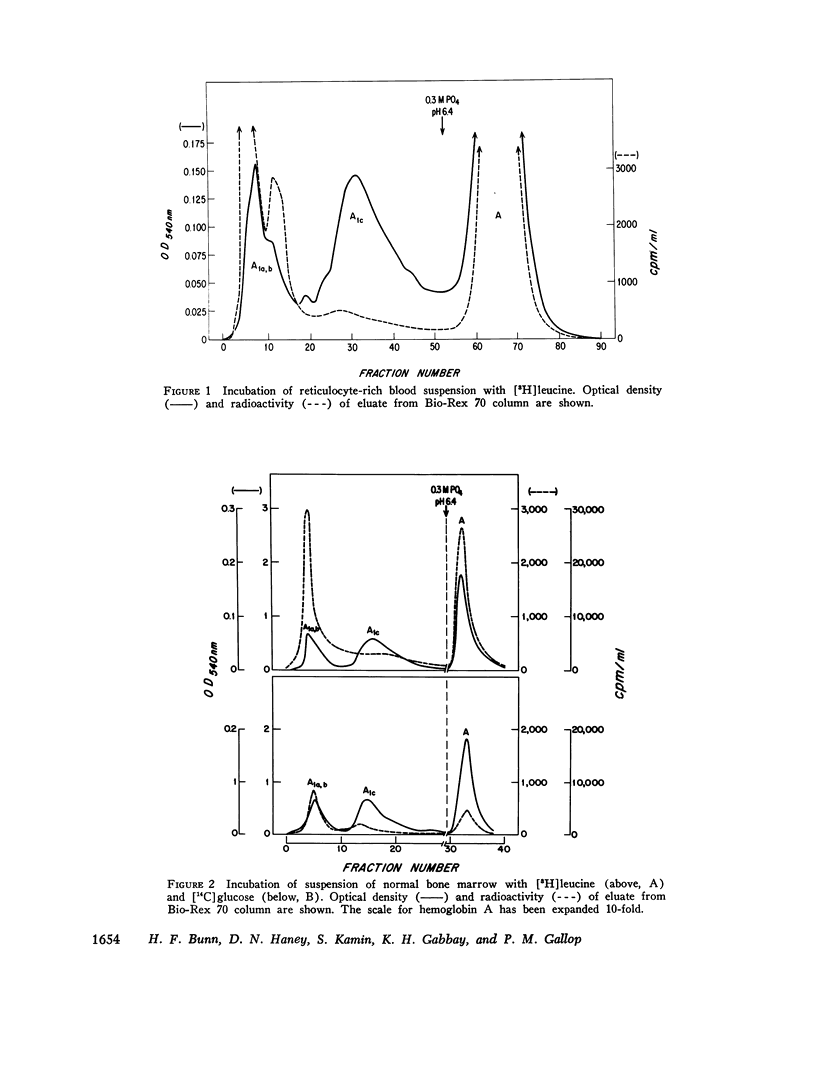
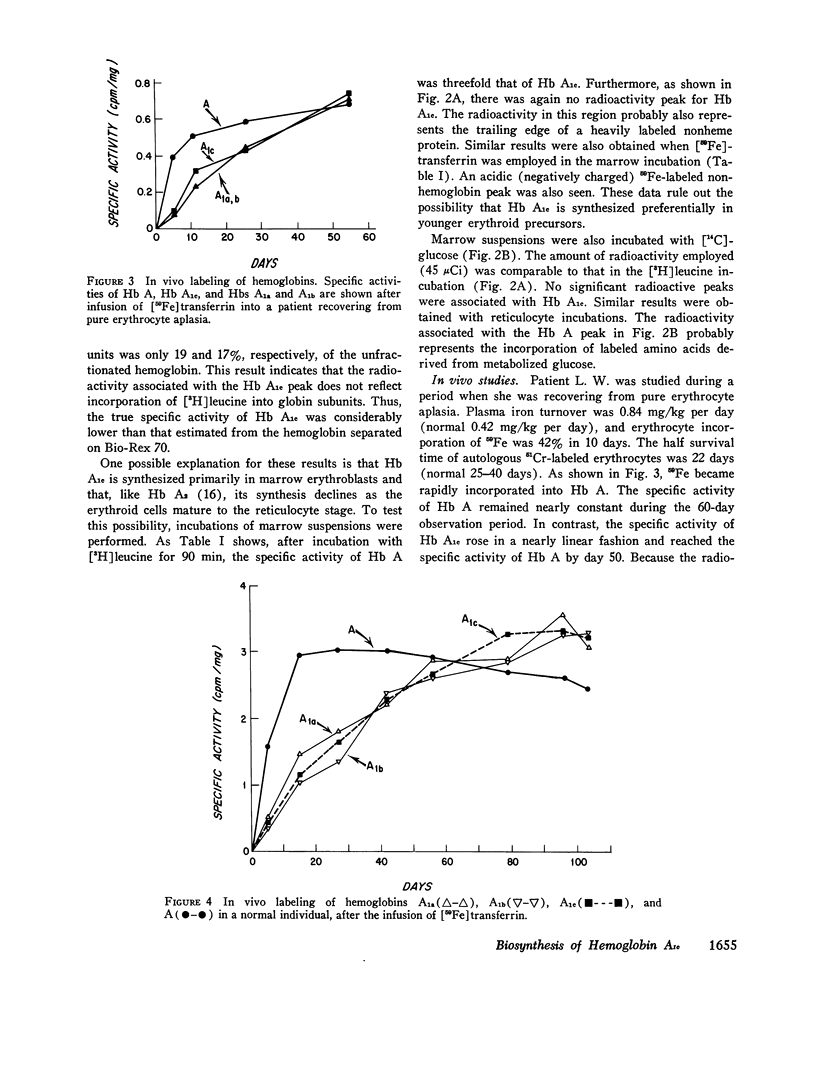
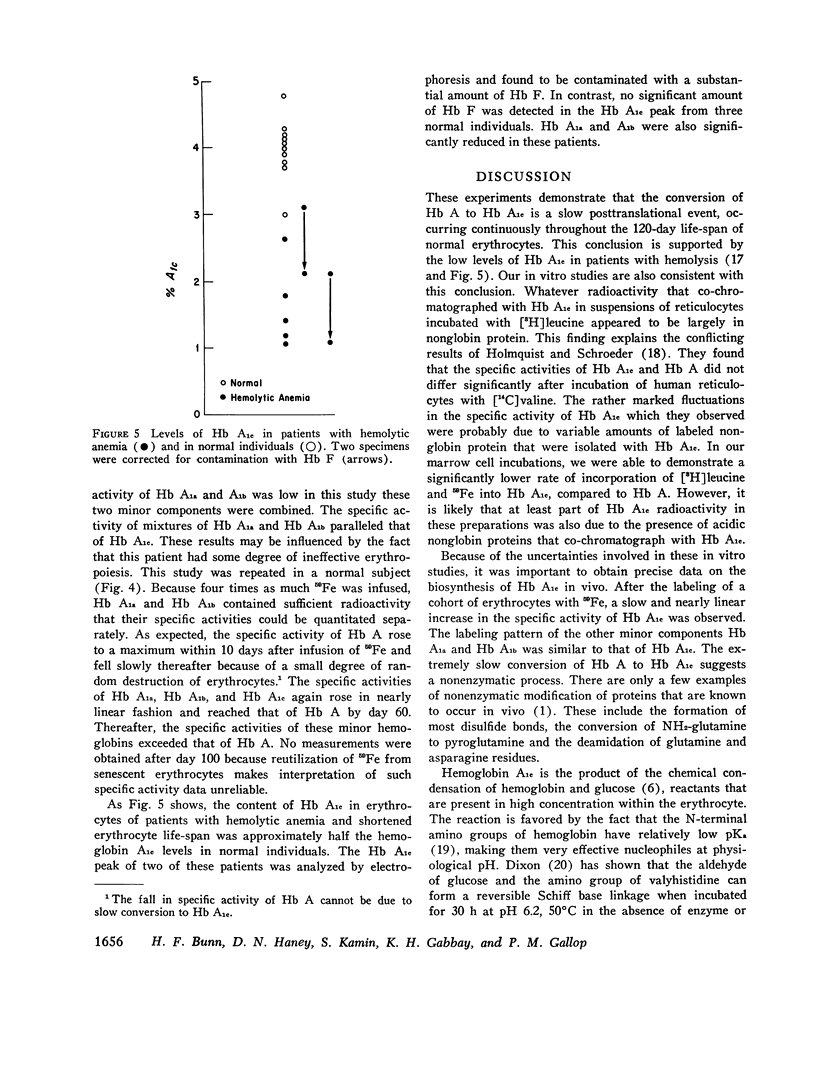
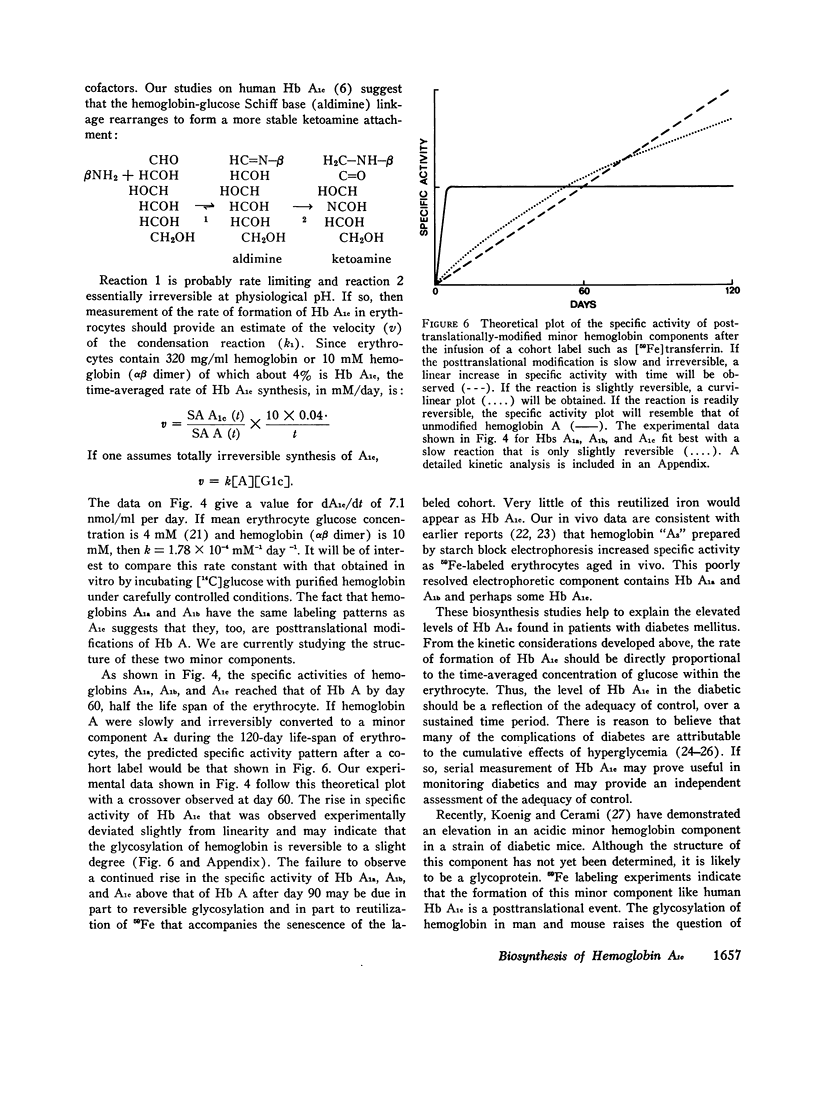
Hemoglobin A1c, the most abundant minor hemoglobin component in human erythrocytes, is formed by the condensation of glucose with the N-terminal amino groups of the beta-chains of Hb A. The biosynthesis of this glycosylated hemoglobin was studied in vitro by incubating suspensions of reticulocytes and bone marrow cells with [3H]leucine or 59Fe-bound transferrin. In all experiments, the specific activity of Hb A1c was significantly lower than that of Hb A, suggesting that the formation of Hb A1c is a posttranslational modification. The formation of Hb A1c in vivo was determined in two individuals who were given an infusion of 59Fe-labeled transferrin. As expected, the specific activity of Hb A rose promptly to a maximum during the 1st week and remained nearly constant thereafter. In contrast, the specific activity of Hb A1c and also of Hbs A1a and A1b rose slowly, reaching that of Hb A by about day 60. These results indicate that Hb A1c is slowly formed during the 120-day life-span of the erythrocyte, probably by a nonenzymatic process. Patients with shortened erythrocyte life-span due to hemolysis had markedly decreased levels of Hb A1c.