Abstract
This review focuses on phytase functionality in the digestive tract of farmed non-ruminant animals and the factors influencing in vivo phytase enzyme activity. In pigs, feed phytase is mainly active in the stomach and upper part of the small intestine, and added phytase activity is not recovered in the ileum. In poultry, feed phytase activities are mainly found in the upper part of the digestive tract, including the crop, proventriculus and gizzard. For fish with a stomach, phytase activities are mainly in the stomach. Many factors can influence the efficiency of feed phytase in the gastrointestinal tract, and they can be divided into three main groups: (i) phytase related; (ii) dietary related and (iii) animal related. Phytase-related factors include type of phytase (e.g. 3- or 6-phytase; bacterial or fungal phytase origin), the pH optimum and the resistance of phytase to endogenous protease. Dietary-related factors are mainly associated with dietary phytate content, feed ingredient composition and feed processing, and total P, Ca and Na content. Animal-related factors include species, gender and age of animals. To eliminate the antinutritional effects of phytate (IP6), it needs to be hydrolyzed as quickly as possible by phytase in the upper part of the digestive tract. A phytase that works over a wide range of pH values and is active in the stomach and upper intestine (along with several other characteristics and in addition to being refractory to endogenous enzymes) would be ideal. © 2014 The Authors. Journal of the Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: phytase activity, digestive tract, pigs, poultry, fish
Introduction
Microbial phytase is the most commonly used exogenous enzyme in the feed for monogastric animals. Phytase can reduce the antinutritional effect of phytate and improve the digestibility of phosphorous (P), calcium, amino acids and energy, as well as reduce the negative impact of inorganic P excretion to the environment. The benefits of using phytase in animal feed are well recognized.
The first generation of commercially available microbial phytases was marketed in 1991.1 Since then, new generations of phytase have been developed and have become commercially available. These different generations of phytase vary in their efficacy.2 The activity of phytase is commonly expressed as FTU, which is defined as the amount of phytase that liberates 1 mmol of inorganic phosphate per minute from 0.0051 mol L−1 sodium phytate at pH 5.5 and at a temperature of 37 °C.3 The pH level in the stomach of animals is far below pH 5.5 and therefore the 'real' activity in vivo is different from the standard phytase activity measurement. In addition, many phytase characteristics, coupled with dietary and animal-related factors, can have an influence on phytase activity in vivo. Adeola and Cowieson2 indicated that the effect of microbial phytase on inorganic P release depends on dietary phytate concentration, the source of phytate, species and age of animals, mineral concentrations in the diet, phytase sources and phytase dosing.
Many literature reviews are available but these are mainly focused on the effect of phytase on nutrient utilization and the performance of farmed animals.2,4–6 To our knowledge, the functionality of phytase in the digestive tract of farmed animals and the factors influencing in vivo phytase enzyme activity have not been critically reviewed. Such a review will provide a better understanding of the function and efficacy of microbial phytase. Therefore the objective of this paper was to focus on the factors influencing in vivo phytase activity in the digestive tract of pigs, poultry and some fish species. Recent literature data on ileal phytate degradation, phosphate release efficiency of different 'generations' of phytases and their associated matrix values will also be discussed.
Mode of Action of Phytase
Phytate and its antinutritional effect
Phytic acid is synthesized from myo-inositol via a series of phosphorylation steps; thus it consists of an inositol ring with six phosphate ester bonds (IP6; Fig. 1).7 Phytic acid is the primary phosphate storage compound in seeds, typically contributing 50–80% of total phosphate in plant seeds.8 It helps control effective germination, allowing for a P release boost when digested by seed phytase upon germination. The salt form of phytic acid is called phytate, and almost all phytic acid is present as a mixed salt (phytin). Phytate P is poorly available to animals and can reduce the digestibility of other nutrients and the performance of animals owing to its antinutritional effect.9
Figure 1.
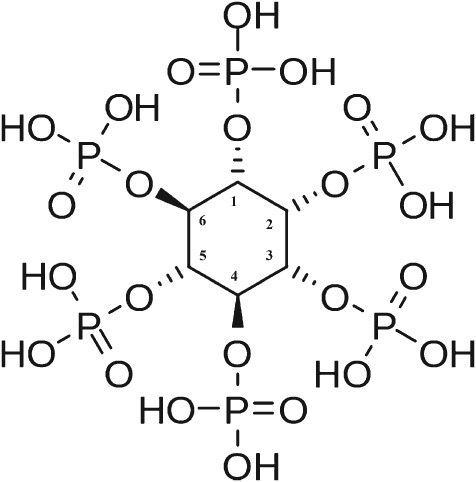
Structure of phytic acid (myo-inositol, 1,2,3,4,5,6-hexakisphosphate (IP6, IUPAC).7
Phytic acid has 12 replaceable reactive sites, carrying strong negative charges in the pH range of the digestive tract of animals. Phytic acid is able to bind di- and trivalent minerals and form very stable complexes, decreasing their availability as well as the availability of phytate P to animals.10 As phytic acid (and phytate) dissociates and is soluble at acidic pH (e.g. stomach), the formation of minerals and phytic acid complexes occurs mainly at the higher small intestinal pH.11 Animal diets contain high amounts of calcium compared to other cationic minerals; thus the phytic acids complex mainly with calcium in the small intestine. In addition, phytic acid can increase endogenous losses of minerals such as sodium in pigs and poultry.12,13 Sodium deficiency can have an impact on the activity of Na–K-ATPase in the gastrointestinal (GI) tract, which is involved in the absorption of nutrients. It has been reported that ingestion of phytic acid reduced the activity of Na–K-ATPase in the GI tract in broilers14 and piglets.15
Phytate also non-selectively binds to proteins and has been shown to inhibit enzymes including trypsin and α-amylase, thus reducing protein digestibility in animals.16,17 Phytic acid can bind protein over a wide pH range. At acidic pH (such as in the stomach) phytic acid binds to basic amino acids such as arginine, histidine and lysine, forming protein–phytate complexes. In the small intestine at a pH above the isoelectric point of proteins, phytic acid can bind protein through cations to form protein–mineral–phytate complexes.18,19 These complexes are insoluble and resistant to enzyme hydrolysis and thus reduce the efficiency of protein utilization. Phytic acid can interact with endogenous enzymes by rendering phytate-bound protein refractory to pepsin digestion, resulting in reduced nutrient digestibility. In addition, phytic acid can increase endogenous amino acid losses,20 due to increased secretion of digestive enzymes and mucins and reduced reabsorption of the endogenously secreted amino acids in the small intestine.9 It has been suggested that Ca phytate may increase the formation of metallic soaps in the gut lumen, resulting in reduced digestion of saturated fats.21,22 These antinutritional effects will consequently result in reduced nutrient utilization, increased maintenance protein and energy costs and reduced energy availability for production.
The antinutritional effects of phytate have long been recognized and are well reviewed.9,10
Different generations of phytases
The first and most extensively studied group of phytases is classified as histidine acid phosphatases (HAPs); other phytase classes are β-propeller phytase (BPPhy, also referred to as alkaline phytase), purple acid phytase and protein tyrosine phosphatase. Currently commercial feed phytases all belong to the HAPs and are acidic phosphatases.1 The first generation of commercialized phytase was a fungal phytase (Aspergillus niger) that was launched in 1991. In 1999, it was discovered that E. coli acid phosphatases were more effective than fungal phytase.23,24 This led to the development of new generations of bacterial phytases, which may be superior in several ways to the first generation of fungal phytases as feed additives.1 These phytases can be divided into 3- and 6-phytases, depending on the carbon site of hydrolysis of phytic acid (position 3 vs. 6, Fig. 1). Some examples of currently commercial available 3- and 6-phytases and their characteristics are presented in Table1. These commercial phytases differ in optimal pH, resistance to endogenous protease and affinity to phytate substrate, which may be the main factors influencing their in vivo efficacy. It has been observed that the new-generation bacterial phytases have a very specific affinity for IP6 and IP5 and possess higher resistance to proteolytic digestion than fungal phytase, which may partly explain their higher efficacy reported in trial studies.2
Table 1.
Some examples of currently commercially available 3- and 6-phytases and their characteristics
| Type† | Protein origin | Expression | pH optima | Temperature optima (°C) | Trade name |
|---|---|---|---|---|---|
| 3 | A. niger* | A. niger | 2; 5–5.5 | 65 | Natuphos® |
| 3 | A. niger* | A. niger, non-recombinant | 6.0 | – | Allzyme® SSF |
| 3 | A. niger* | Trichoderma reesei | 2.5 | – | Finase® P/L |
| 6 | Escherichia coli* | Schizosaccharomyces pombe (ATCC 5233) | 4.5 | 55 | Phyzyme® XP |
| 6 | Escherichia coli* | Pichia pastoris | 4.5 | – | Quantum® |
| 6 | Escherichia coli | Trichoderma reesei | — | – | Quantum Blue® |
| 6 | Escherichia coli* | Pichia pastoris | 3.4, 5.0 | 58 | OptiPhos® |
| 6 | Peniophora lycii* | Aspergillus oryzae | 4–4.5 | 50–55 | Ronozyme® |
| 6 | Citrobacter braakii | Aspergillus oryzae | — | — | Ronozyme Hiphos® |
| 6 | Buttiauxella spp. | Trichoderma reesei | 3.5–4.5# | 60# | Axtra® PHY |
Adapted from Lei et al.1 with modifications;
3- or 6-phytase; —, no information available;
personal communication (C Evans).
Phytase mode of action
Phytase (myo-inositol hexakisphosphate phosphohydrolase) catalyzes the stepwise removal of phosphate from phytic acid or its salt phytate.25,26 The removal of the phosphate group starts with a fully phosphorylated phytic acid (IP6), followed by penta- (IP5), tetra- (IP4), tri- (IP3), di- and mono-esters of inositol in descending order of preference. This means that the phytases first hydrolyze all of the available fully phosphorylated phytic acid to penta-esters of inositol before they hydrolyze the latter to tetra-esters of inositol and so on.25,26 In an ideal situation, a complete hydrolysis will result in a myo-inositol and phosphate (plus amino acids, minerals and other nutrients which are linked to phytic acid). However, in the in vivo situation, hydrolysis will be incomplete and therefore normally result in a mixture of inositol-phosphate esters (e.g. IP5, IP4, IP3).
In a recent publication, Yu et al.26 reported the protein binding capacity of IP6 and its esters (IP1–5, where the number indicates how many phosphates are linked to the inositol ring). In this study, IP6 was enzymatically hydrolyzed by either a fungal or an E. coli phytase, and the binding capacity of the resulting molecules to soy protein was tested. This included an appraisal of the various IP5 positional isomers (i.e. phosphate at different C positions of the inositol ring). The protein binding capacity was measured by a turbidity test, i.e. a high turbidity value (low solubility and high absorbance) shows a higher binding capacity. The results showed that IP6 has strong binding capacity to soy protein, while the absorbance of the system decreased rapidly with a decrease in phosphorylation from IP6 to IP3 (Fig. 2). IP1–4 have very low protein binding capacities, while IP5 is still active but to a much lesser degree than IP6 in binding soy proteins. In this study, it was observed that an intermediate product of E. coli 6-phytase (IP5 (1, 2, 3, 4, 5)) showed significantly less protein aggregation than the 2 IP5 isomers (IP5 (1, 3, 4, 5, 6) and IP5 (1, 2, 4, 5, 6)) generated by A. niger 3-phytase (6.6- vs. 4.6 fold turbidity reduction respectively). This demonstrated that there are differences between phytase sources, and the position of first hydrolyzation can have a significant impact on protein binding capacity. In this study, it was observed that the binding capacity to Fe3+ decreased proportionally from IP6 to IP3. For maximal alleviation of pepsin inhibition, IP6 needs to be broken down to IP1–2.26
Figure 2.
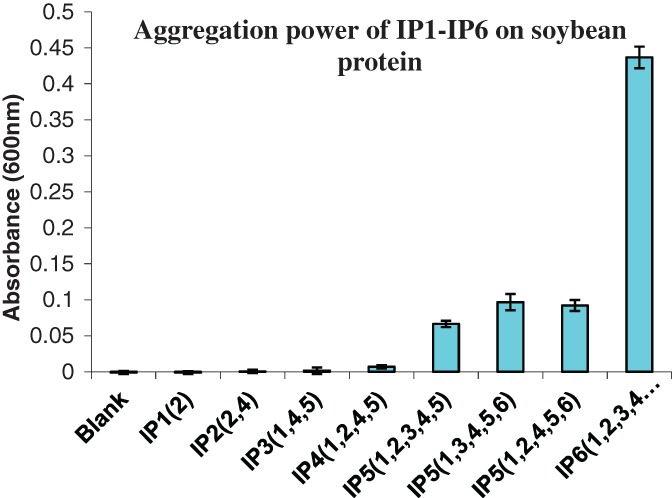
The aggregation of soy protein by myo-inositol phosphate esters (IP1–6) and IP5 positional isomers. Each data point is the average of four measurements with SD. Source: Yu et al.26
The phytate binding to Ca is also related to phytate ester composition, with a disproportionate decrease in the capacity of phytate to bind Ca, e.g. IP3 has approximately 11% of the binding affinity of IP6.2 Thus the rapid removal of IP6 and IP5 in the stomach will significantly reduce the binding of Ca in the small intestine.
Morales et al.27 reported that the negative effect of IP6 on binding of proteins occurs mainly in the acid environment (e.g. stomach). The authors observed that sodium phytate reduced protein solubility by up to 80% when casein was used as substrate and incubated with fish acid protease (pH 2.5, 16 °C, 180 min), whereas no reduction was found in protein solubility during intestinal digestion (pH 8.5). Similar results were found when soybean meal (SBM) was used as the substrate. A change of amino acid release of 60% was observed with an E. coli phytase during acid digestion, whereas no effect was observed during intestinal digestion.
Based on these data, it can be concluded that an early and thorough hydrolysis of phytate by phytase in the upper digestive tract is essential for an improved digestion of phosphorus, minerals (e.g. Ca, Fe) and protein. These data imply also that to eliminate the antinutritional effects of phytate IP6 needs to be hydrolyzed as completely as possible by phytase in the upper part of the digestive tract.
Factors Influencing Phytase Activity
Many factors can have an influence on in vivo phytase activities, including phytase-related factors such as optimal pH range, type of phytases and resistance to protease. Animal-related factors include species, age of animals and retention time. Dietary-related factors such as phytate content, calcium levels and ingredient composition (e.g. type of substrate and intrinsic phytase activity) are also very relevant.
