Abstract
Introduction
Oncological implications of laparoscopic resection in primary hepatic malignancy are not well defined. Laparoscopic liver resection (LLR) for hepatocellular carcinoma (HCC) in comparison to an open liver resection (OLR) in peri-operative and long-term oncological outcomes are described from a single North American institution.
Methods
From 2006 to 2013, all forty-three LLR patients for HCC were evaluated. Each patient was matched to two OLR patients for age at operation, maximal tumour size and tumour number.
Results
When compared with OLR, LLR had a lower severity of complication (0% versus 27%, P = 0.050) and lower 30-day readmission rate (2.3% versus 18.6%, P = 0.010). The length of stay (LOS) was shorter in LLR patients (5 versus 7 days, P < 0.001) and the estimated blood loss was also lower in LLR (300 versus 700 ml, P = 0.004). Admission to intensive care unit (ICU), emergency room (ER) visits and complication rates were similar. Overall, recurrence-free and intra-hepatic recurrence-free survival were comparable between LLR and OLR.
Discussion
LLR confers the widely-accepted benefits of laparoscopic surgery, namely severity of complication, 30-day readmission rate, LOS and blood loss. Further studies are required to examine intra- and extra-hepatic recurrence after LLR. LLR for HCC should be considered for appropriately selected patients in centres with requisite volume and expertise.
Introduction
Hepatocellular carcinoma (HCC) is the most common solid tumour in the world and the third leading cause of cancer-related death.1 In recent years, there has been a clear increase in HCC incidence in North America as a result of a multitude of factors including trends in the prevalence of predisposing conditions including non-alcohol-related fatty liver disease as well as hepatitis B and C infections.2,3 Curative options for HCC include surgical resection and liver transplantation. In the majority of North American centres, liver transplantation is reserved for patients with advanced cirrhosis and early HCC that meet regional transplantation guidelines.4 In contrast, hepatic resection may be considered as a primary therapy in patients with HCC and well-preserved liver function. Indeed, a resection may also be performed in patients with cirrhosis with well-persevered hepatic function who have been deemed unsuitable for, or declined, a liver transplantation.5
The surgical management of HCC is complicated by the concomitant management of two disease processes, the primary malignancy and the underlying liver disease. To date, an open liver resection (OLR) has been the accepted standard operative approach for resectable HCC. Owing to the presence of underlying liver disease, patients with HCC undergoing OLR are at a high risk of developing significant post-operative complications compared with open liver resections for other indications.6 A laparoscopic liver resection (LLR) offers a less-invasive alternative to OLR and may therefore be of particular benefit in this patient population. LLR has been slow to gain widespread traction because of the relative technical complexity and dearth of formal training; however, recent data reveal that an increasing number of centres are implementing LLR for both benign and malignant liver lesions.7 Emerging data suggest that LLR is safe,8 however, whereas its role in the treatment of benign and metastatic disease is well described, its application to primary hepatic malignancy is not well defined and the oncological outcomes are not clear. The aim of this study was to compare the outcomes of HCC patients with LLR versus OLR on a 2-to-1 matched-case basis.
Patients and methods
Study design and patient selection
Ethical approval for this study was granted by the institutional research ethics board at the University Health Network. A prospectively maintained database of all hepatic resections was interrogated to identify all patients who underwent a primary liver resection for HCC. Forty-three patients who underwent a liver resection for HCC were identified during the period from 30 May 2007 to 18 October 2013. Previous studies have demonstrated that variables including tumour size, tumour number and age are independent risk factors for survival, based on multivariate analysis.9–11 Thus, each patient was matched to two patients who received OLR according to the age at operation within 15 years, tumour size within 2.5 cm and tumour number was matched for solitary or multifocal tumours. All resections were performed by a specialist hepato-pancreatobiliary (HPB) surgeon at a university teaching centre.