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Haney D. N., Gabbay K. H., Gallop P. M. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochem Biophys Res Commun. 1975 Nov 3;67(1):103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Dixon H. B. A reaction of glucose with peptides. Biochem J. 1972 Aug;129(1):203–208. doi: 10.1042/bj1290203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay K. H. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annu Rev Med. 1975;26:521–536. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Paz M. A. Posttranslational protein modifications, with special attention to collagen and elastin. Physiol Rev. 1975 Jul;55(3):418–487. doi: 10.1152/physrev.1975.55.3.418. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Bogardt R. A., Jr, Gurd F. R. Determination of the pK values for the alpha-amino groups of human hemoglobin. J Biol Chem. 1975 Jun 25;250(12):4398–4404. [PubMed] [Google Scholar]
- HORTON B. F., HUISMAN T. H. STUDIES ON THE HETEROGENEITY OF HAEMOGLOBIN. VII. MINOR HAEMOGLOBIN COMPONENTS IN HAEMATOLOGICAL DISEASES. Br J Haematol. 1965 May;11:296–304. doi: 10.1111/j.1365-2141.1965.tb06589.x. [DOI] [PubMed] [Google Scholar]
- HUISMAN T. H., MEYERING C. A. Studies on the heterogeneity of hemoglobin. I. The heterogeneity of different human hemoglobin types in carboxymethylcellulose and in amberlite IRC-50 chromatography qualitative aspects. Clin Chim Acta. 1960 Jan;5:103–123. doi: 10.1016/0009-8981(60)90098-x. [DOI] [PubMed] [Google Scholar]
- Holmquist W. R., Schroeder W. A. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2489–2503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Holquist W. R., Schroeder W. A. The in vitro biosynthesis of hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2504–2512. doi: 10.1021/bi00872a003. [DOI] [PubMed] [Google Scholar]
- Jensen M., Nathan D. G., Bunn H. F. The reaction of cyanate with the alpha and beta subunits in hemoglobin. Effects of oxygenation, phosphates, and carbon dioxide. J Biol Chem. 1973 Dec 10;248(23):8057–8063. [PubMed] [Google Scholar]
- KUNKEL H. G., BEARN A. G. Minor hemoglobin components of normal human blood. Fed Proc. 1957 Sep;16(3):760–762. [PubMed] [Google Scholar]
- Koenig R. J., Cerami A. Synthesis of hemoglobin AIc in normal and diabetic mice: potential model of basement membrane thickening. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3687–3691. doi: 10.1073/pnas.72.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Sutherland D. E., Najarian-S, Michael A. F., Brown D. M. Studies of the rate of regression of the glomerular lesions in diabetic rats treated with pancreatic islet transplantation. Diabetes. 1975 Mar;24(3):280–285. doi: 10.2337/diab.24.3.280. [DOI] [PubMed] [Google Scholar]