Phytase enzyme-related factors
Optimal pH range
Phytase activity is measured as a phytase unit. In the official standardized phytase activity measurement, 1 unit is the amount of phytase that liberates 1 mmol of inorganic phosphate per minute from 0.0051 mol L−1 sodium phytate at pH 5.5 and at a temperature of 37 °C.3 Phytase units are commonly abbreviated as FTU, though other abbreviations including FYT, U and PU have also been used. In this review, only the abbreviation of FTU will be used.
As mentioned above, the most effective way to reduce the antinutritional effect of phytate is to fully hydrolyze phytate as quickly as possible in the upper part of the digestive tract.26 However, the pH in the stomach is considerably lower than pH 5.5, the pH used in the standardized phytase activity measurement. Therefore, the 'real' phytase activities of different commercial phytases vary considerably in vivo, owing to their diverse optimal pH characteristics. The optimal pH range will provide an indication of the effectiveness of a phytase in the stomach and upper part of the small intestine.
Relative activities of different commercial phytases have been published. Figure 3 shows some examples of the pH curves of different phytases reported in two studies.27,28
Figure 3.
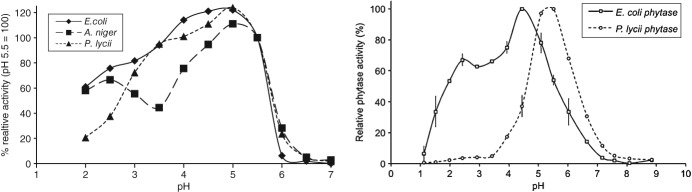
Relative activity of different commercial phytases. Left-hand figure compares three commercial phytases (A. niger, E. coli and P. lycii) when using the activity at pH 5.5 as 100%. Source: Kumar et al.28 Right-hand figure compares two commercial phytases (E. coli and P. lycii); the maximum phytase activity recorded was considered as 100%. Source: Morales et al.27
Although the different methodologies used in these studies may have a slight impact on the pH curves, it can be concluded from these studies that the efficiency of different commercial phytases varies over a pH range of 2.5–4.5 (the most relevant pH range in vivo). Figure 3 indicates that E. coli phytases are more active at a lower pH range (e.g. pH 2.5–4.5) than fungal phytases. In addition, the phytase activity curve at different pH values can vary for different E. coli phytases as well, due to the production technology and bacterial expression.
Tran et al.29 compared the activity of four commercial phytases (two E. coli phytases, A. niger phytase and P. lycii phytase) in a pH range of 2.0–8.5 using IP6–lysozyme complex as the substrate. It was observed that E. coli phytase variant 1 (Schizosaccharomyces pombe) showed the highest activity even when compared with E. coli variant 2 (Pichia pastoris) in the pH range 2–5.5. Aspergillus niger phytase showed low activity in the pH range 2.5–5, with two optimal peaks of activity at pH 5.5 and 2.0. The P. lycii phytase showed optimal activity around pH 4–5, which is comparable to the pH curve determined by Kumar et al.28 The activity of these different commercial phytase products in the pH range 2.5–5.5 is compared in Table2, the values being derived from figures in the study of Tran et al.29 Clearly, there are large differences in relative activity between these phytases (Table2).
Table 2.
Activity (OD600 × 10−3 min−1) of different commercial phytases at different pH values measured on IP6–lysozyme substrate complex
| pH | E. coli 1 | E. coli 2 | A. niger | P. lycii |
|---|---|---|---|---|
| 2.5 | 130 | 84 | 38 | 13 |
| 3.5 | 122 | 52 | 32 | 26 |
| 4.5 | 124 | 59 | 54 | 54 |
| 5.5 | 88 | 52 | 90 | 48 |
Reactions were carried out in 50 mmol L−1 glycine–HCl (pH 2.5–3.5) and 50 mmol L−1 sodium acetate (pH 3.5–5.5) containing 0.3 mmol L−1 IP6 and 0.23 mmol L−1 lysozyme in a total volume of 120 µL at 37 °C. The enzyme dose for each reaction was 0.1 FTU mL−1 based on inorganic P release from IP6 in conventional phytase activity assay. Values are derived from figures from the original paper.29
Phytase resistance to endogenous protease
Phytase is a protein molecule that can be hydrolyzed by endogenous protease in the digestive tract of animals. Kumar et al.28 evaluated the response of phytase derived from P. lycii, A. niger and E. coli to endogenous protease. These three phytases were incubated in a buffer containing protease for 2 h and the residual phytase was measured at pH 5. It was observed that the phytase derived from E. coli had greater protease resistance than P. lycii and A. niger phytase (Table3). The authors suggested that this might partially explain differences in bio-efficiency between commercial phytases in monogastric animals.
Table 3.
Percentage residual activities of different types of commercial phytases when treated with endogenous proteases for 2 h28
| E. coli | A. niger | P. lycii | |
|---|---|---|---|
| Pepsin | 76.7a | 31.4b | 5.42c |
| Trypsin | 23.0a | 0.45b | 1.25b |
| Chymotrypsin | 65.8a | 2.95b | 5.77b |
Means within a row without the same letter are significantly different (P <0.05).
Similarly, the high resistance of E. coli phytase to protease was also observed by Morales et al.27 Two commercial (E. coli and Peniophora lycii) phytases were incubated with pepsin or a gastric crude extract from rainbow trout.27 After 1 h incubation, the activity of P. lycii phytase reduced rapidly, while E. coli phytase maintained its activity over the 4 h incubation time (Fig. 4). There was no difference between pepsin and the gastric crude extract from trout. The results indicate that there is a large variation in the resistance of different commercial phytases to protease in the stomach, as indicated by the gastric crude extract from trout used in this incubation. The authors concluded that under the acidic pepsin gastric environment of fish with a stomach like that of rainbow trout E. coli phytase would be more active and stable than phytase from P. lycii. These in vitro results are in agreement with in vivo studies, where higher phytase activity in digesta of broilers was observed with E. coli phytase compared to P. lycii phytase.30 Also higher efficacy was reported with E. coli phytase compared to P. lycii phytase in both poultry and swine.31
Figure 4.
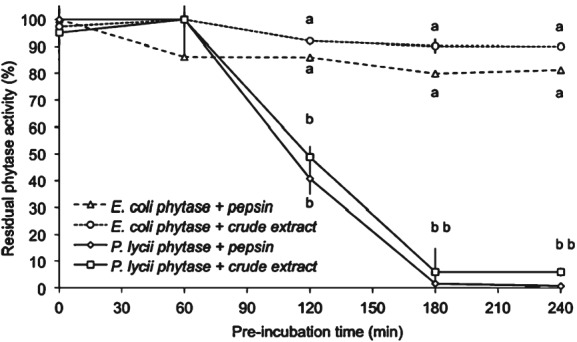
Residual phytase activity of E. coli and P. lycii phytase after incubation with pepsin or a gastric crude extract from trout stomach for up to 4 h. The incubation was performed by adding 1 FTU phytase to a protease solution with 5000 U pepsin or gastric crude extract from fish at pH 2.0 and 16 °C. Different letters indicate significant difference (P <0.05). Adapted from Morales et al.27
Phytase specific activity versus targeted substrates
Target substrates can also have some impact on phytase activity. Tran et al.29 carried out an assay at pH 3.0 and 37 °C to determine the relative activity of four commercial phytases on the hydrolyzation of IP6 from IP6–soy protein or IP6–lysozyme complex compared to sodium phytate. All phytases were added on an equal FTU basis (according to the standard measurement). As shown in Table4, at pH 3 phytase activity differs significantly between the commercial phytases and is related to target substrates. Compared to the standard substrate IP6–Na used in phytase activity measurement, higher variations in activities between different phytase sources were observed when soy protein and IP6–lysozyme were used as target substrates. Escherichia coli phytase showed increased activity with soy protein and IP6–lysozyme complex compared to IP6–Na, while A. niger phytase had reduced relative activity on these substrates. For E. coli phytases, the activity was higher with IP6–soy protein or IP6–lysozyme complex than IP6 sodium. Escherichia coli phytases hydrolyzed IP6 several fold faster than the fungal phytase at pH 3, and the efficacy varies due to different types of substrates.29 However, there was also a large difference in relative activity between the two E. coli phytases due to bacterial expression. Thus the affinity of phytase to different types of substrates can be one of the factors that influence the efficacy of the phytase.
Table 4.
Relative activity (%) of different phytases measured at pH 3 when using IP6 sodium, IP6–soy protein and IP6–lysozyme complex as substrates29
| IP6–Na+ | IP6–soy protein | IP6–lysozyme complex | |
|---|---|---|---|
| E. coli 1 (S. pombe) | 100 | 164 | 229 |
| E. coli 2 (P. pastoris) | 103 | 138 | 152 |
| A. niger | 37 | 32 | 23 |
| P. lycii | 10 | 25 | 13 |
The assay was carried out in a total volume of 120 µL in 50 mmol L−1 glycine–HCl (pH 3.0) at 37 °C for the different phytases added at a dose of 0.1 FTU mL−1. The reaction rate was measured as inorganic P release (µmol inorganic P mL−1 min−1) by stopping the reaction at different time intervals and analyzing inorganic P on Konelab. Activity of E. coli 1 phytase (0.096 µmol inorganic P mL−1 min−1) on IP6–Na + was set at 100%. Activities of the phytase on the other substrates were reported relative to the activity of E. coli 1 phytase on IP6–Na+.
Dietary-related factors
Phytate type and levels: enzyme:substrate ratio
In standard phytase activity measurement, sodium phytate is used as a substrate; however, the hydrolysis rate of phytate from natural feed ingredients by phytase is different from chemically synthesized sodium phytate. This difference has been demonstrated in many studies.32,33 Due to the difference in composition, levels and location of phytate (IP6), as well as the contribution of intrinsic phytase in some cereals and oilseeds, the rate of hydrolysis of phytate by microbial phytase can vary to a large extent in these plant-based ingredients.32
In broilers and laying hens, it was observed that the rate of hydrolysis of IP6 and total P retention differed significantly between feed ingredients (Table5).32 In this study, semi-purified diets were formulated so that the only phytate and total P sources were from the feed ingredients tested. The phytase used was a product produced from fungal A. niger at an inclusion level of 600 FTU kg−1 in broiler feed and 300 FTU kg−1 in layer feed.
Table 5.
In vivo hydrolysis of IP6 in different feed ingredients by exogenous phytase (data derived from Leske and Coon32)
| Ingredients | Total P (g kg−1)a | IP6 P (g kg−1)a | % IP6 P/total P | Hydrolysis of IP6 (%) |
Increase |
Total P retention (%) |
Diff. (% points) | IP6-P releaseb by 100 FTU phytase | % IP6-P released by 100 FTU phytase | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − phytase | + phytase | % points | IP-P release** | − phytase | + phytase | |||||||
| Broilers | ||||||||||||
| SBM | 2.15 | 1.12 | 52.1 | 34.9 | 72.4 | 37.5 | 0.42 | 27 | 58 | 31.0 | 0.070 | 6.25 |
| Corn | 1.77 | 1.43 | 80.8 | 30.8 | 59 | 28.2 | 0.40 | 34.8 | 40.9 | 6.1 | 0.067 | 4.66 |
| Rice bran | 5.96 | 4.8 | 80.5 | 33.2 | 48 | 14.8 | 0.71 | 15.5 | 26.5 | 11.0 | 0.118 | 2.47 |
| Canola meal | 3.51 | 2.41 | 68.7 | 36.7 | 55.8 | 19.1 | 0.46 | 39.4 | 45.7 | 6.3 | 0.077 | 3.18 |
| Barley | 2.22 | 1.68 | 75.7 | 32.2 | 71.3 | 39.1 | 0.66 | 40.3 | 55.5 | 15.2 | 0.110 | 6.55 |
| Wheat | 2.22 | 1.98 | 89.2 | 30.7 | 46.8 | 16.1 | 0.32 | 16 | 33.8 | 17.8 | 0.053 | 2.69 |
| Wheat midds | 3.96 | 3.5 | 88.4 | 29.1 | 52.2 | 23.1 | 0.81 | 31.9 | 43.4 | 11.5 | 0.135 | 3.86 |
| Laying hens | ||||||||||||
| SBM | 2.73 | 1.41 | 51.6 | 25.7 | 62.4 | 36.7 | 0.52 | 36.8 | 53.4 | 16.6 | 0.173 | 12.29 |
| Corn | 1.83 | 1.34 | 73.2 | 23 | 52 | 29.0 | 0.39 | 28.6 | 44.7 | 16.1 | 0.130 | 9.70 |
| Rice bran | 11.3 | 9.14 | 80.9 | 36.1 | 50.9 | 14.8 | 1.35 | 35.9 | 43 | 7.1 | 0.450 | 4.92 |
Analyzed value in test diets as fed basis. Birds were placed in the cages and fed experimental diets for 3 days, then excreta were collected for 48 and 72 h for the broiler and layer trial, respectively.
IP6-P release, expressed as g kg−1, is calculated as percentage increase in IP6-P hydrolysis multiplied by IP6-P content (g kg−1) in the feed.
The accessibility of phytate by phytase differs between different ingredients in broilers. For example, 600 FTU phytase released 0.42 and 0.46 g kg−1 IP6-P from SBM and canola meal respectively; however, as canola meal contained a higher amount of IP6 than SBM in the diet (2.41 vs. 1.12 g kg−1), this results in a higher percentage of IP6 degradation in SBM (37.5%) compared to canola (19%). The highest IP6-P release was observed with wheat middlings in broilers, e.g. 0.81 g kg−1 IP6-P, which was related to the high IP6 content. The addition of the A. niger phytase at 600 FTU kg−1 degraded phytate in a range from 15% to 39% in the tested ingredients in broilers. Therefore the IP6 levels in the ingredients (e.g. the enzyme:substrate ratio) may also play a role in the efficiency of IP6-P release.
When the IP6-P release is calculated per 100 FTU phytase and expressed as g kg−1 or percentage of IP6 in the diet (see Table5), IP6-P release by 100 FTU phytase in laying hens is clearly higher than in broilers. Compared to broilers, laying hen total P retention was greater for SBM without phytase supplementation. However, hydrolysis of IP6 of SBM without phytase supplementation by broilers was greater than in laying hens. This may be related to the difference in intestinal microbial balance (maturity) and digesta retention time between layers and broilers.
Morales et al.33 evaluated the effect of a microbial phytase on the increment in the bioavailability of P and protein in different plant ingredients by simulating conditions in the gastric environment of a fish. An in vitro assay was carried out which simulated the rainbow trout stomach (pH 3.0; 16 °C; 2000 U total acid protease from stomach extract), and eight plant-based ingredients were tested. The results showed that P and AA release as a percentage of control varied significantly between the plant ingredients tested (Table6).