Upon diagnosis of HCC, all patients were staged with multiphasic computed tomography (CT) of the chest and abdomen. If necessary, contrast-enhanced ultrasound (CEUS) and or magnetic resonance (MR) imaging were employed to confirm the diagnosis of HCC as per the American Association for the Study of Liver Diseases (AASLD) guidelines.4 All HCC patients were discussed at weekly multidisciplinary conferences consisting of HPB surgical oncologists, hepatologists, medical and radiation oncologists and interventional radiologists. In general, a liver resection was recommended for solitary lesions greater than 2 cm with well-preserved liver function as defined by Child–Pugh Class (A/B) and evidence of limited portal hypertension (platelet count > 100 000/μl, or hepatic venous pressure gradient <10 mmHg). Ablation was recommended as a definitive treatment for small, solitary HCC ≤2 cm. Liver transplantation was recommended for patients with multifocal HCC or decompensated cirrhosis. Patients with resectable multifocal lesions who were ineligible for, or declined transplantation, were offered a surgical resection.
Surgical technique
The technique employed for LLR has been described previously.12 In general, the approach to both OLR and LLR was similar. A major anatomical resection was reserved for larger tumours or where major vascular relations mandated a formal anatomical resection. For the purpose of parenchymal sparing, a non-anatomical and segmental resection was performed when an adequate margin could confidently be predicted. Inflow occlusion was obtained in all LLR and OLR lobectomies before parenchymal transection. Standard vascular stapling devices were used in both OLR and LLR when required. Water-jet dissection was used for parenchymal transection in all OLR and major (> 3 segments) LLR patients (Helix Hydrojet, ERBE and AMT Electrosurgery). Ultrasonic shears were used for parenchymal transection in all laparoscopic patients.13
Clinical outcomes
Patient demographics, including gender, age at resection and Child–Pugh classification, were recorded. Peri-operative outcomes included complication rate, severity of complications based on Clavien–Dindo classification,14 type of hepatic resection, estimated blood loss, admission to the intensive care unit (ICU), 30-day readmission rate, emergency room (ER) visits within 3 months, resection margin, length of stay (LOS), incision to closure time and conversion rate. Histological analysis of resected HCC specimens was also assessed, including underlying liver disease, WHO histological grade, microvascular invasion, liver fibrosis based on Laennec classification,15 tumour number and maximal tumour diameter.
Follow-up, survival and recurrence
After resection, patients were followed every 3 months in the first two post-operative years and then at 4-month intervals for post-operative years 3–5 with contrast-enhanced CT imaging of the abdomen and chest and or ultrasound (US). Suspected recurrence was further investigated with contrast enhanced CT, CEUS or MRI to confirm the diagnosis of HCC per AASLD criteria. After 5 years, patients returned to normal screening with US performed at 6-monthly intervals as per AASLD guidelines.4
The overall survival (OS) was calculated from the day of surgery until the day of death or last contact. The recurrence-free survival of patients who recurred was defined as the time from the day of surgery to the day of imaging study that confirmed tumour recurrence. For patients who did not develop recurrent disease, the day of surgery to the day of death or last contact was used.
Statistical analysis
Descriptive statistics were reported as median and range for continuous variables and as a number and percentage for discrete variables. The chi-square test or Fisher's exact test, where appropriate, was conducted to compare discrete variables between groups. The Mann–Whitney U-test was conducted for continuous variables, such as tumour margin and tumour diameter. Overall survival and recurrence-free survival were calculated by the Kaplan–Meier method and differences were compared by the log-rank test. The Cox-regression test was used for univariate and multivariate analysis using a confidence interval of 95%. Statistical significance was defined as P < 0.05. Statistical analysis was carried out using SPSS software (version 20; SPSS, Chicago, IL, USA).
Results
Demographics and peri-operative outcomes are shown in Table 1. Six out of 43 patients required conversion from a laparoscopic to open resection. All of these occurred in the first 3 years of the study and there were no conversions in subsequent years. Five of the six patients were converted as a result bleeding, whereas one patient was converted because of unanticipated anatomical considerations. Three patients in the LLR group had evidence of recent rupture but no evidence of peritoneal disease at the time of resection.
Table 1.
