Abstract
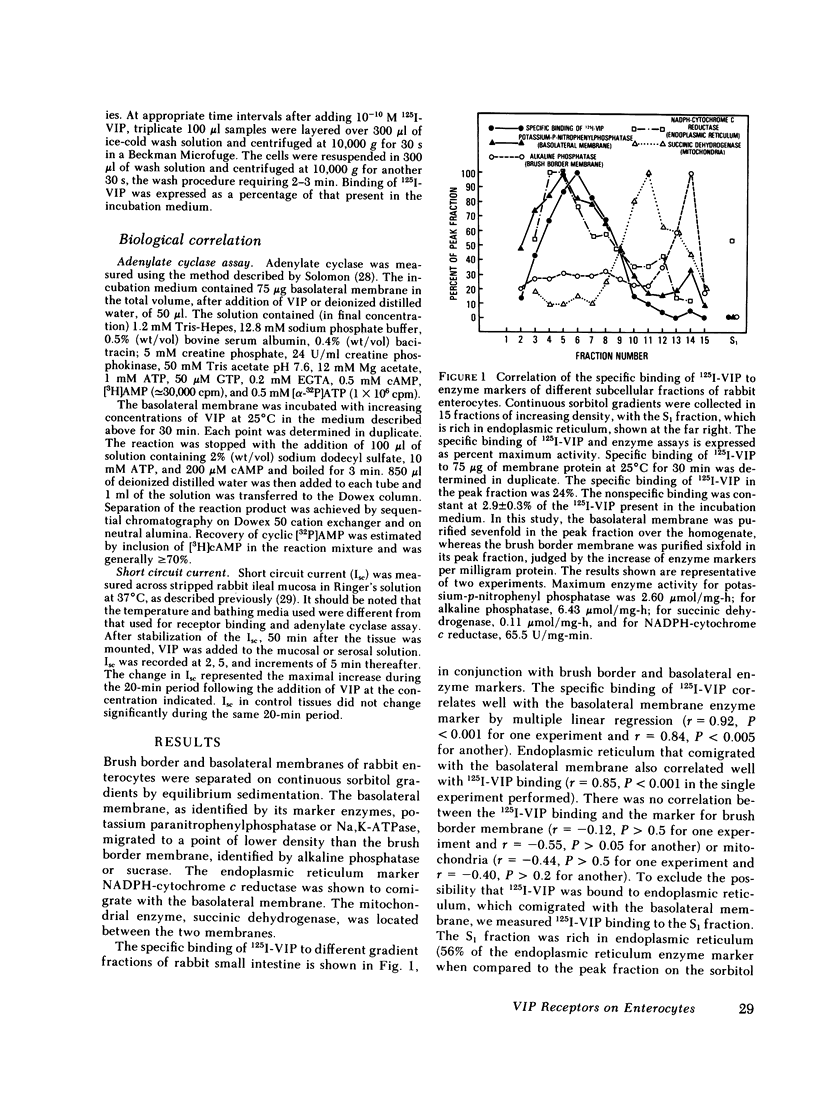
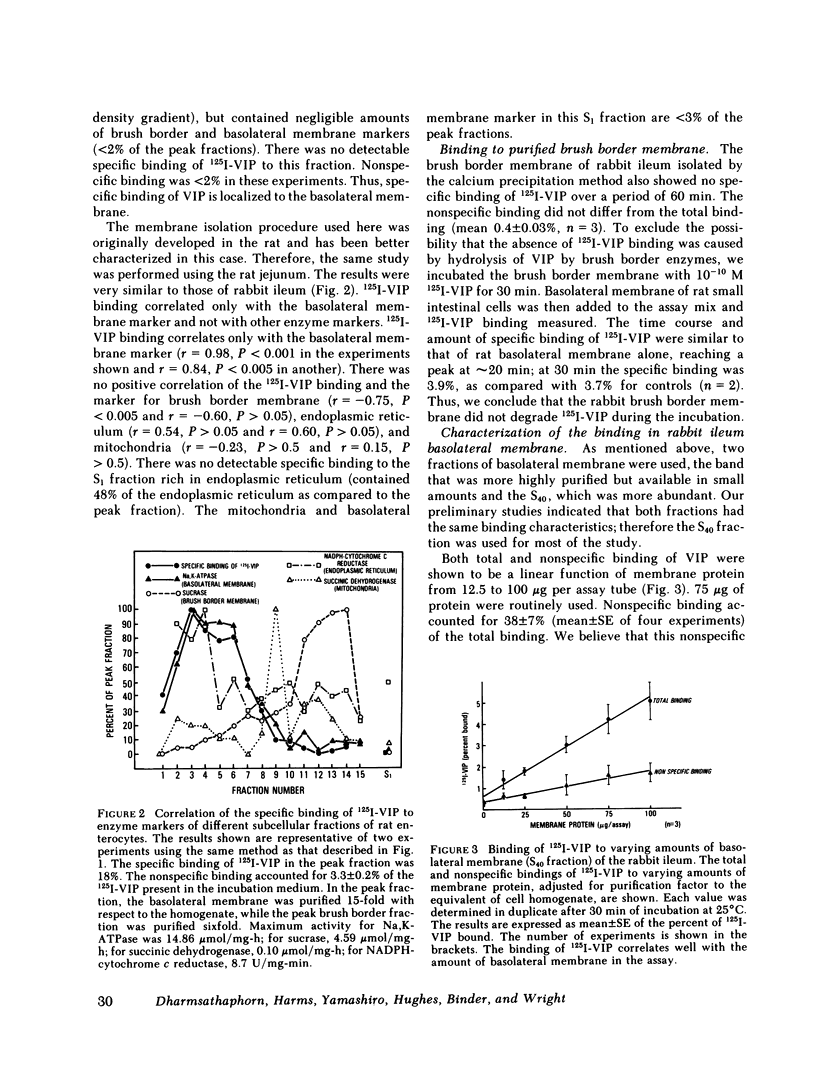
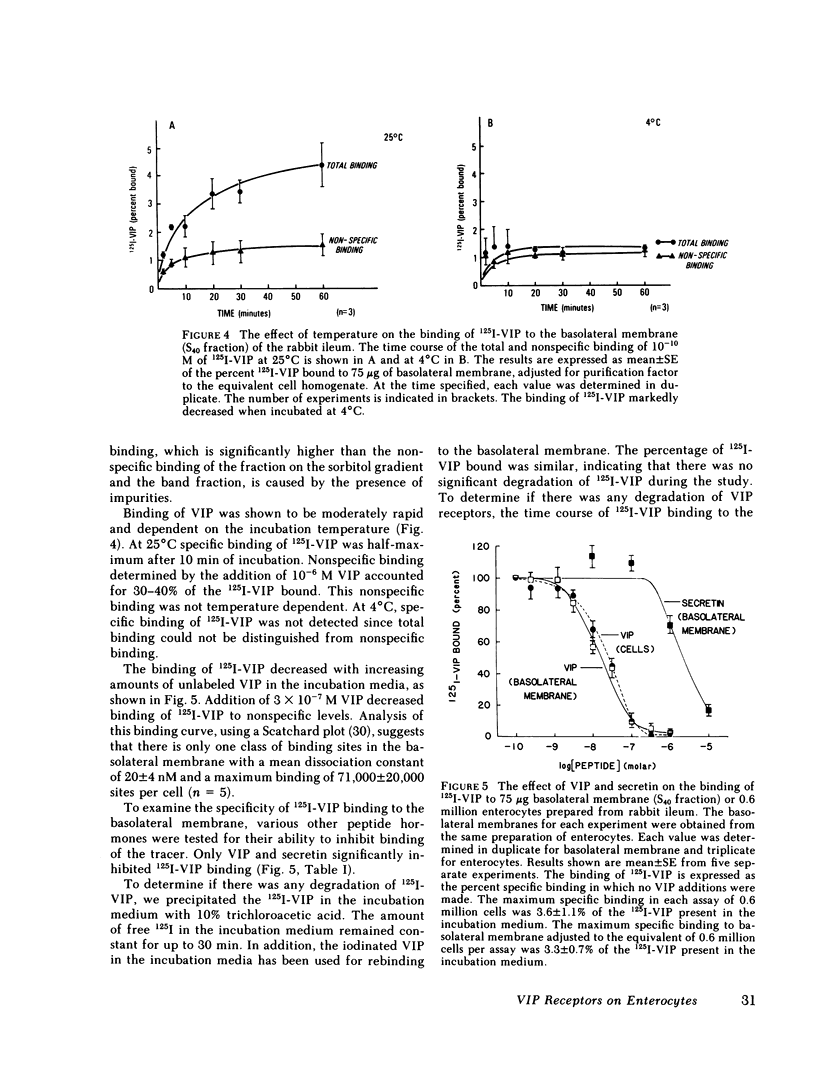
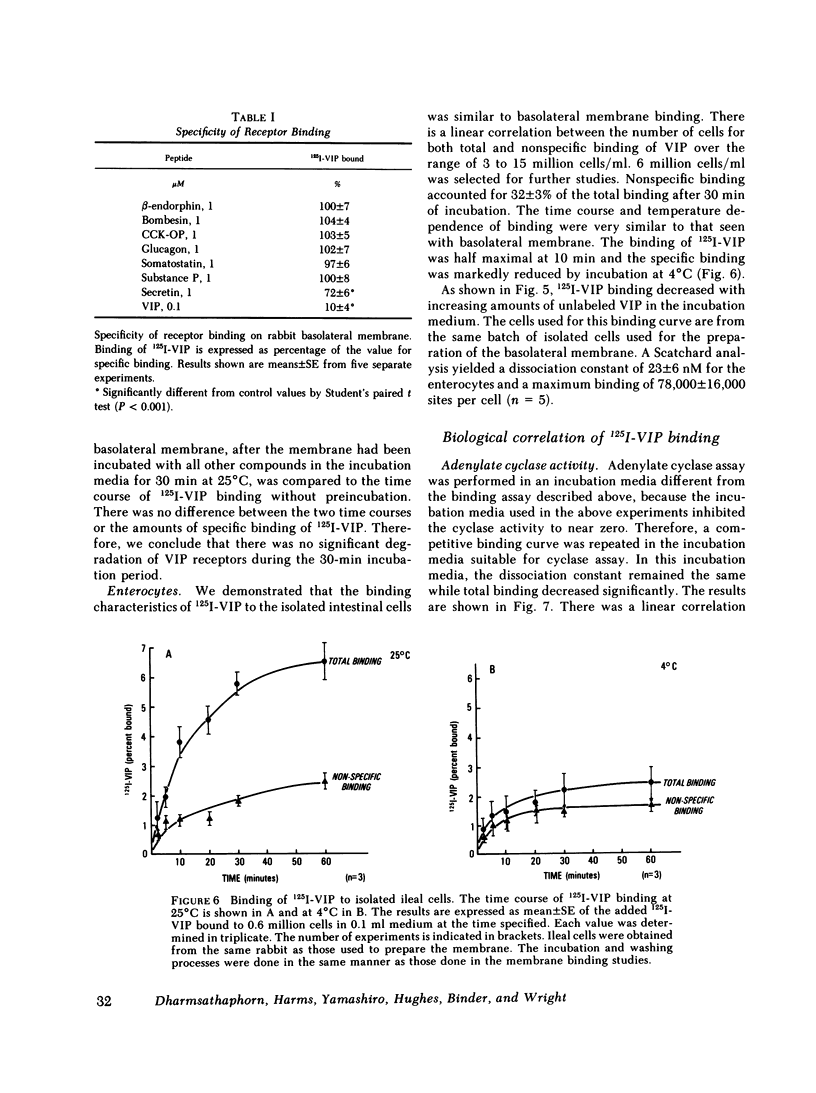
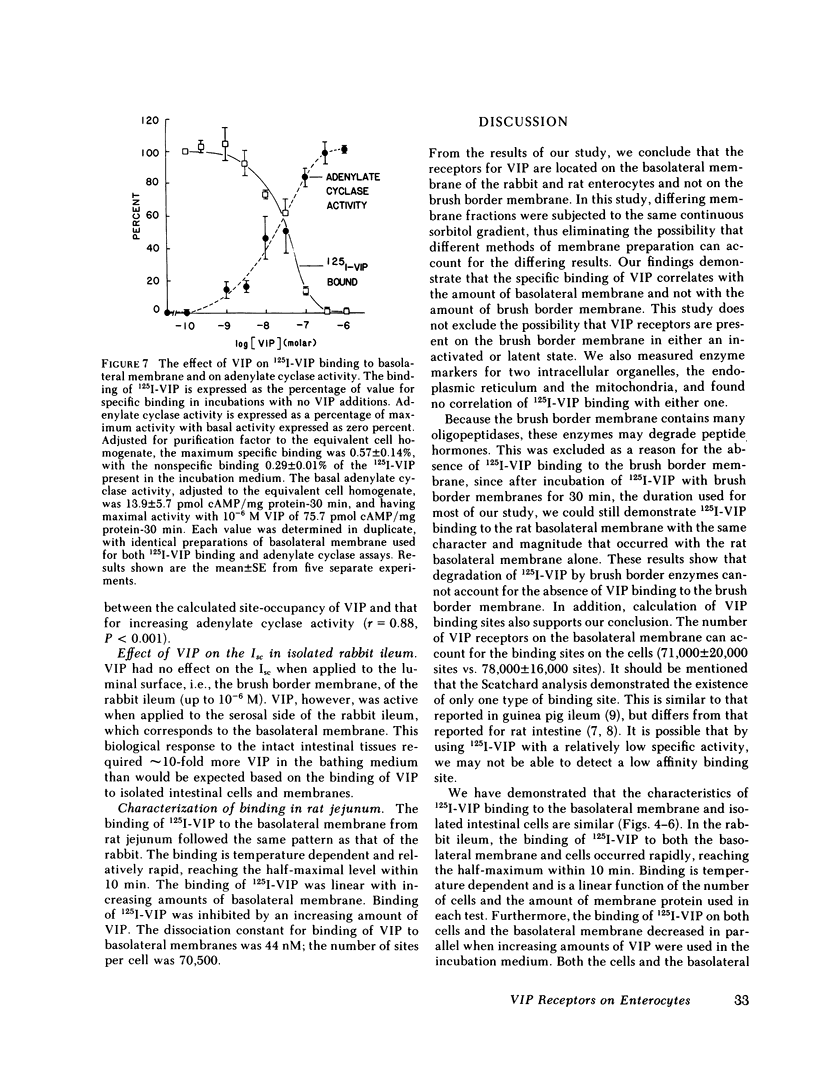
Binding of radioiodinated vasoactive intestinal polypeptide (VIP) to intestinal cell membranes of the rabbit ileum and rat jejunum was investigated. Specific binding of 125I-labeled VIP could be demonstrated only on the basolateral membrane and not on the brush border membrane. This corresponded with the lack of an effect on ion transport when VIP was applied to the mucosal side of an in vitro preparation of rabbit ileum. VIP altered ion transport only when it was applied to the serosal side. The binding of 125I-VIP was specific and dependent upon incubation temperature. There was a close correlation between the potency of VIP for inhibition of 125I-VIP binding and that for increasing adenylate cyclase activity. These observations demonstrate that VIP receptors are located on the basolateral membrane.