Table 6.
Mean protein solubility increment (%, SDS-PAGE analysis), total soluble P released (mg g−1 DM) and total amino acids released (mg g−1 DM) from selected plant-derived ingredients after 240 min of incubation under simulated gastric conditions in fish in the presence or absence of an E. coli phytase
| Plant ingredients | Phytase treatment | % increase protein solubility | Total soluble P release (mg g−1 DM) | % increase over control | total amino acid release (mg g−1 DM) | % increase over control |
|---|---|---|---|---|---|---|
| Soybean meal | Control | 5.6 | 12.2 | |||
| Phytase | 54 | 14.2 | 153.6 | 14.2 | 16.4 | |
| Pea meal | Control | 3.6 | 11.2 | |||
| Phytase | 140 | 7.5 | 108.3 | 12.3 | 9.8 | |
| Broad bean meal | Control | 3.4 | 10.7 | |||
| Phytase | 189 | 10 | 194.1 | 11.9 | 11.2 | |
| Chickpea protein isolate | Control | 5.9 | 12 | |||
| Phytase | 25 | 17 | 188.1 | 12.5 | 4.2 | |
| Lupin meal | Control | 7.6 | 11.2 | |||
| Phytase | 12 | 11.9 | 56.6 | 11.5 | 2.7 | |
| Canola meal | Control | 6.7 | 9.4 | |||
| Phytase | 9 | 16.4 | 144.8 | 10.5 | 11.7 | |
| Wheat middling | Control | 7.4 | 8 | |||
| Phytase | 31 | 10.9 | 47.3 | 8.2 | 2.5 | |
| Wheat flour | Control | 3.6 | 6.9 | |||
| Phytase | 5 | 3.6 | 0 | 6.9 | 0 |
Source: Morales et al.33
In another study by the same authors,34 it was observed that the increment of protein solubility after phytase treatment was related to the protein fractions present. The protein fractions in which solubilization was most positively affected by the presence of phytase were convicilin, vicilin and legumin present in the seeds of pea and broad bean (369%, 181%, 113% increase respectively), as well as glycinin and conglycinin present in soybean (with a 56% and 51% increase respectively). Increased protein solubility will result in improved protein digestibility and therefore a higher release of AA. Glycinin and conglycinin are storage proteins in SBM which are antigenic and can cause an allergic response; this can result in intestinal damage, especially in young animals such as weaning piglets.35 It may be speculated that the increment in solubility of glycinin and conglycinin may increase protein digestibility of these soy proteins and reduce their antigenic effect in the intestine.
Cheng and Hardy36 observed that the effect of phytase on phytate P digestibility in rainbow trout was closely related to the type of ingredients and phytate P levels. Addition of 500 FTU phytase (A. niger) improved apparent digestibility of phytate P by 85, 81.6, 64.5 and 29.7 percentage points for barley, wheat, wheat middlings and canola meal respectively. The phytate P levels in barley, wheat, wheat middlings and canola meal-based diets were 0.6, 0.4, 2.1 and 2.7 g kg−1 respectively. Barley and wheat showed high digestibility, which may be mainly due to low dietary phytate P content. A canola meal-based diet had higher phytate P content than wheat middlings but showed much lower phytate P digestibility, indicating relatively low phytate P availability in canola meal compared to wheat middlings. The low accessibility of phytate in canola meal is in agreement with the observation of Newkirk and Classen,37 that a portion of the phytate in canola meal is resistant to phytase hydrolysis.
However, this is in contrast to the in vitro results,33 in which total soluble P release by phytase was higher in canola meal than wheat middlings, which may be due to the different type of phytases used and the ingredient processing methods. In addition, the in vitro method simulates the stomach digesta environment, while the in vivo study measured total tract phytate P digestibility. This may partially explain why different results were observed between the in vitro and in vivo studies.
Wheat or wheat by-products contain more endogenous phytase than canola meal;38 however, as the IP6-P release was calculated as the increase in phytate P digestibility in the test diet (with phytase) compared to control (without phytase), the impact of endogenous phytase is excluded as both test and control diets contained the same amount of endogenous phytase.
Other dietary factors
Many other dietary factors such as Ca:P ratio and inorganic P content in the diet may have an impact on the inorganic P release rate from phytate by phytase. As discussed above, phytate can bind to cations such as Fe3+ and Ca2+ in the small intestine, reducing the solubility of phytate and thereby reducing its accessibility by phytase. Animal feed contains high amounts of calcium; therefore calcium content in the diet can have a large impact on phytate P utilization and phytase efficacy. In addition, it has been observed that particle size of limestone can have an influence on the efficacy of phytase due to the high solubility of Ca in fine limestone.39
When no limestone was added to the diets (Ca content below 1.5 g kg−1 in the diet), Leytem et al.40 observed that ileal phytate P digestibility was 81% and 89% in barley and corn, respectively, in broilers without addition of microbial phytase. Similarly, Tamim et al.41 reported that in the absence of dietary Ca broilers were able to degrade 69.2% phytate P by the terminal ileum; however, this was reduced to 25.4% when dietary Ca level was increased to 0.5%. Plumstead et al.42 observed that increasing Ca from 4.7 to 11.6 g kg−1 in broiler diets linearly decreased ileal phytate P digestibility by 71%. In laying hens, van der Klis et al.43 reported that increasing dietary Ca from 30 to 40 g kg−1 reduced phytate P degradation from about 33 % to 9% in the diet without phytase supplementation. In the diet supplemented with 500 FTU kg−1 phytase, the reduction in degradation of phytate P was from about 76% to 65%. Similarly, Seynaeve et al.44 found that the addition of inorganic phosphate (MCP) and limestone reduced phytase activity in the small intestine. Cao et al.6 suggested that a Ca:P ratio in a range of 1.1–1.4:1 is optimal for phytase activity in fish feed. Increasing the Ca:P ratio may have a negative impact on phytase activities.45,46 Qian et al.45 reported that reducing dietary Ca:P ratio from 2:1 to 1.2:1 increased phytase efficiency by approximately 16% and improved performance and digestibility in weaning piglets. Similarly, Liu et al.46 concluded that lowering Ca:P ratio from 1.5:1 to 1:1 improved performance and P utilization in pigs fed low-P corn–SBM-based diets supplemented with phytase. Plumstead et al.42 report that the optimum ratio of Ca:nPP (non-phytate P) that resulted in the highest P retention and lowest P excretion was 2.53:1, 2.40:1 and 2.34:1 for diets with 0.28%, 0.24% and 0.10% phytate P. Increasing dietary Ca levels reduced the extent of phytate P hydrolysis and P digestibility and the optimum Ca:nPP ratio was reduced when diets contained less phytate P.
However, Adeola et al.47 observed that in weaning piglets fed a corn–SBM diet decreasing Ca:P ratio from 1.8 to 1.2 improved body weight gain and feed efficiency. Addition of 1000 FTU kg−1 E. coli phytase increased weight gain and feed efficiency regardless of Ca:P ratios. No interaction between phytase and Ca:P ratio was found. Similar results were reported in broilers, where addition of 1000 FTU kg−1 Buttiauxella phytase increased phytate P, total P and energy digestibility regardless of Ca:available-P ratios.48 Increasing dietary Ca: available-P ratio in the absence of phytase reduced bone ash, but in the presence of phytase bone ash was increased at higher Ca:available-P ratios. Phytate degradation was reduced at higher Ca :available-P ratio; however, no interaction between Ca:available-P ratio and phytase was found on phytate P degradation. In this study, phytase treatment degraded up to 88% of phytate at the terminal ileum. These data indicate that the phytases that efficiently hydrolyze phytate in the proximal intestinal tract may be less prone to inhibition by higher dietary Ca levels, as Ca binding to phytate occurs mainly in the small intestine when the pH level is above 5. Therefore the negative effect of Ca on phytate degradation may be reduced by using a phytase that is highly efficient at low pH and with a high dosage.
Literature studies on calcium and phytase interactions have been intensively reviewed by Selle et al.49
In addition to Ca, other dietary minerals can also have an impact on phytase efficacy. Augspurger et al.50 reported that pharmacological levels of zinc supplementation in the diet (1500 mg kg−1 in pigs and 800 mg kg−1 in chickens) reduced the phosphorus-releasing efficacy of phytase in young pigs and chickens; however, supplementation of 200 mg kg−1 Cu did not affect the response of chickens to phytase. Thus the effect may be related to the absolute supplementation levels of Zn and Cu and the duration of feeding, which may need to be investigated further.
Other factors such as organic acids and Na level may also have some impact on phytase activity in vivo. It was reported that citric acid and its salts, often used in blends of organic acids, increased phytate P utilization and the efficacy of microbial phytase in hydrolyzing phytic acid in vitro51 and in vivo in broilers.52,53 The addition of organic acids might be more beneficial in stomachless fish species that have a higher pH level in the intestine. For example, Phromkunthong et al.54 reported that the addition of citric acid (0.22%) increased the efficacy of a P. lycii phytase in common carp. Kemme et al.55 observed a significant interaction between lactic acid and phytase on apparent total tract digestibility in growing-finishing pigs fed a corn and SBM-based diet.
It was observed that Na concentration and dietary electrolyte balance (DEB) levels can have an impact on the effect of phytase.56 Diets with the highest DEB and Na concentrations were the least responsive to microbial phytase. It was suggested that greater dietary Na concentrations may reduce the antinutritive effect of phytate and the nutrient digestibility response to phytase.2
Phytase can be used in combination with other enzymes. It was observed that xylanase increases the permeability of the aleurone layer of wheat, which is the site of phytic acid storage.57 Thus the combination of xylanase with phytase may produce a greater effect than the enzymes used individually. The interactions between carbohydrases and phytase have been intensively reviewed5 and therefore will not be discussed in detail in this review.
Animal-related factors: phytase activity in the digestive tract
Animal-related factors including species and ages of animals can have an effect on the efficacy of added phytase in the GI tract. There are limited literature studies available on the determination of phytase activities in the digestive tract of animals, with or without dietary phytase supplementation.
Pigs
In pigs, literature data showed that without microbial phytase supplementation in the feed the main phytase activity was observed in the colon. With microbial phytase supplementation, the main active site was the stomach and upper part of the small intestine.
Yi and Kornegay58 investigated the site of phytase activity in the GI tract of young pigs fed a diet containing A. niger fungal phytase and found that it was higher in the digesta of the stomach than the digesta from the upper small intestine. In pigs fed a diet containing 1050 FTU kg−1 microbial phytase, the phytase activity in the digesta was 579, 348 and 53 FTU kg−1 dry matter (DM) in the stomach, upper and lower part of the small intestine respectively. Phytase activity in the digesta of pigs fed a basal diet was around 30 FTU kg−1 in the stomach and small intestine. These data suggested that the stomach is the site of the highest added microbial phytase activity because of a more favorable pH. A similar conclusion was made by Kemme et al.,59 Lantzsch et al.60 and Mroz et al.61 that the stomach is the major site of phytase action in pigs. Jongbloed et al.62 found no exogenous phytase activity in the ileal digesta.
Pagano et al.63 observed that in young piglets receiving a diet supplemented with E. coli phytase the highest phytase activities were observed in the stomach and upper jejunum, whereas no activities were detected in the digesta of the lower jejunum or ileum. Pigs fed phytase-supplemented feed showed dose-dependent phytase activity in the upper part of the digestive tract and a reduction in total colonic digesta P and Ca concentration. However, the basal diet showed basically no phytase activity in the stomach and small intestine, but high phytase activity and soluble P concentration in the colon (Fig. 5). This resulted in a high soluble P and Ca concentration in the colon of pigs fed the basal diet. However, supplementation of 500 FTU phytase increased plasma P concentration significantly compared to the control group (7.95 vs. 5.2 mg dL−1), indicating that absorption of the inorganic P released by endogenous phytase in the colon is low. The high endogenous phytase activity in the colon may not improve P utilization but can increase soluble P excretion.
Figure 5.
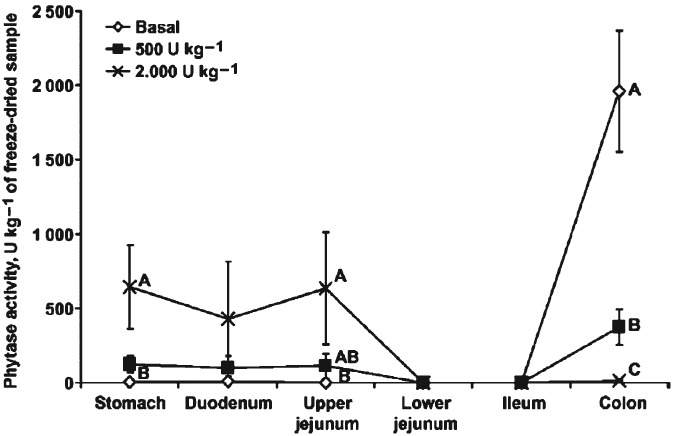
Phytase activity of digesta from various segments of the gut of pigs fed a basal diet or diets supplemented with 500 and 2000 FTU phytase kg−1. A–C: for each segment, means not sharing a common letter differ (P <0.05). Graph adapted from Pagano et al.63
This is in agreement with the observation by Seynaeve et al.44 that endogenous phytase activity in the large intestine does not improve P utilization efficiency but converts organic P to inorganic P form in the feces. In a basal diet without phytase supplementation, the breakdown of IP6 at the terminal ileum was only 16.2%, while the breakdown was tripled in pigs fed a diet supplemented with 500 FTU kg−1 phytase (A. niger phytase). Phytase supplementation reduced ileal digesta total P (P =0.09) and IP6-P (P <0.05) compared with the non-supplemented groups. In ileal digesta, phytate P content was, on average, 42% lower in the phytase-treated group when compared to the control group. However, fecal IP6-P was low in both phytase-treated and non-treated groups, indicating a high endogenous phytase activity in the large intestine of the control group, resulting in higher inorganic P in the feces. Phytase treatment reduced total P as well as inorganic P in the feces (on average, 36% lower than control group).
In the pig's stomach, the pH is normally at 2–2.5 when it is relatively empty and increases after feeding. Newly weaned piglets have a low capacity to secrete hydrochloric acid (HCl); therefore the pH level in the stomach can be higher than in growing pigs. In the first part of the small intestine the pH ranges from 3.5 to 5.5.59 Jongbloed et al.62 observed that the pH of digesta in the duodenum (approximately 25 cm posterior to the pylorus) was about 6 at feeding, decreased to 5 at 1 h after feeding and remained at about 4 from 2 to 5 h after feeding. Therefore a microbial phytase that has a high activity at low pH will be more effective in pigs.