- Paulsen E. P. Hemoglobin A1 C in childhood diabetes. Metabolism. 1973 Feb;22(2):269–271. doi: 10.1016/0026-0495(73)90170-4. [DOI] [PubMed] [Google Scholar]
- RANNEY H. M., KONO P. Studies of the incoporation of Fe59 into normal and abnormal hemoglobins. J Clin Invest. 1959 Mar;38(3):508–515. doi: 10.1172/JCI103828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIEDER R. F., WEATHERALL D. J. STUDIES ON HEMOGLOBIN BIOSYNTHESIS: ASYNCHRONOUS SYNTHESIS OF HEMOGLOBIN A AND HEMOGLOBIN A2 BY ERYTHROCYTE PRECURSORS. J Clin Invest. 1965 Jan;44:42–50. doi: 10.1172/JCI105125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta. 1968 Oct;22(2):296–298. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Biochemistry of the renal glomerular basement membrane and its alterations in diabetes mellitus. N Engl J Med. 1973 Jun 21;288(25):1337–1342. doi: 10.1056/NEJM197306212882506. [DOI] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Vogler N. J., Kilo C. Early capillary basement membrane changes in subjects with diabetes mellitus. Adv Metab Disord. 1973;2(Suppl):363–371. doi: 10.1016/b978-0-12-027362-1.50044-3. [DOI] [PubMed] [Google Scholar]