Full text
PDF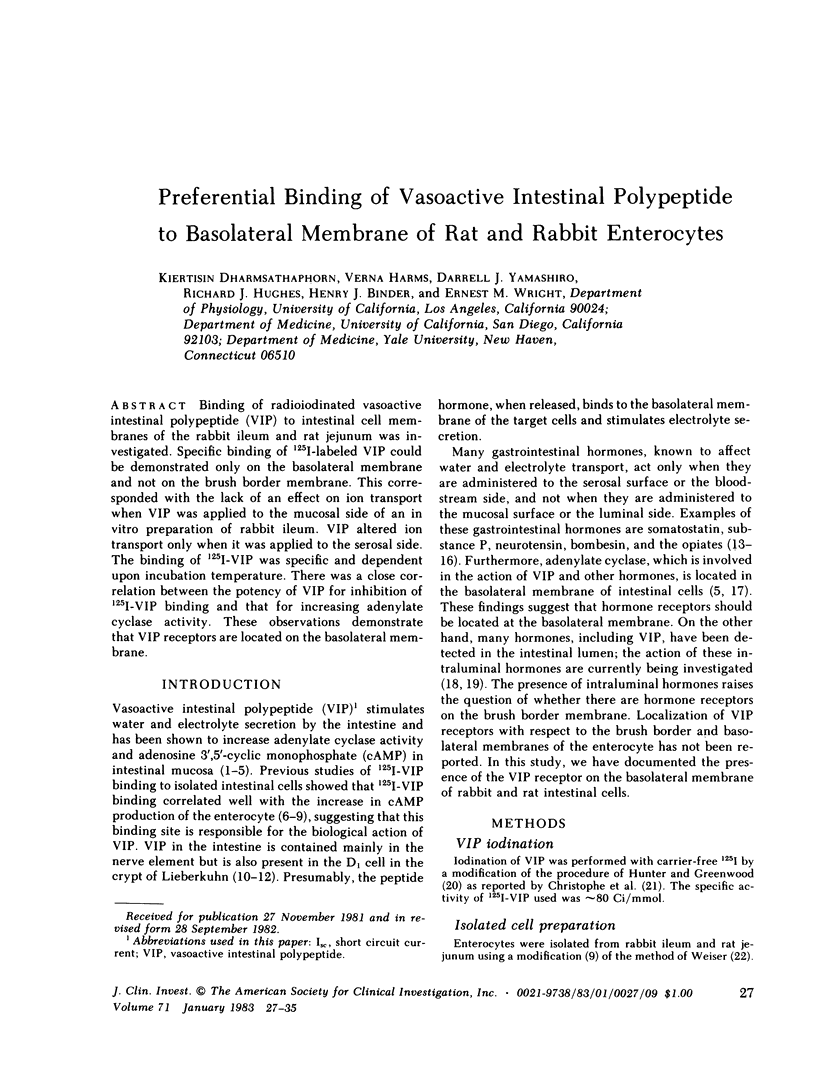



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder H. J., Lemp G. F., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on small intestinal epithelial cells. Am J Physiol. 1980 Mar;238(3):G190–G196. doi: 10.1152/ajpgi.1980.238.3.G190. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Powell D. W., Tai Y. H., Curran P. F. Electrolyte transport in rabbit ileum. Am J Physiol. 1973 Oct;225(4):776–780. doi: 10.1152/ajplegacy.1973.225.4.776. [DOI] [PubMed] [Google Scholar]
- Broyart J. P., Dupont C., Laburthe M., Rosselin G. Characterization of vasoactive intestinal peptide receptors in human colonic epithelial cells. J Clin Endocrinol Metab. 1981 Apr;52(4):715–721. doi: 10.1210/jcem-52-4-715. [DOI] [PubMed] [Google Scholar]
- Christophe J. P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. J Biol Chem. 1976 Aug 10;251(15):4629–4634. [PubMed] [Google Scholar]
- Davis G. R., Santa Ana C. A., Morawski S. G., Fordtran J. S. Effect of vasoactive intestinal polypeptide on active and passive transport in the human jejunum. J Clin Invest. 1981 Jun;67(6):1687–1694. doi: 10.1172/JCI110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Walsh J. H. Evidence for and significance of the projection of VIP neurons from the myenteric plexus to the taenia coli in the guinea pig. Gastroenterology. 1981 Jun;80(6):1557–1561. [PubMed] [Google Scholar]
- Garrahan P. J., Pouchan M. I., Rega A. F. Potassium activated phosphatase from human red blood cells. The mechanism of potassium activation. J Physiol. 1969 Jun;202(2):305–327. doi: 10.1113/jphysiol.1969.sp008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guandalini S., Kachur J. F., Smith P. L., Miller R. J., Field M. In vitro effects of somatostatin on ion transport in rabbit intestine. Am J Physiol. 1980 Feb;238(2):G67–G74. doi: 10.1152/ajpgi.1980.238.2.G67. [DOI] [PubMed] [Google Scholar]
- Guerrero J. M., Prieto J. C., Elorza F. L., Ramirez R., Goberna R. Interaction of vasoactive intestinal peptide with human blood mononuclear cells. Mol Cell Endocrinol. 1981 Feb;21(2):151–160. doi: 10.1016/0303-7207(81)90052-6. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J., Field M., Rivier J. Neurohumoral control of ileal electrolyte transport. I. Bombesin and related peptides. J Pharmacol Exp Ther. 1982 Mar;220(3):449–455. [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J., Field M., Rivier J. Neurohumoral control of ileal electrolyte transport. II. Neurotensin and substance P. J Pharmacol Exp Ther. 1982 Mar;220(3):456–463. [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Prieto J. C., Amiranoff B., Dupont C., Hui Bon Hoa D., Rosselin G. Interaction of vasoactive intestinal peptide with isolated intestinal epithelial cells from rat. 2. Characterization and structural requirements of the stimulatory effect of vasoactive intestinal peptide on production of adenosine 3':5'-monophosphate. Eur J Biochem. 1979 May 15;96(2):239–248. doi: 10.1111/j.1432-1033.1979.tb13034.x. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay J. S., Linaker B. D., Turnberg L. A. Influence of opiates on ion transport across rabbit ileal mucosa. Gastroenterology. 1981 Feb;80(2):279–284. [PubMed] [Google Scholar]