Poultry
In poultry, the main activity site for added microbial phytase is in the crop and upper part of the digestive tract, which is similar to pigs.
In laying hens, phytase activity in the digesta was studied with a wheat, corn and SBM-based diet without microbial phytase supplementation.64 The diet contained phytate and non-phytate P at 2 and 4.37 g kg−1 respectively. The phytase activity in 1 kg basal diet hydrolyzed 160.2 µmol phytic acid (Na phytate) per minute to phosphate, inositol and lower inositol phosphate. It was observed that the highest specific activity of phytase (per gram digesta) was in the caecum. There was no significant difference in phytase activity between the crop, stomach, small intestinal content or mucosa (Table7). In general, the phytase activity level was high in caecum, intermediate in the small intestine and low in the crop and stomach when no microbial phytase was supplemented.
Table 7.
Phytase activity (µmol phytic acid h−1) in the digestive tract of laying hens fed wheat–corn–soybean meal-based diet without microbial phytase supplementation
| Segment | Specific, g−1 digesta | Total, per segment |
|---|---|---|
| Crop | 10.2a | 98a |
| Stomach | 9.2a | 97a |
| Small intestine | 14.6a | 359b |
| Small intestinal mucosa | 11.5a | 227ab |
| Sum pre-caecal | 781 | |
| Caeca | 135.4b | 663c |
| Sum total | 1444 |
Means within a column not sharing a common letter differ significantly (P < 0.05).
Source: Marounek et al.64
The results from this study indicated that without microbial phytase supplementation the phytase activity is mainly observed in caecum (similar to pigs). In this study, phytate digestibility and P retention were measured at ileal and total tract level. It was observed that ileal phytate digestibility was lower than total tract digestibility (20% and 18% vs. 33% and 35% in laying hens and broiler breeders respectively), which may be due to the relative amounts of phytase activity in the caeca. However, the total tract retention of P was lower than the intestinal retention (22% and 19% vs. 52% and 42% in laying hens and broiler breeders respectively), indicating that degradation of phytate in the caecum did not contribute to the total P retention. This study shows that it is crucial to add microbial phytase in the diet to increase the hydrolysis of phytate in the upper part of the digestive tract, to reduce the negative effect of phytate on nutrient digestion and to improve P utilization in poultry.
With the pH ranging from 5.2 to 5.8 in the crop and a pH of 2.8 in the proventriculus, these GI segments were expected to be the major sites of exogenous phytase activity in poultry.65 It was observed that exogenous phytase activity (P. lycii) progressively declined along the small intestine, with no detectable activity in the ileum in broilers. The low activity in the lower part of the small intestine may be due to the activity of endogenous digestive protease which is able to break down exogenous phytase. However, as discussed above, different phytases differ in their resistance to endogenous protease. For example, a significantly higher phytase activity was observed with E. coli phytase than P. lycii phytase in all sections of the digestive tract of broilers, when expressed in FTU kg−1 DM intake (Table8).30 In this study, phytase activity was determined in broilers at 22 days of age fed mash diets with or without microbial phytase. The broilers were fed a negative control diet with low P or the control diet supplemented with either an E. coli phytase or a P. lycii phytase at 1000 FTU kg−1 from 8 to 22 days of age. It was observed that when expressed in FTU kg−1 DM intake, the exogenous phytase activity was highest in the crop, followed by the proventriculus and gizzard, with very low activity in the ileum (Table8). Phytase activity in the digesta of broilers fed the basal diet without phytase supplementation was very low throughout the digestive tract. Supplementation of E. coli phytase significantly increased phytase activity in the crop, proventriculus and gizzard, jejunum and ileum, whereas P. lycii phytase improved phytase activity only in the crop. Liebert et al.66 determined phytase activity in the digestive tract of chickens (3–5 weeks of age) fed control diet and diets with 500 or 1000 FTU kg−1 phytase (A. niger) supplementation. Basically no phytase activity (<50 FTU kg−1) was found in feed and gut contents of chicken fed the control diet. In phytase-supplemented diets, the main phytase activity was found in the crop (250–575 FTU kg−1 freeze-dried sample) and stomach (100–225 FTU kg−1 freeze-dried sample). No activity (<50 FTU kg−1) was detected in the small intestine. These data showed that different type of phytases may differ in their activity in the digestive tract.
Table 8.
Phytase activity in diet and digesta of broiler chicks fed diets with or without added microbial phytase from 8 to 22 days of age; activity measured at day 22
| NC: Low-P diet | NC +1000 FTU E. coli phytase kg−1 | NC +1000 FTU P. lycii phytase kg−1 | |
|---|---|---|---|
| Feed, FTU kg−1 | 14 | 825 | 1152 |
| Digesta, FTU kg−1 DM intake | |||
| Crop | 67c | 649a | 404b |
| Proventriculus and gizzard | 28b | 406a | 63b |
| Jejunum | 29b | 554a | 25b |
| Ileum | 16b | 91a | 6b |
Means within a row not sharing a common letter differ significantly (P < 0.05).
Source: Onyango et al.30
When phytate is incompletely hydrolyzed in the stomach, it may result in reprecipitated phytate in the small intestine. Liebert et al.66 measured phytate P concentration in the digestive tract in chickens and observed that supplementation of 1000 FTU kg−1 phytase in the diet reduced phytate P content in the crop, stomach and small intestine compared to the control diet. At the end of the small intestine, phytate P disappearance was up to 65%, while it was only 15–23% in non-phytase-supplemented diets. Among different digestive sections, the phytate P content was lower in the crop and stomach and increased in the small intestine in all treatments, but to a lesser degree in the phytase-treated group, indicating reprecipitated phytate in the small intestine due to increased pH. Similar results were also reported by Onyango et al.30
Fish
In fish species with a stomach, the phytase activity is mainly in the upper part of the digestive tract, which is similar to pigs and poultry.67
The dephosphorylation starts already during diet preparation. In diets produced by pelleting at low temperature, it was observed that total phytate and IP6 content reduced significantly with increasing phytase inclusion from 0 to 8000 FTU kg−1 (A. niger phytase) (Fig. 6). Nearly 36% of phytate was broken down at a high phytase inclusion level (8000 FTU kg−1) after diet preparation, whereas 19% was degraded at 500 FTU kg−1 phytase inclusion.67 In this study, no inorganic P was added to the diets. Similar results were reported by Schäfer et al.:68 addition of 500 and 1000 FTU kg−1 phytase released about 20% and 40% of the phytin-bound P, respectively, during diet preparation.
Figure 6.
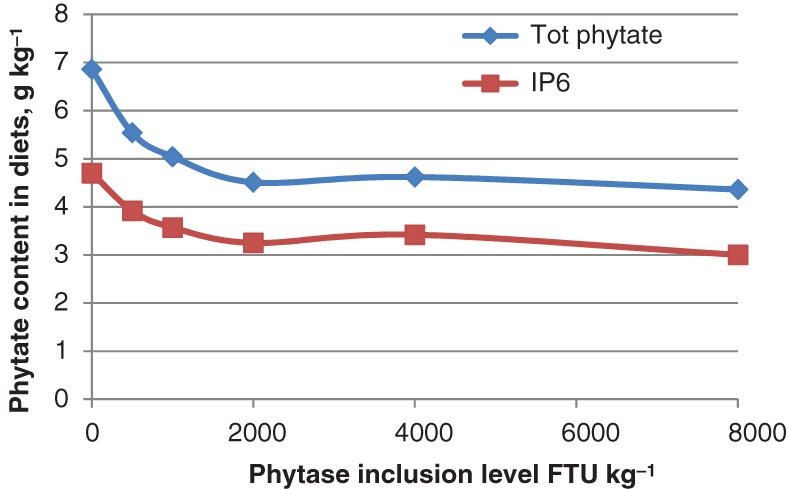
Effect of microbial phytase inclusion on dephosphorylation of phytate during preparation of cold pelleted diet.67
It was observed that the phytate degradation rate in the stomach of channel catfish was related to phytase inclusion levels. At 2 h after feeding, 1 g of stomach content contained 92%, 68%, 50%, 9% and 6% of the total phytate in 1 g of diet in fish fed 500, 1000, 2000, 4000 and 8000 FTU kg−1 phytase-supplemented feed respectively.67 At 8 h after feeding, total phytate content in the stomach was less than 6% of the initial level in fish fed 1000–8000 FTU kg−1 phytase-supplemented diets, and 15% in 500 FTU kg−1 phytase diet, whereas in the control diet total phytate content in the stomach was not reduced at 8 h after feeding. A level of 2000 FTU kg−1 of A. niger phytase was needed to degrade 50% of phytate in the stomach within 2 h and 1000 FTU kg−1 phytase broke down more than 90% of phytate in the stomach in 8 h. Consequently, fecal phytate content was very low in the phytase-treated groups but high in the control group (around 1 mg g−1 vs. 12.5 mg g−1).
Phytase activity is influenced by pH and temperature in the digesta of fish. Morales et al.27 tested the effect of phytase on P bio-accessibility simulating different fish digestion temperatures (6, 16 °C) and gastric pH conditions (pH 2, 3, 4). The phytase activity was measured by soluble P release and residual IP6-P from native IP6 during in vitro digestion of SBM with a gastric extract from fish and with 2500 FTU kg−1 E. coli phytase. The authors observed that phytase activity is more effective at low pH and higher temperature. It can therefore be assumed that to produce a significant effect a higher amount of phytase will be needed in cold-water fish than in warm-water fish.
The pH value in the intestine of stomachless carp is in a range 6.17–7.73,68 which may not be the optimal environment for some commercial phytases, and higher phytase inclusion levels may be needed. However, there might be some activities of phytase in the digesta, where the pH is about 6. Schäfer et al.68 evaluated the impact of adding an A. niger phytase at 500 and 1000 FTU kg−1 on growth performance and P digestibility of carp. Based on growth performance data, it was concluded that 500 or 1000 FTU can replace 1.9 g P from monocalcium phosphate. However, part of the phytate P was already degraded during diet preparation (release of 0.5–0.8 g phytate P kg−1). Apparent fecal digestibility of P was 32% and 49.4% in a low-P diet without and with 500 FTU kg−1 phytase supplementation, respectively, indicating that phytate was partially degraded in the digestive tract by the addition of microbial phytase.
Phytase Efficacy Assessment
A large number of studies have evaluated the effects of dietary phytase on the digestibility and utilization of phosphorus in farm animals. These studies demonstrated that, in general, addition of phytase in the diet improved digestibility of P and reduced P excretion in pigs,69–71 in poultry21,72,73 and in fish.74–76
However, different results on the degree of improvement in phytate P digestibility by a standard 500 FTU phytase dose have been reported in different studies. This may be related to experimental methodologies, efficacy of phytase and other factors influencing phytase activities, as discussed above.
Ileal phytate P degradation
As the added microbial phytase is active mainly in the stomach and upper part of the small intestine, ileal phytate P degradation data will be useful to assess the efficacy of phytases. However, owing to limitations in measuring phytate in the ileal digesta, the ileal phytate P digestibility data are not commonly reported in literature studies. Some available literature data on ileal phytate P degradation in poultry and pigs are discussed below.
Ileal phytate P degradation can be influenced by dietary total P levels. In chickens, Liebert et al.66 measured ileal phytate P digestibility with or without exogenous phytase. It was observed that addition of 1000 FTU kg−1 A. niger phytase reduced phytate P content mainly in the crop and stomach, which corresponded to the higher phytase activity in these sections. Phytate P disappearance at the end of the small intestine was 16.1% and 62.5% in birds fed diets without and with phytase supplementation, respectively, at a dietary total P level of 4.8 g kg−1, whereas the values were 23% and 65.4%, at a dietary total P level of 3.6 g kg−1. Thus the addition of 1000 FTU kg−1 A. niger phytase degraded 42–46% phytate above the control level at the end of the small intestine and this was related to the dietary total P levels.
Dietary calcium level can also have an impact on ileal phytate P degradation. Tamim et al.41 determined ileal phytate P degradation at two dietary Ca levels (0 or 0.5% added Ca). They found that in control diets without added phytase phytate degradation was 69% and 25.4% respectively at 0 and 0.5% added Ca. With addition of 500 FTU kg−1 A. niger or P. lycii phytase, the ileal phytate degradation was 79.5% and 76.2% respectively in diets without added Ca, and 58.9% and 44.9% respectively in diets with 0.5% added Ca. Although a high phytate P degradation rate was found in dietary treatments without Ca addition, this is not representative of a commercial diet. When 0.5% Ca was added, the ileal phytate degradations were 20% and 34% above control due to the addition of 500 FTU kg−1 A. niger or P. Lycii phytase respectively. However, Amerah et al.48 reported that ileal phytate degradations were not influenced by dietary Ca:available-P ratio between 1.43 to 3.57, with ileal phytate P digestibility being between 75% and 88% in broilers fed diets supplemented with 1000 FTU kg−1 Buttiauxella phytase (analyzed level of 831 FTU kg−1). Phytate degradation was in the range 40–51% in the control diets. The different response between these studies may be explained in that in the latter study a high proportion of phytate was degraded in the upper part of the digestive tract and this would reduce the Ca phytate formulation in the lower part of the GI tract, thus reducing the impact of Ca on phytate P degradation. This has already been discussed in the 'Other dietary factors' section.
There was also a gender effect on ileal phytate P degradation. With supplementation of a graded level of A. niger phytase from 500 to 2000 FTU kg−1, Wu et al.77 observed that ileal phytate P degradation was in the range 59–79% (31–51% above control) in male broilers and 52–72% (25–44% above control) in female broilers.
Dietary ingredient composition can also have an impact on ileal phytate P digestibility. In laying hens, van der Klis et al.43 reported that addition of 250 and 500 FTU kg−1 A. niger phytase to a P-deficient corn–SBM–sunflower meal-based diet degraded 59% and 72% phytate respectively (37% and 50% above control) in the first experiment and 50% and 66% respectively (42% and 58% above control) in the second experiment. It was suggested that different inclusion levels of sunflower meal and phytate P level in the diet may partially explain the different results from the two experiments. In pigs, Jongbloed et al.62 reported that the phytic acid degradation rate by phytase was related to the type of feed and thus to dietary phytate P content. Supplementation of 1500 FTU (A. niger) phytase kg−1 diet increased apparent ileal phytic acid digestibility from 9.6% to 59.7% in a corn–SBM-based diet and from −1.4% to 74% in an SBM–tapioca-based diet. Phytic acid (IP6) content was 29.5 versus 12.5 (corn–SBM diet) and 20.8 versus 5.6 g kg−1 DM (SBM–tapioca diet) in ileal digesta for treatments without and with phytase supplementation respectively. The authors estimated that 60–74% of phytic acid was degraded before the ileum by 1500 FTU dietary microbial phytase, whereas only up to 10% was hydrolyzed in control groups. Ileal total P digestibility increased by 18.5 and 29.8 percentage points due to addition of phytase in these two diets. An interesting result observed in this study was that phytate P digestibility was lower at the ileal level compared to duodenal level regardless of dietary treatment. The reason for this was not clear. It was suggested that the method used for determining phytate P digestibility and the difference in liquid/DM phase flow rate in duodenum might have resulted in overestimation of phytate P degradation. It was also speculated that there might be de novo synthesis of myo-inositol phosphates in the small intestine of pigs (as indirectly confirmed by IP5 being found in the blood of birds). More research is needed to test this hypothesis.
Other factors such as experimental design and phytase sources and levels may also have an impact on ileal phytate P degradation measurements. Kemme et al.55 estimated that in growing/finishing pigs fed a corn–SBM-based diet supplementation of 900 FTU A. niger phytase degraded about 38% phytate. Rutherfurd et al.78 reported that addition of 1000 and 2000 FTU kg−1 of a Citrobacter braakii phytase to a pig diet resulted in increased ileal phytate degradation by 29 and 32 percentage points, respectively, compared to a negative control. Interestingly, when using the negative control as reference, phytate degradation by this exogenous phytase occurred not in the stomach but in the jejunum, which might be related to the pH optimum of this phytase. A recent publication79 determined ileal phytate degradation in pigs fed a corn–SBM-based diet supplemented with 500 and 1000 FTU kg−1 E. coli phytase. The ileal phytate degradation was 58.4 and 64.1% for 500 and 1000 FTU kg−1 E. coli phytase inclusion. The ileal phytate degradation was 11% in the negative control diet; thus the phytate degradation by this phytase was from 48% to 53% above control. In contrast, Onyango et al.30 reported that addition of an E coli phytase to a low-P diet did not significantly affect ileal total P digestibility but improved total tract total P digestibility; this may indicate some limitations around the accuracy of determination of ileal total P digestibility. Phytate P degradation was not reported in this study.
Some limited literature data on ileal phytate degradation rate by phytase are summarized in Table9. When excluding the data generated at extreme low dietary Ca concentrations, the data in Table9 indicate that a standard 500 FTU kg−1 phytase degrades about 45–60% phytate (20–48% above control) by the end of the small intestine in pigs and broilers; thus the degradation is incomplete. With a phytase inclusion level above 1000 FTU kg−1, the ileal phytate degradation was in the range 56–88% (29–75% above control). Clearly, large variations in ileal phytate P degradation have been reported in the literature studies, which may be related to dietary phytate P, total P levels, Ca levels and forms, method of analysis of phytate P, ingredient composition, phytase types, adaptation time, age and species (breed and gender) of animals. It was observed that there was an upregulation on P utilization when broilers adapted to a P-deficient diet;80 this would have an impact on phytate degradation rate in control diet. Chung et al.81 statistically analyzed some literature studies in broilers fed corn/soybean meal-based diets supplemented with different types and levels of phytase, and summarized that factors such as dietary Ca and P concentration as well as bird breed and age had a great impact on P and Ca retention.
Table 9.
Some examples of ileal phytate degradation with/without exogenous phytase
| Phytate digestibility |
||||||||
|---|---|---|---|---|---|---|---|---|
| Phytase type | Phytase inclusion (FTU kg−1) | PP, TP, Caa (g kg−1) | − phytase | + phytase | % above controlb | Species | Diet | Reference |
| A. niger | 1500 | 2.1, 3.3, 5.0 | 9.6 | 59.7 | 50.1 | Pigs (37 kg) | Corn–SBM | Jongbloed et al.62 |
| 1500 | 2.1, 4.1, 5.4 | −1.4 | 74 | 75.4 | SBM–tapioca–hominy feed | |||
| A. niger | 900 | 2.6, 3.0, 5.5 | 14.9 | 53.2 | 38.3 | Pigs (37 kg) | Corn–SBM | Kemme et al.55 |
| A. niger | 500 | 2.3, 4.1–5.7, 5–10 | 16.2 | 51.4 | 35.2 | Pigs | Corn–tapioca–SBM | Seynaeve et al.44 |
| E. coli | 500 | 2.3, 3.5, 4.4 | 11.1 | 58.6 | 47.5 | Pigs (20 kg) | Corn–SBM | Zeng et al.79 |
| 1000 | 11.1 | 64.1 | 53 | |||||
| Citrobacter braakii | 1107 | 3, 4, 3.9 | 39.1 | 67.7 | 28.6 | Pigs (22 kg) | Corn–SBM–wheat bran–rapeseed meal | Rutherfurd et al.78 |
| 2215 | 39.1 | 70.8 | 31.7 | |||||
| A. niger | 1000 | 2.2, 4.8, — | 16.1 | 62.5 | 46.4 | Chicken | Corn–SBM | Liebert et al.66 |
| 1000 | 2.2, 3.6, — | 23 | 65.4 | 42.4 | ||||
| A. niger | 500 | 2.8, 4.8, — | 28 | 59.3 | 31.3 | Male broilers | Wheat–SBM–canola | Wu et al.77 |
| 1000 | 28 | 63.5 | 35.5 | |||||
| 1500 | 28 | 69.7 | 41.7 | |||||
| 2000 | 28 | 79.1 | 51.1 | |||||
| 500 | 2.8, 4.8, — | 27.2 | 51.9 | 24.7 | Female broilers | Wheat–SBM–canola | Wu et al.77 | |
| 1000 | 27.2 | 55.6 | 28.4 | |||||
| 1500 | 27.2 | 70.9 | 43.7 | |||||
| 2000 | 27.2 | 71.5 | 44.3 | |||||
| A. ficuum | 500 | 3.1, 4.0, 1.8 | 69.2 | 79.5 | 10.3 | Male broilers | Corn–SBM | Tamim et al.41 |
| P. lycii | 500 | 69.2 | 76.2 | 7 | ||||
| A. ficuum | 500 | 3.1, 4.0, 6.8 | 25.4 | 58.9 | 33.5 | |||
| P. lycii | 500 | 25.4 | 44.9 | 19.5 | ||||
| Buttiauxella | 1000 | 3.2, 5.1, 5.1 | 51.4 | 88.4 | 37 | Male broilers | Corn–SBM | Amerah et al.48 |
| 1000 | 3.2, 5.1, 6.8 | 40.4 | 75.2 | 34.8 | ||||
| 1000 | 3.2, 5.1, 9.1 | 43.7 | 76.2 | 32.5 | ||||
| 1000 | 3.2, 5.1, 13 | 39.8 | 75.9 | 36.1 | ||||
| A. niger | 250 | 2.7c | 21.6 | 54.2 | 32.6 | Laying hen,24 weeks | Corn–SBM–hominy feed–sunflower meal | van der Klis et al.43 |
| 250 | 2.7, 3.5, 30–40 | 21.7 | 59 | 37.3 | ||||
| 500 | 2.7, 3.5, 30–40 | 21.7 | 71.7 | 50 | ||||
| 250 | 2.4, 3.2, 35 | 8.1 | 49.6 | 41.5 | Corn–SBM–sunflower meal | |||
| 500 | 2.4, 3.2, 35 | 8.1 | 66.1 | 58 | ||||
PP, phytate P; TP, total P; —, data not reported.
Percentage points increase in phytate degradation above control, due to phytase addition.
With addition of 0.5 g kg−1 MCP-P.
A standardized method should be established to determine efficacy of different phytases in vivo when using ileal phytate P degradation as the criterion.
Total tract P digestibility and retention, bone ash and performance
Total tract P digestibility, bone ash and growth performance are commonly used parameters to assess the efficacy of phytase. Similar to ileal P digestibility, there are many factors influencing the total tract P digestibility measurement. The most important factors are the source and level of inorganic P used and the endogenous microbial phytase levels in the large intestine. Literature studies on the effect of phytase on P digestibility in broilers have been reviewed by Woyengo and Nyachoti.5 It was summarized that with corn–SBM-based diets addition of 500–1000 FTU kg−1 phytase improved P digestibility by 12–21 percentage points. In wheat–SBM-based diets the increase was in the range from 7 to 14 percentage points due to addition of 500–600 FTU kg−1 phytase.
In literature studies, bone ash and performance data have been commonly used for the estimation and validation of inorganic P release by phytase, as these parameters give additional useful mechanistic information. For example, Hoppe et al.82 reported that addition of 500 FTU kg−1 phytase released 42% of phytate P, based on bone ash content, P and Ca retention in pigs fed a grain–SBM-based diet. The estimated phytate P release was 15% and 57% of the phytate P with 250 and 1000 FTU kg−1 phytase addition respectively. Cromwell et al.83 observed that the addition of 250, 500 and 1000 FTU kg−1 phytase released 12%, 22% and 43% phytate P, respectively, in growing pigs fed an SBM-based semi-purified diet, using bone shear force as the response criterion. However, Yi et al.84 reported that 250, 500, 750 and 1000 FTU phytase could release 0.52, 0.83, 1.08 and 1.21 g kg−1 inorganic P as defluorinated phosphate in the diet for young pigs. When the inorganic P release was converted to MCP, it was calculated as 0.41, 0.69, 0.86 and 0.96 g kg−1 inorganic P respectively.
As discussed above, a phytase that is active in the stomach and upper part of the small intestine will reduce the binding of phytate to dietary protein and eliminate its antinutritional effect as well as reducing endogenous amino acid losses.20 Therefore not only phytate P release but also amino acid digestibility and energy (ME) efficiency may be used as additional parameters to determine the effectiveness of phytase. For example, Santos et al.85 observed that addition of an E. coli-derived phytase increased ME value by 65–195 kcal kg−1 in 21-day broilers fed diets containing 500–1000 FTU kg−1, while the increase was about 195 kcal kg−1 in 22–42-day broilers fed diets containing 750 and 1000 FTU kg−1 phytase, when diets were formulated with reduced ME, Ca and P. Onyango et al.30 reported that addition of an E coli phytase in a low-P diet improved total tract P digestibility, retention of P, Ca, N and a number of amino acids, tibia ash and weight gain. Amerah et al.48 observed that supplementation of 1000 FTU kg−1 Buttiauxella phytase improved digestibility of P, amino acids and energy, which was co-related to improved body weight gain and feed efficiency in broilers. The effect of microbial phytase on P utilization and amino acid digestibility has been intensively reviewed.86,87
In summary, phytate P digestibility can be measured at either ileal or fecal levels. Total tract P digestibility can be used to determine P retention as it has fewer practical limitations. However, determination of phytate P release should be measured at the ileal level because in the hindgut phytate is degraded by the intestinal microflora, but the released P is not absorbed. Direct measurement of the hydrolysis of phytate P at the ileal level will give a good indication of the effectiveness of a phytase feed additive. In addition, tibia ash, bone strength and performance data are useful parameters for validation of matrix values (e.g. inorganic phosphorus, Ca, energy and digestible amino acids) for phytase.
Method of analysis and phytase recovery
Other factors that may have a big impact on the determination of phytase efficiency are method of analysis and the analyzed phytase levels in the diets, which is not measured or not reported in most of the literature studies. One example is from the study of Kerr et al.88 in finishing pigs, in which four commercial phytases were tested. The authors analyzed the phytase activity in these phytases and found that the activities differed significantly according to the methods of analysis. When using the official AOAC3 method as a standard, one product (Phyzyme) confirmed the activity indicated by the producer (5000 FTU g−1), whereas the activities of other phytases were 1.5–4.65 times higher compared to the activity declared by the producers (Table10).
Table 10.
Phytase activity (FTU g−1) of phytase samples measured by different methodsa and its effect on inorganic P release estimations
| Phytasesb | Natuphos | Phyzyme | Ronozyme P | OptiPhos |
|---|---|---|---|---|
| Activity by producer (FTU g−1) | 5000 | 5000 | 2500 | 2000 |
| Analyzed by different methods (FTU g−1)c | ||||
| AOAC (2000) | 7300 | 5000 | 2800 | 9300 |
| Danisco | 4800 | 4300 | 2800 | 9300 |
| Phytex | 4400 | 1200 | 1500 | 1800 |
| Roche | 4800 | 3700 | 2600 | 6200 |
| Inorganic P released by 500 FTU (g kg−1) | ||||
| Based on activity by producer | 0.34 | 0.39 | 0.28 | 1.02 |
| Based on activity analyzed by AOAC method | 0.24 | 0.39 | 0.25 | 0.22 |
1 FTU is defined as the quantity of enzyme required to liberate 1 µmol inorganic P min−1, at pH 5.5, from an excess of 15 µmol L−1 sodium phytate at 37 °C kg−1 feed.3
Natuphos (BASF, Mt Olive, NY, USA); Phyzyme (Dupont/Danisco Animal Nutrition, Marlborough, UK); OptiPhos (Enzyvia LLC, Sheridan, IN, USA); Ronozyme P (DSM Nutritional Products Inc., Parsippany, NJ, USA).
Each phytase premix was analyzed by the same lab with different methods (Eurofins Scientific Inc., Des Moines, IA, USA).
Data from Kerr et al.88
In this study, phytase was included in the diets based on the activity provided by the producers, but not the analyzed values. As shown in Table10, the inorganic P released by 500 FTU kg−1 of the phytase products can be significantly different when using analyzed activity based on the official AOAC method compared to the expected values based on the activity indicated by producers. Clearly, in trial studies to compare different commercial phytase products, it is essential to determine the activity in the products and the activities in the feed using an official method. The importance of measuring phytase activity after feed formulation has also been stressed by Onyango et al.30 The authors found much lower phytase activity in mash diets than expected dosages; thus analyzed values were used in this study to calculate inorganic P equivalent values.
Challenges in Determining Phytase Matrix Values
With continued increases in feed ingredient and feed-grade phosphate prices, it is crucial to use feed phosphate resources efficiently and to maximize efficient use of phytase. To do so, basic 'know-how' on phytate and intrinsic phytase levels, the factors influencing phytase activity and in vivo efficiency of different generations of phytase source need to be considered.
Variation in phytate and intrinsic phytase content in feed materials
It is well known that, like all other nutrients, the total P and phytate content in feed ingredients varies to a large extent. Table11 gives an example of average phytate values and intrinsic phytase levels in commonly used plant ingredients in animal feed.8,49,87,89 The values in Table11 are average values reported in the literature. However, the phytate content and phytase activity can vary to a large extent between crops from different seasons and regions, due to changes in climate and environment.
Table 11.