- Mircheff A. K., Wright E. M. Analytical isolation of plasma membranes of intestinal epithelial cells: identification of Na, K-ATPase rich membranes and the distribution of enzyme activities. J Membr Biol. 1976 Sep 17;28(4):309–333. doi: 10.1007/BF01869703. [DOI] [PubMed] [Google Scholar]
- Mircheff A. K., van Os C. H., Wright E. M. Pathways for alanine transport in intestinal basal lateral membrane vesicles. J Membr Biol. 1980 Jan 31;52(1):83–92. doi: 10.1007/BF01869009. [DOI] [PubMed] [Google Scholar]
- Parkinson D. K., Ebel H., DiBona D. R., Sharp G. W. Localization of the action of cholera toxin on adenyl cyclase in mucosal epithelial cells of rabbit intestine. J Clin Invest. 1972 Sep;51(9):2292–2298. doi: 10.1172/JCI107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Garaud J. C., Bloom S. R. Cellular localization of a vasoactive intestinal peptide in the mammalian and avian gastrointestinal tract. Gut. 1974 Sep;15(9):720–724. doi: 10.1136/gut.15.9.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen L. C., Binder H. J. Alteration of large intestinal electrolyte transport by vasoactive intestinal polypeptide in the rat. Gastroenterology. 1977 Oct;73(4 Pt 1):790–796. [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Sheerin H. E., Field M., Said S. I. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest. 1974 Sep;54(3):536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talor Z., Emmanouel D. S., Katz A. I. Insulin binding and degradation by luminal and basolateral tubular membranes from rabbit kidney. J Clin Invest. 1982 May;69(5):1136–1146. doi: 10.1172/JCI110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Pert C. B. Vasoactive intestinal polypeptide: specific binding to rat brain membranes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):660–664. doi: 10.1073/pnas.76.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman D. B., Gardner J. D., Zfass A. M., Makhlouf G. M. Effects of vasoactive intestinal peptide, secretin, and related peptides on rat colonic transport and adenylate cyclase activity. Gastroenterology. 1977 Sep;73(3):518–523. [PubMed] [Google Scholar]
- Walling M. W., Mircheff A. K., Van Os C. H., Wright E. M. Subcellular distribution of nucleotide cyclases in rat intestinal epithelium. Am J Physiol. 1978 Nov;235(5):E539–E545. doi: 10.1152/ajpendo.1978.235.5.E539. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wright E. M., van Os C. H., Mircheff A. K. Sugar uptake by intestinal basolateral membrane vesicles. Biochim Biophys Acta. 1980 Mar 27;597(1):112–124. doi: 10.1016/0005-2736(80)90155-8. [DOI] [PubMed] [Google Scholar]