Phytate content and intrinsic phytase activity in commonly used feed ingredients
| Feed ingredient | Total P (g kg−1) | Phytate P (g kg−1) | % phytate P/total P | Phytase (FTU kg−1) |
|---|---|---|---|---|
| Corn | 2.4–2.62 | 1.7–2.05 | 72–85.4 | 24–25 |
| Soybean meal | 6.49–6.66 | 3.88–4.53 | 60–68 | 10–95 |
| Full fat Soybean | 5.55 | 3.08 | 55.5 | 40 |
| Wheat | 2.0–3.08 | 1.6–2.2 | 72–80 | 255–840 |
| Barley | 2.6–3.21 | 1.69–1.96 | 61–67 | 130–595 |
| canola meal | 8.76–9.72 | 6.45–7.4 | 66–76.4 | 5–35 |
| Rapeseed meal | 11.8 | 7 | 59 | — |
| Wheat Bran | 10.96 | 8.36 | 76.3 | 1700–3090 |
| Wheat middling | 8.45 | 7.8 | 92 | 2500 |
| Sunflower meal | 9.05 | 7.48–7.7 | 82.8–85 | <10 |
In vitro analysis of feed ingredients is a useful tool to provide precise data on total P, phytate P and available/digestible P in feed formulations.90 As a basic rule, phytate content in the feed should be considered for choosing appropriate phytase inclusion levels.
In addition, the presence of intrinsic phytase in some feed ingredients also needs to be considered. It is well known that the intrinsic phytase activity in feed is closely related to feed processing conditions. Feed processed at high temperature (such as pelleting and extrusion) will reduce the intrinsic phytase activity to a minimum level. However, in mash diets, feed ingredients, such as wheat bran and other wheat by-products, may contribute significant amounts of intrinsic phytase. The stability of intrinsic phytase may differ among types of grains. An in vitro study showed that intrinsic phytases of wheat and rye were resistant to pepsin, but barley phytase was susceptible to pepsin and its stability decreased to 57% at 5 mg mL−1 pepsin concentration.91 In addition, the optimum pH for intrinsic phytase activity is in the range 4.0–6.0;92 thus the intrinsic phytase has lower efficacy compared to exogenous phytase.
Matrix values
Phytase was initially included in animal feed to release inorganic P from phytate in order to reduce P excretion to the environment. In practice, an additional advantage of phytase on amino acid digestibility and energy utilization has been observed and increasingly recognized. Thus phytase feed matrix values used in practice include values for available P, Ca, amino acids and energy. However, the matrix values differ for different types of phytase (e.g. different generations of development) and different animal species and are influenced by dietary composition.
In most of the literature studies, phytate P release has been determined by replacement of inorganic P from the positive control diet to measure whether addition of phytase to a P-deficient diet can recover some parameters such as bone ash, P retention or body weight gain when compared to the positive control. Table12 gives some examples of phytase equivalencies to inorganic P from literature studies. Clearly, as indicated in Table12, there are large variations in inorganic P equivalency of phytase determined from literature studies, as discussed above, which can be related to dietary total P, inorganic P, phytate P and Ca levels, feed ingredient composition, age and species of animals, adaptation periods and the assessment criteria.
Table 12.
Some examples of inorganic P equivalent values for different types of phytases in different animal species
| Species | Phytase | Response | Inorganic P source | Inorganic P (iP) equivalency | References |
|---|---|---|---|---|---|
| Swine | E. coli | Bone ash | MSP | 500 FTU =0.77 g iP | 47 |
| E. coli | WG | MSP | 500 FTU =0.49 g iP | 93 | |
| E. coli | Bone ash | MSP | 500 FTU =1 g iP | 93 | |
| E. coli | Fibula ash | MPP | 400 FTU =1.08 g iP | 31 | |
| A. niger | Fibula ash | MPP | 400 FTU =0.81 g iP | 31 | |
| A. niger | WG, bone ash | DCP | 500 FTU =0.87–0.96 g iP | 70 | |
| A. niger | ADC P | Defluorinated phosphate | 777 FTU =1 g iP | 84 | |
| P. lycii | Fibula ash | MPP | 400 FTU =0.43 g iP | 31 | |
| P. lycii | Bone ash | MSP | 500 FTU =0.572 g iP | 47 | |
| Buttiauxella | digestible P | MCP/DCP | 250 FTU =1.3 g dig. P | 94 | |
| Buttiauxella | digestible P | MCP/DCP | 500 FTU =1.5 g dig. P | 94 | |
| Buttiauxella | digestible P | MCP/DCP | 1000 FTU =1.6 g dig. P | 94 | |
| Broilers | E. coli | Tibia, toe ash | MSP | 1000 FTU =0.93–1.10 g iP | 95 |
| E.coli | WG | MSP | 500 FTU =0.72 g iP | 93 | |
| E. coli | Bone ash | MSP | 500 FTU =1.19 g iP | 93 | |
| E.coli | WG | DCP | 500 FTU =1.7 g iP | 96 | |
| E.coli | WG, bone ash | MPP | 500 FTU =1.25 g iP | 31 | |
| P. lycii | WG, bone ash | MPP | 500 FTU =0.28 g iP | 31 | |
| E.coli1 | Tibia ash | MSP | 500 FTU =0.77 g iP | 30 | |
| E.coli | Tibia ash | MSP | 750 FTU =1.13 g iP | 30 | |
| E.coli | Tibia ash | MPP | 500 FTU =1.6 g iP | 97 | |
| P. lycii | Tibia ash | MPP | 500 FTU =0.5 g iP | 97 | |
| A. niger | Tibia ash | MPP | 500 FTU =0.6 g iP | 97 | |
| A. niger | WG | DCP | 885 FTU =1.7 g iP | 96 | |
| A. niger | WG, bone ash | TCP | 939 FTU =1 g nPP | 98 | |
| A. niger | WG, bone ash | MPP | 500 FTU =0.32 g iP | 31 | |
| A. niger | WG, toe ash | 500 FTU =0.84 g iP | 99 | ||
| A. niger | WG, toe ash | 1000 FTU =1.05 g iP | 99 | ||
| Buttiauxella | FCR | 250 FTU =1.7 avp P | 100 | ||
| Catfish | A.niger | WG | DCP | 250 FTU =0.75% DCP | 101 |
| Carp | A.niger | WG | MCP | 500/1000 FTU =1.9 g iPa | 68 |
| Rainbow trout | A.niger | Phytate P digestibility | 500 FTU releases 0.84–1.54 g kg−1 phytate P | 36 | |
| Grass carp | neutral phytase | WG | MCP | 500 FTU replaces 1% MCP | 102 |
| Carp | P. lycii | WG | DCP | 500 FTU replaces 0.5% DCP | 103 |
| Common carp | P. lycii | WG, dig | MSP | 750 FTU (+0.22% citric acid) replaces 0.55% MSP | 54 |
| Tilapia | E coli | WG, FCR, bone mineralization | DCP | 500 FTU replaces 3.5 g available P | 104 |
WG, weight gain; FCR, feed conversion ratio; ADC, apparent digestibility coefficient; MSP, monosodium phosphate; MPP, monopotassium phosphate; MCP, monocalcium phosphate; MDCP, monodicalcium phosphate; DCP, dicalcium phosphate; TCP, tricalcium (defluorinated) phosphate.
The phytate P was partially released during diet preparation (0.5–0.8 g phytate P kg−1).
Selle et al.105 observed that feed utilization efficiency as a response to dietary phytase was positively correlated with dietary phytate content in weaning piglets fed a phosphorus-adequate diet. In addition, the type of phytase and method of analysis may also contribute to the variations. Literature studies have shown that E. coli phytases exhibit greater efficiency compared to fungal phytases.31,47,97 However, there are also differences between different E. coli phytase products due to different expression organisms used. For example, as indicated in Tables2 and 4, E. coli expressed in S. pombe has higher activity than E. coli expressed in P. pastoris when using IP6 soy protein as substrate measured at pH 3.
In common practice, phytase is added to most of the poultry and pig feeds at a standard inclusion level of 500 FTU kg−1 (300 FTU kg−1 in the layer's diet), the activity being based on the standard measurement at pH 5.5. Owing to the differences in phytase activity in an in vivo environment, as discussed above, the matrix values for different types of phytase may differ. The inorganic P equivalent reported in the literature31,96 is in the range 0.3–1.76 g kg−1 based on the addition of claimed 500 FTU kg−1 phytase (but activity measurements may differ significantly due to methods of analysis). Feed enzyme suppliers recommend P equivalence values of more than 1.0 g P at 500 FTU kg−1 phytase inclusion; however, as shown in Table12, P equivalence values determined in some of the studies showed a value below1.0 g P. It can be argued that consideration should be given when using inorganic P matrix values, as many factors can have an influence on phytase efficacy, as discussed above.
The data in Tables9 and 12 indicate that normally less than 50% of dietary phytate P is hydrolyzed by 500 FTU phytase. High dosage levels of phytase (e.g. 1000–2000 FTU kg−1) may be needed to break down >60% of phytate in the upper part of the digestive tract, to reduce the antinutritional effects of phytate and improve the efficiency of utilization of organic P in plant-based ingredients.
In addition, the matrix value can also differ depending on the species and age of the animal and can also be influenced by dietary composition. It is important to be aware that there is no fixed matrix value of microbial phytase in all feed formulations. To use microbial phytase more efficiently and economically the matrix values for phytase should be empirically derived and based on the specific diet composition, its application and using models that have been developed to strategically inform the end user of the biological and economic value of the enzyme product.106
With increasing demands from the feed industry, more efficient phytase products are being developed over time. Some new-generation phytases have recently been made available to the feed industry, including an E. coli phytase expressed in Trichoderma reesei and a Citrobacter braakii phytase expressed in Aspergillus oryzae. Another recently marketed new-generation phytase is derived from Buttiauxella spp. and expressed in Trichoderma reesei. Studies have shown that the new-generation (Buttiauxella) phytase has improved efficacy compared to E. coli phytase.100,107 As discussed above, if the phytase can degrade phytate rapidly and efficiently in the upper part of the GI tract, more phytate-bound amino acids will be released (extra effect beyond phosphorus release) and this leads to improved amino acid digestibility. This hypothesis has been confirmed in a recent study, in which it was found that supplementation of 1000 FTU kg−1 Buttiauxella phytase improved digestibility of all amino acids in broilers by about 10 percentage points on average, which was positively correlated with the degree of phytate degradation.48 Development of high-efficacy phytases will result in both improved economic value for the feed industry and environmental benefits due to further reductions in P excretion.
Implications and Future Research on Phytase
Many factors can have an influence on in vivo feed phytase activities, which can be divided into phytase, dietary and animal-related factors. The mode of action of the factors influencing phytase activity is illustrated in Fig. 7.
Figure 7.
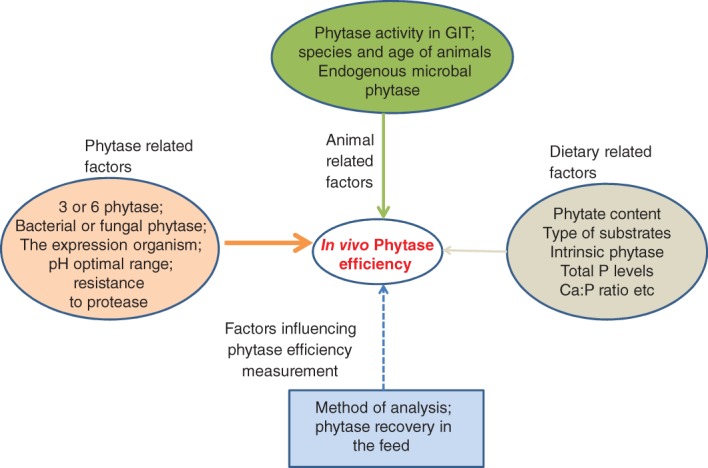
Illustration of factors influencing in vivo phytase activity and factors influencing phytase efficiency measurement.
New generations of phytases have been developed over the last decade and a large body of research has been carried out to determine the matrix values for different types of phytase. There is increased knowledge on using phytase in animal nutrition. However, as many factors can influence phytase activities in vivo, further research is still needed to optimize the use of phytase.
In the first place, the authors would like to emphasize that the official method for phytase activity measurement is done at pH 5.5, which is not reflecting the optimal pH in the upper digestive tract where the phytase activity takes place. Therefore, when selecting a commercial phytase product, it is essential to know the optimum pH levels of that phytase. A new method to measure phytase activity that reflects optimal pH in the stomach chyme (i.e. at pH 3) should be urgently developed to compare the 'effective' phytase activity of different phytase products. Additionally, in standard phytase activity measurement Na phytate is used as the substrate. However, the 'real' substrate in the diet is natural phytate from different plant ingredients and therefore, in the new method, a natural phytate source, such as IP6 from soybeans, could be used as a substrate.
Currently, the microbial phytase at 500 FTU kg−1 inclusion level hydrolyzes about 45–60% phytate (20–48% above control) by the end of the small intestine in pigs and broilers. A phytase which has high activity at low pH and a wide pH range would be expected to hydrolyze phytate more quickly and completely in the upper part of the digestive tract, and thus more efficiently reduce the antinutritional effect of phytate.
Limited information is available on phytase for cold-water fish species. A phytase which is active at low temperature may be more efficient for cold-water species such as salmon and trout.
Different assessment criteria can be used to determine the equivalent inorganic P value of feed phytase, e.g. ileal total P and phytate P digestibility, total tract P retention, weight gain and bone ash. In literature studies, large variations in the equivalent inorganic P value of phytase have been reported and this may be related to animal species, method of analysis, feed composition (level of phytate and Ca in the diet), adaptation time and type of phytase used. One of the important factors to be considered in trial studies with phytase is the analyzed phytase activity (or percent recovery) in the feed, as the analyzed values can be very different from the calculated values. Using calculated values may result in bias in the estimation of inorganic P equivalent.
In conclusion, it is generally accepted that using phytase can reduce feed costs and improve the efficiency of utilization of phosphate and other nutrients in plant-based feed ingredients, resulting in economic and environmental benefits. However, a better understanding of in vivo phytase activity is important so as to use phytase more economically and efficiently. A phytase that works over a wide range of pH and is active up to the stomach and upper intestine (along with several other characteristics, such as being refractory to endogenous enzymes) would be the ideal phytase for animal feed. In addition, matrix values for phytase should be calculated with consideration of the specific diet composition and its applications.
Acknowledgments
The authors would like to express their thanks to Dr Shukun Yu from DuPont Industrial Biosciences for review and comments on the manuscript.
References
- 1.Lei XG, Weaver JD, Mullaney E, Ullah AH, Azain MJ. Phytase, a new life for an 'old' enzyme. Annu Rev Anim Biosci. 2013;1:283–309. doi: 10.1146/annurev-animal-031412-103717. [DOI] [PubMed] [Google Scholar]
- 2.Adeola O, Cowieson AJ. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- 3.AOAC. Official Methods of Analysis of AOAC International. 17th edn. Arlington, VA: Association of Official Analytical Chemists; 2000. Method 2000.12: Phytase activity in feed: colorimetric enzymatic method. [Google Scholar]
- 4.Selle PH, Ravindran V. Review: Microbial phytase in poultry nutrition. Anim Feed Sci Technol. 2007;135:1–41. [Google Scholar]
- 5.Woyengo TA, Nyachoti CM. Review: Supplementation of phytase and carbohydrases to diets for poultry. Can J Anim Sci. 2011;91:177–192. [Google Scholar]
- 6.Cao L, Wang W, Yang C, Yang Y, Diana J, Yakupitiyage A, et al. Review: Application of microbial phytase in fish feed. Enzyme Microb Technol. 2007;40:497–507. [Google Scholar]
- 7.IUPAC-IUB. Biochem J. 1989;258:1–2. Numbering of atoms in myo-inositol: recommendations 1988. Nomenclature Committee of the International Union of Biochemistry. ) [Google Scholar]
- 8.Kumar V, Sinha AK, Makkar HPS, De Boeck G, Becker K. Phytate and phytase in fish nutrition. J Anim Physiol Anim Nutr. 2012;96:335–364. doi: 10.1111/j.1439-0396.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- 9.Woyengo TA, Nyachoti CM. Review: Anti-nutritional effects of phytic acid in diets for pigs and poultry: current knowledge and directions for future research. Can J Anim Sci. 2013;93:9–21. [Google Scholar]
- 10.Angel R, Tamim NM, Applegate TJ, Dhandu AS, Ellestad LE. Phytic acid chemistry: influence on phytin-phosphorous availability and phytase efficiency. J Appl Poult Res. 2002;11:417–480. [Google Scholar]
- 11.Maenz DD. Enzymatic and other characteristics of phytases as they relate to their use in animal feeds. In: Bedford MR, Partridge GG, editors. Enzymes in Farm Animal Nutrition. Wallingford, UK: CABI; 2001. pp. 61–84. ed. by, ) [Google Scholar]
- 12.Cowieson AJ, Acamovic T, Bedford MR. The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from broiler chickens. Br Poult Sci. 2004;45:101–108. doi: 10.1080/00071660410001668923. [DOI] [PubMed] [Google Scholar]
- 13.Woyengo TA, Cowieson AJ, Adeola O, Nyachoti CM. Ileal digestibility and endogenous flow of minerals and amino acids: responses to dietary phytic acid in piglets. Br J Nutr. 2009;102:428–433. doi: 10.1017/S0007114508184719. [DOI] [PubMed] [Google Scholar]
- 14.Liu N, Ru YJ, Li FD, Cowieson AJ. Effect of diet containing phytate and phytase on the activity and messenger ribonucleic acid expression of carbohydrase and transporter in chickens. J Anim Sci. 2008;86:3432–3439. doi: 10.2527/jas.2008-1234. [DOI] [PubMed] [Google Scholar]
- 15.Woyengo TA. Gut secretions and nutrient absorption responses to dietary phytic acid and phytase in piglets. Winnipeg: University of Manitoba; 2010. . PhD thesis, ) [Google Scholar]
- 16.Singh M, Krikorian AD. Inhibition of trypsin activity in vitro by phytate. J Agric Food Chem. 1982;30:799–800. [Google Scholar]
- 17.Deshpande SS, Cheryan M. Effects of phytic acid, divalent cations, and their interactions on α-amylase activity. J Food Sci. 1984;49:516–519. [Google Scholar]
- 18.Cheryan M, Rackis JJ. Phytic acid interactions in food systems. Crit Rev Food Sci Nutr. 1980;13:297–335. doi: 10.1080/10408398009527293. [DOI] [PubMed] [Google Scholar]
- 19.Kies AK, De Jonge LH, Kemme PA, Jongbloed AW. Interaction between protein, phytate, and microbial phytase: in vitro studies. J Agric Food Chem. 2006;54:1753–1758. doi: 10.1021/jf0518554. [DOI] [PubMed] [Google Scholar]
- 20.Cowieson AJ, Ravindran V, Selle PH. Influence of dietary phytic acid and source of microbial phytase on ileal endogenous amino acid flows in broiler chickens. Poult Sci. 2008;87:2287–2299. doi: 10.3382/ps.2008-00096. [DOI] [PubMed] [Google Scholar]
- 21.Ravindran V, Cabahug S, Ravindran G, Selle PH, Bryden WL. Response of broiler chickens to microbial phytase supplementation as influenced by dietary citric acid and non-phytate phosphorus levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention. Br Poult Sci. 2000;41:193–200. doi: 10.1080/00071660050022263. [DOI] [PubMed] [Google Scholar]
- 22.Zaefarian F, Romero JF, Ravindran V. Influence of a microbial phytase on the performance and the utilisation of energy, crude protein and fatty acids of young broilers fed on phosphorus-adequate maize- and wheat-based diets. Br Poult Sci. 2013;54:653–660. doi: 10.1080/00071668.2013.830209. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez E, Han Y, Lei XG. Cloning, sequencing, and expression of an Escherichia coli phosphatase/phytase gene (AppA2) isolated from pig colon. Biochem Biophys Res Commun. 1999;257:117–123. doi: 10.1006/bbrc.1999.0361. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez E, Porres JM, Han Y, Lei XG. Different sensitivity of recombinant Aspergillus niger phytase (r-PhyA) and Escherichia coli pH 2.5 aid phosphatase (r-AppA) to trypsin and pepsin in vitro. Arch Biochem Biophys. 1999;365:262–257. doi: 10.1006/abbi.1999.1184. [DOI] [PubMed] [Google Scholar]
- 25.Wyss M, Brugger R, Kronenberger A, Rémy R, Fimbel R, Oesterhelt G, et al. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolase): catalytic properties. Appl Environ Microbiol. 1999;65:367–373. doi: 10.1128/aem.65.2.367-373.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S, Cowieson A, Gilbert C, Plumstead P, Dalsgaard S. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J Anim Sci. 2012;90:1824–1832. doi: 10.2527/jas.2011-3866. [DOI] [PubMed] [Google Scholar]
- 27.Morales GA, Moyano FJ, Marquez L. In vitro assessment of the effects of phytate and phytase on nitrogen and phosphorus bioaccessibility within fish digestive tract. Anim Feed Sci Technol. 2011;170:209–221. [Google Scholar]
- 28.Kumar V, Miasnikov A, Sands JS, Simmins PH. Proceedings of the Australian Pig Science Association. 2003. In vitro activities of three phytases under different pH and protease challenges; p. 164. [Google Scholar]
- 29.Tran TT, Hatti-Kaul R, Dalsgaard S, Yu S. A simple and fast kinetic assay for phytases using phytic acid–protein complex as substrate. Anal Biochem. 2011;410:177–184. doi: 10.1016/j.ab.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Onyango EM, Bedford MR, Adeola O. Efficacy of an evolved Escherichia coli phytase in diets for broiler chicks. Poult Sci. 2005;84:248–255. doi: 10.1093/ps/84.2.248. [DOI] [PubMed] [Google Scholar]
- 31.Augspurger NR, Webel DM, Lei XG, Baker DH. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J Anim Sci. 2003;81:474–483. doi: 10.2527/2003.812474x. [DOI] [PubMed] [Google Scholar]
- 32.Leske KL, Coon CN. A bioassay to determine the effect of phytate phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens. Poult Sci. 1999;78:1151–1157. doi: 10.1093/ps/78.8.1151. [DOI] [PubMed] [Google Scholar]
- 33.Morales GA, de Rodrigañez MS, Marquez L, Díaz M, Moyano FJ. 15th International Symposium on Fish Nutrition and Feeding. Molde, Norway: 2012. Effect of phytase on the solubility of protein fractions in selected plant ingredients for fish feeds using SDS-PAGE image analysis: book abstract; p. 48. , 4–7 June ( ) [Google Scholar]
- 34.Morales GA, de Rodrigañez MS, Marquez L, Díaz M, Moyano FJ. 15th International Symposium on Fish Nutrition and Feeding. Molde, Norway: 2012. Effect of phytase on the hydrolysis of protein and phosphorus in selected plant ingredients simulating fish gastric environment: book abstract; p. 47. 4–7 June ( ) [Google Scholar]
- 35.Li DF, Nelssen JL, Reddy PG, Blecha F, Klemm RD, Giesting DW, et al. Measuring suitability of soya bean products for early-weaned pigs with immunological criteria. J Anim Sci. 1991;69:3299–3307. doi: 10.2527/1991.6983299x. [DOI] [PubMed] [Google Scholar]
- 36.Cheng ZJ, Hardy RW. Effects of microbial phytase supplementation on apparent digestibility of barley, canola meal, wheat and wheat middlings, measured in vivo using rainbow. Oncorhynchus mykiss. Aquacult Nutr. 2002;8:271–277. [Google Scholar]
- 37.Newkirk RW, Classen HL. In vitro hydrolysis of phytate in canola meal with purified and crude sources of phytase. Anim Feed Sci Technol. 1998;72:315–327. [Google Scholar]
- 38.Eeckhout W, de Paepe M. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim Feed Sci Technol. 1994;47:19–29. [Google Scholar]
- 39.Manangi MK, Coon CN. The effect of calcium carbonate particle size and solubility on the utilization of phosphorus from phytase for broilers. Int J Poult Sci. 2007;6:85–90. [Google Scholar]
- 40.Leytem AB, Willing BP, Thacker PA. Phytate utilization and phosphorus excretion by broiler chickens fed diets containing cereal grains varying in phytate and phytase content. Anim Feed Sci Technol. 2008;146:160–168. [Google Scholar]
- 41.Tamim NM, Angel R, Christman M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult Sci. 2004;83:1358–1367. doi: 10.1093/ps/83.8.1358. [DOI] [PubMed] [Google Scholar]
- 42.Plumstead PW, Leytem AB, Maguire RO, Spears JW, Kwanyuen P, Brake J. Interaction of calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult Sci. 2008;87:449–458. doi: 10.3382/ps.2007-00231. [DOI] [PubMed] [Google Scholar]
- 43.Van der Klis JD, Versteegh HAJ, Simons PCM, Kies AK. The efficacy of phytase in corn–soybean meal-based diets for laying hens. Poult Sci. 1997;76:1535–1542. doi: 10.1093/ps/76.11.1535. [DOI] [PubMed] [Google Scholar]
- 44.Seynaeve M, Janssens G, Hesta M, van Nevel C, De Wilde RO. Effects of dietary Ca/P ratio, P level and microbial phytase supplementation on nutrient digestibilities in growing pigs: precaecal, post-ileal and total tract disappearances of OM, P and Ca. J Anim Physiol Anim Nutr. 2000;83:36–48. [Google Scholar]
- 45.Qian H, Kornegay ET, Veit HP. Effects of supplemental phytase and phosphorus on histological, mechanical and chemical traits of tibia and performance of turkeys fed on soyabean-meal-based semi-purified diets high in phytate phosphorus. Br J Nutr. 1996;76:263–272. doi: 10.1079/bjn19960030. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Bollinger DW, Ledoux DR, Veum TL. Lowering the dietary calcium to total phosphorus utilization in low-phosphorus corn–SBM diets supplemented with microbial phytase for growing-finishing pigs. J Anim Sci. 1998;76:808–813. doi: 10.2527/1998.763808x. [DOI] [PubMed] [Google Scholar]
- 47.Adeola O, Olukosi OA, Jendza JA, Dilger RN, Bedford MR. Response of growing pigs to Peniophora lycii- and Escherichia coli-derived phytases or varying ratios of calcium to total phosphorus. Anim Sci. 2006;82:637–644. [Google Scholar]
- 48.Amerah AM, Plumstead PW, Barnard LP, Kumar A. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult Sci. 2014;93:906–915. doi: 10.3382/ps.2013-03465. [DOI] [PubMed] [Google Scholar]
- 49.Selle PH, Cowieson AJ, Ravindran V. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest Sci. 2009;124:126–141. [Google Scholar]
- 50.Augspurger NR, Spencer JD, Webel DM, Baker DH. Pharmacological zinc levels reduce the phosphorus-releasing efficacy of phytase in young pigs and chickens. J Anim Sci. 2004;82:1732–1739. doi: 10.2527/2004.8261732x. [DOI] [PubMed] [Google Scholar]
- 51.Maenz DD, Engele-Schaan CM, Newkirk RW, Classen HL. The effect of minerals and mineral chelators on the formation of phytase-resistant and phytase susceptible forms of phytic acid in solution and in a slurry of canola meal. Anim Feed Sci Technol. 1999;81:177–192. [Google Scholar]
- 52.Boling SD, Webel DM, Mavromichalis I, Parsons CM, Baker DH. The effects of citric acid on phytate-phosphorus utilization in young chicks and pigs. J Anim Sci. 2000;78:682–689. doi: 10.2527/2000.783682x. [DOI] [PubMed] [Google Scholar]
- 53.Brenes A, Viveros A, Arija I, Centeno C, Pizarro M, Bravo C. The effect of citric acid and microbial phytase on mineral utilization in broiler chicks. Anim Feed Sci Technol. 2003;110:201–219. [Google Scholar]
- 54.Phromkunthong W, Nuntapong N, Gabaudan J. Interaction of phytase Ronozyme® P(L) and citric acid on the utilization of phosphorus by common carp (Cyprinus carpio. Songklanakarin J Sci Technol. 2010;32:547–554. [Google Scholar]
- 55.Kemme PA, Jongbloed AW, Mroz Z, Kogut J, Beynen AC. Digestibility of nutrients in growing-finishing pigs is affected by Aspergillus niger phytase, phytate and lactic acid levels. 2. Apparent total tract digestibility of phosphorus, calcium and magnesium and ileal degradation of phytic acid. Livest Prod Sci. 1999;58:119–127. [Google Scholar]
- 56.Ravindran V, Cowieson AJ, Selle PH. Influence of dietary electrolyte balance and microbial phytase on growth performance, nutrient utilization, and excreta quality of broiler chickens. Poult Sci. 2008;87:677–688. doi: 10.3382/ps.2007-00247. [DOI] [PubMed] [Google Scholar]
- 57.Parkkonen T, Tervila-Wilo A, Hopeakoski-Nurminen M, Morgan A, Poutanen K, Autio K. Changes in wheat microstructure following in vitro digestion. Acta Agric Scand, Sect B. 1997;47:43–47. [Google Scholar]
- 58.Yi Z, Kornegay ET. Sites of phytase activity in the gastrointestinal tract of young pigs. Anim Feed Sci Technol. 1996;61:361–368. [Google Scholar]
- 59.Kemme PA, Jongbloed AW, Mroz Z, Beynen AC. Diurnal variation in degradation of phytic acid by plant phytase in the pig stomach. Livest Prod Sci. 1998;54:33–44. [Google Scholar]
- 60.Lantzsch HJ, Hillenbrand S, Scheuermann SE, Menke KH. Comparative study of phosphorus utilization from wheat, barley and corn diets by young rats and pigs. J Anim Physiol Anim Nutr. 1992;67:123–132. [Google Scholar]
- 61.Mroz Z, Jongbloed AW, Kemme P. Apparent digestibility and retention of nutrients bound to phytate complexes as influenced by microbial phytase and feeding regimen in pigs. J Anim Sci. 1994;72:126–132. doi: 10.2527/1994.721126x. [DOI] [PubMed] [Google Scholar]
- 62.Jongbloed AW, Mroz Z, Kemme PA. The effect of supplementary Aspergillus niger phytase in diets for pigs on concentration and apparent digestibility of dry matter, total phosphorus, and phytic acid in different sections of the alimentary tract. J Anim Sci. 1992;70:1159–1168. doi: 10.2527/1992.7041159x. [DOI] [PubMed] [Google Scholar]
- 63.Pagano AR, Roneker KR, Lei XG. Distribution of supplemental Escherichia coli AppA2 phytase activity in digesta of various gastrointestinal segments of young pigs. J Anim Sci. 2007;85:1444–1452. doi: 10.2527/jas.2006-111. [DOI] [PubMed] [Google Scholar]
- 64.Marounek M, Skřivan M, Rosero O, Rop O. Intestinal and total tract phytate digestibility and phytase activity in the digestive tract of hens fed a wheat–maize–soyabean diet. J Anim Feed Sci. 2010;19:430–439. [Google Scholar]
- 65.Yu B, Jan YC, Chung TK, Lee TT, Chiou PWS. Exogenous phytase activity in the gastrointestinal tract of broiler chickens. Anim Feed Sci Technol. 2004;117:295–303. [Google Scholar]
- 66.Liebert F, Wecke C, Schoner FJ. Wenk C and Boessinger M. Proceedings of the 1st Symposium on Enzymes in Animal Nutrition. Switzerland: Kartause Ittingen; 1993. Phytase activities in different gut contents of chickens as dependent on levels of phosphorus and phytase supplementations; pp. 202–205. , ed. by, 13–16 October ( ) [Google Scholar]
- 67.Yan W, Reigh RC, Xu Z. Effects of fungal phytase on utilization of dietary protein and minerals, and dephosphorylation of phytic acid in the alimentary tract of channel catfish Ictalurus punctarus fed an all-plant-protein diet. J World Aquacult Soc. 2002;33:10–22. [Google Scholar]
- 68.Schäfer A, Koppe WM, Meyer-Burgdorff KH, Günter KD. Effects of a microbial phytase on the utilization of native phosphorus by carp in a diet based on SBM. Water Sci Technol. 1995;31:149–155. [Google Scholar]
- 69.Adeola O, Lawrence BV, Sutton AL, Cline TR. Phytase-induced changes in mineral utilization in zinc-supplemented diets for pigs. J Anim Sci. 1995;73:3384–3391. doi: 10.2527/1995.73113384x. [DOI] [PubMed] [Google Scholar]
- 70.Harper AF, Kornegay ET, Schell TC. Phytase supplementation of low-phosphorus growing-finishing pig diets improves performance, phosphorus digestibility, and bone mineralization and reduces phosphorus excretion. J Anim Sci. 1997;75:3174–3186. doi: 10.2527/1997.75123174x. [DOI] [PubMed] [Google Scholar]
- 71.Sands JS, Ragland D, Baxter C, Joern BC, Sauber TE, Adeola O. Phosphorus bioavailability, growth performance, and nutrient balance in pigs fed high available phosphorus corn and phytase. J Anim Sci. 2001;79:2134–2142. doi: 10.2527/2001.7982134x. [DOI] [PubMed] [Google Scholar]
- 72.Sebastian S, Touchburn SP, Chavez ER, Lague PC. Efficacy of supplemental microbial phytase at different dietary calcium levels on growth performance and mineral utilization of broiler chickens. Poult Sci. 1996;75:1516–1523. doi: 10.3382/ps.0751516. [DOI] [PubMed] [Google Scholar]
- 73.Ahmad T, Rasool S, Sarwar M, Haq AU, Hassan ZU. Effect of microbial phytase produced from a fungus Aspergillus niger on bioavailability of phosphorus and calcium in broiler chickens. Anim Feed Sci Technol. 2000;83:103–114. [Google Scholar]
- 74.Jackson LS, Li MH, Robinson EH. Use of microbial phytase in channel catfish Ictalurus punctatus diets to improve utilization of phytate phosphorus. J World Aquacult Soc. 1996;27:309–313. [Google Scholar]
- 75.Storebakken T, Shearer KD, Roem AJ. Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture. 1998;161:365–379. [Google Scholar]
- 76.Vielma J, Lall SP, Koskela J, Schöner FJ, Mattila P. Effects of dietary phytase and cholecalciferol on phosphorus bioavailability in rainbow trout (Oncorhynchus mykiss. Aquaculture. 1998;163:309–323. [Google Scholar]
- 77.Wu YB, Ravindran V, Hendriks WH, Morel PCH, Pierce J. Evaluation of a microbial phytase, produced by solid-state fermentation, in broiler diets. II. Influence on phytate hydrolysis, apparent metabolizable energy, and nutrient utilization. J Appl Poult Res. 2004;13:561–569. [Google Scholar]
- 78.Rutherfurd SM, Chung TK, Moughan PJ. Effect of microbial phytase on phytate P degradation and apparent digestibility of total P and Ca throughout the gastrointestinal tract of the growing pig. J Anim Sci. 2014;92:189–197. doi: 10.2527/jas.2013-6923. [DOI] [PubMed] [Google Scholar]
- 79.Zeng ZK, Wang D, Piao XS, Li PF, Zhang HY, Shi CX, Yu SK. Effects of adding super dose phytase to the phosphorus-deficient diets of young pigs on growth performance, bone quality, minerals and amino acids digestibilities. Asian-Australas J Anim Sci. 2014;27:237–246. doi: 10.5713/ajas.2013.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan F, Angel R, Ashwell C, Mitchell A, Christman M. Evaluation of the broiler's ability to adapt to an early moderate deficiency of phosphorus and calcium. Poult Sci. 2005;84:1232–1241. doi: 10.1093/ps/84.8.1232. [DOI] [PubMed] [Google Scholar]
- 81.Chung TK, Rutherfurd SM, Thomas DV, Moughan PJ. Effect of two microbial phytases on mineral availability and retention and bone mineral density in low-phosphorus diets for broilers. Br Poult Sci. 2013;54:362–373. doi: 10.1080/00071668.2013.783902. [DOI] [PubMed] [Google Scholar]
- 82.Hoppe PP, Schoner FJ, Wiesche H, Schwarz G, Safer A. Phosphorus equivalency of Aspergillus niger-phytase for piglets fed a grain–soybean-meal diet. J Anim Physiol Anim Nutr. 1993;69:225–234. [Google Scholar]
- 83.Cromwell GL, Stahly TS, Coffey RD, Monegue HJ, Randolph JH. Efficacy of phytase in improving the bioavailability of phosphorus in soybean meal and corn–soybean meal diets for pigs. J Anim Sci. 1993;71:1831–1840. doi: 10.2527/1993.7171831x. [DOI] [PubMed] [Google Scholar]
- 84.Yi Z, Kornegay ET, Ravindran V, Lindemann MD, Wilson JH. Effect of Natuphos phytase in improving the bioavailabilities of phosphorus and other nutrients in soybean meal-based semi-purified diets for young pigs. J Anim Sci. 1996;74:1601–1611. doi: 10.2527/1996.7471601x. [DOI] [PubMed] [Google Scholar]
- 85.Santos FR, Hruby M, Pierson EEM, Remus JC, Sakomura NK. Effect of phytase supplementation in diets on nutrient digestibility and performance in broiler chicks. J Appl Poult Res. 2008;17:191–201. [Google Scholar]
- 86.Selle PH, Ravindran V, Bryden WL, Scott T. Influence of dietary phytate and exogenous phytase on amino acids digestibility in poultry: a review. J Poult Sci. 2006;43:89–103. [Google Scholar]
- 87.Selle PH, Ravindran V, Caldwell RA, Bryden WL. Phytate and phytase: consequence for protein utilisation. Nutr Res Rev. 2000;13:255–278. doi: 10.1079/095442200108729098. [DOI] [PubMed] [Google Scholar]
- 88.Kerr BJ, Weber TE, Miller PS, Southern LL. Effect of phytase on apparent total tract digestibility of phosphorus in corn–soybean meal diets fed to finishing pigs. J Anim Sci. 2010;88:238–247. doi: 10.2527/jas.2009-2146. [DOI] [PubMed] [Google Scholar]
- 89.Ravindran V, Cabahug S, Ravindran G, Bryden WL. Influence of microbial phytase on apparent ileal amino acid digestibility in feedstuffs for broilers. Poult Sci. 1999;78:699–706. doi: 10.1093/ps/78.5.699. [DOI] [PubMed] [Google Scholar]
- 90.van der Klis JD, Pedersen C. Proceedings of the 1st International Phytase Summit. Washington, DC: 2010. In vitro tools to assess the efficacy of phytase and phosphorus availability; pp. 41–44. , 28–30 September ( ) [Google Scholar]
- 91.Esmaeilipour O, Van Krimpen MM, Jongbloed AW, De Jonge LH, Bikker P. Effects of temperature, pH, and pepsin on the stability of intrinsic phytase of rye, wheat, and barley. Poult Sci. 2011;90:36. [Google Scholar]
- 92.Von Sheuermann SE, Lantzoch HJ, Marke KH. In vitro and in vivo experiments on the hydrolysis of phytate: activity of plant phytase. J Anim Physiol Anim Nutr. 1988;60:64–75. [Google Scholar]
- 93.Jendza JA, Dilger RN, Sands JS, Adeola O. Efficacy and equivalency of an Escherichia coli-derived phytase for replacing inorganic phosphorus in the diets of broiler chickens and young pigs. J Anim Sci. 2006;84:3364–3374. doi: 10.2527/jas.2006-212. [DOI] [PubMed] [Google Scholar]
- 94.Bento MHL, Pedersen C, Plumstead PW, Salmon L, Nyachoti CM, Bikker P. Dose response of a new phytase on dry matter, calcium, and phosphorus digestibility in weaned piglets. J Anim Sci. 2012;90:245–247. doi: 10.2527/jas.53988. [DOI] [PubMed] [Google Scholar]
- 95.Adedokun SA, Sands JS, Adeola O. Determining the equivalent phosphorus released by an Escherichia coli derived phytase in broiler chicks. Can J Anim Sci. 2004;84:437–444. [Google Scholar]
- 96.Sands JS, Partridge GG, Hruby M, Perez-Maldonado RA. Proceedings of the 17th Australian Poultry Science Symposium. Australia: Sydney; 2005. A comparison of two phytase sources in diets for broilers; pp. 242–245. . 7–9 February ( ) [Google Scholar]
- 97.Pillai PB, O'Connor-Dennie T, Owens CM, Emmert JL. Efficacy of an Escherichia coli phytase in broilers fed adequate or reduced phosphorus diets and its effect on carcass characteristics. Poult Sci. 2006;85:1737–1745. doi: 10.1093/ps/85.10.1737. [DOI] [PubMed] [Google Scholar]
- 98.Kornegay ET, Radcliffe JS, Denbow DM. Influence of Natuphos® phytase on calcium bioavailability in plant ingredients and development of calcium equivalency values for swine and poultry. In: Coelho MB, Kornegay ET, editors. Phytase in Animal Nutrition and Waste Management. Mount Olive, NJ: BASF Corporation; 1996. pp. 419–434. ed. by, ) [Google Scholar]
- 99.Denbow DM, Ravindran V, Kornegay ET, Yi Z, Hulet RM. Improving phosphorus availability in soybean meal for broilers by supplemental phytase. Poult Sci. 1995;74:7831–1842. doi: 10.3382/ps.0741831. [DOI] [PubMed] [Google Scholar]
- 100.Kumar A, Bold RM, Plumstead PW. Comparative efficacy of Buttiauxella and E. coli phytase on growth performance in broilers. Poult Sci. 2012;90(Suppl 1) [Google Scholar]
- 101.Robinson EH, Li MH, Manning BB. Comparison of microbial phytase and dicalcium phosphate for growth and bone mineralization of pond-raised channel catfish. Ictalurus punctatus. J Appl Aquacult. 2002;12:81–88. [Google Scholar]
- 102.Liu LW, Luo YL, Hou HL, Pan J, Zhang W. Partial replacement of monocalcium phosphate with neutral phytase in diets for grass carp. Ctenopharyngodon idellus. J Appl Ichthyol. 2013;29:520–525. [Google Scholar]
- 103.Sardar P, Randhawa HS, Abid M, Prabhakar SK. Effect of dietary microbial phytase supplementation on growth performance, nutrient utilization, body compositions and haemato-biochemical profiles of Cyprinus carpio (L.) fingerlings fed soyprotein-based diet. Aquacult Nutr. 2007;13:444–456. [Google Scholar]
- 104.Peron A, Rema P, Conceiçao L, Frouel S. Phytase supplementation improves performance, nutrient digestibility and bone mineralization of Nile tilapia, Oral presentation. Ho Chi Minh City, Vietnam: Asian–Pacific Aquaculture Conference; 2013. 9–13 December ( ) [Google Scholar]
- 105.Selle PH, Cadogan DJ, Bryden WL. Effects of phytase supplementation of phosphorous-adequate, lysine-deficient, wheat-based diets on growth performance of weaner pigs. Aust J Agric Res. 2003;54:323–330. [Google Scholar]
- 106.Plumstead P. Research in phytate and phytase chemistry supports a higher efficacy of new generation bacterial phytases. AFMA MATRIX. 2007:12–13. December ( ) [Google Scholar]
- 107.Plumstead PW, Kwakernaak C, van der Klis JD. Use of a slope ratio assay to determine comparative efficacy of E. coli vs. Buttiauxella phytases in broilers. Poult Sci. 2012;91(Suppl 1) [Google Scholar]