Patient demographics, peri- and post-operative course of patients who underwent laparoscopic and open liver resections for hepatocellular carcinoma
| LLR n = 43 (%) | OLR n = 86 (%) | Combined n = 129 (%) | P-value | |
|---|---|---|---|---|
| Gender | 0.110 | |||
| Male | 29 (67.4) | 69 (80.2) | 98 (76.0) | |
| Female | 14 (32.6) | 17 (19.8) | 31 (24.0) | |
| Age at resection (years; median, range) | 62.0 (30–86) | 63.0 (34–84) | 62.9 (30–86) | 0.521 |
| Underlying liver disease | 0.100 | |||
| HBV | 19 (44.2) | 52 (60.5) | 71 (55.0) | |
| HCV | 13 (30.2) | 18 (20.9) | 31 (24.0) | |
| Other (NASH, alcohol, undetermined) | 11 (25.6) | 16 (18.6) | 27 (20.9) | |
| Child–Pugh | 0.789 | |||
| A | 41 (97.6) | 81 (97.6) | 122 (97.6) | |
| B | 1 (2.4) | 2 (2.4) | 3 (2.4) | |
| Not available | 1 | 3 | 4 | |
| Clavien–Dindo Classification | 0.050 | |||
| Non-severe (Grade 1–2) | 10 (100.0) | 24 (72.7) | 34 (79.1) | |
| Severe (Grade 3–4) | 0 (0.0) | 9 (27.3) | 9 (20.9) | |
| No complication | 32 | 52 | 84 | |
| Not available | 1 | 1 | 2 | |
| Type of hepatic resection | ||||
| 1–2 segments | 21 (48.8) | 45 (52.3) | 66 (51.2) | 0.740 |
| 2 + segments | 22 (51.2) | 41 (47.7) | 63 (48.8) | |
| Resection margin | 1.000 | |||
| Positive | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Negative | 43 (100.0) | 86 (100.0) | 129 (100.0) | |
| Not available | 0 | 0 | 0 | |
| Estimated blood loss (ml; median, range) | 300 (0–6500) | 700 (0–3500) | 600 (0–6500) | 0.004 |
| Incision to closure time (min; median, range) | 170 (88–536) | 197 (70–365) | 194 (88–536) | 0.610 |
| Complications | 0.067 | |||
| Yes | 10 (23.3) | 34 (39.5) | 44 (34.1) | |
| No | 33 (76.7) | 52 (60.6) | 85 (65.9) | |
| Conversion | ||||
| Yes | 6 (14.0) | NA | NA | NA |
| No | 37 (86.0) | |||
| Admission to ICU | 0.657 | |||
| Yes | 3 (7.0) | 8 (9.3) | 11 (8.5) | |
| No | 40 (93.0) | 78 (90.7) | 118 (91.5) | |
| 30-day readmission | 0.010 | |||
| Yes | 1 (2.3) | 16 (18.6) | 17 (13.2) | |
| No | 42 (97.7) | 70 (81.4) | 112 (86.8) | |
| ER visit in 3 months | 0.267 | |||
| Yes | 2 (4.7) | 9 (10.5) | 11 (8.5) | |
| No | 41 (95.3) | 77 (89.5) | 118 (91.5) | |
| Length of stay (days; median, range) | 5 (3–51) | 7 (5–77) | 7 (3–77) | <0.001 |
OLR, open liver resection; HBV, hepatitis B virus;HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis; ICU, intensive care unit.
There were 18/43 and 33/86 cirrhotic patients in the LLR and OLR groups, respectively (Table 2). There was no statistical difference between these groups with respect to age at resection, type of resection, complication rate, ER visits within 3 months, resection margin, gender, 30-day readmission rate and severe complications. However, compared with the OLR group, the LLR group had a lower rate of ICU admission (0% versus 12%, P = 0.012), lower estimated blood loss (250 versus 800 ml, P = 0.015) and shorter LOS (6 versus 7 days, P = 0.018)
Table 2.
Histological analysis of hepatocellular carcinoma in patients who underwent laparoscopic and open liver resections for hepatocellular carcinoma
| LLR n = 43 (%) | OLR n = 86 (%) | Combined n = 129 (%) | P-value | |
|---|---|---|---|---|
| Histological grade | 0.541 | |||
| Well differentiated | 2 (5.0) | 5 (6.0) | 7 (5.6) | |
| Moderately differentiated | 29 (72.5) | 64 (76.2) | 93 (75.0) | |
| Poorly differentiated | 9 (22.5) | 15 (17.9) | 24 (19.4) | |
| Not available | 3 | 2 | 5 | |
| Microvascular invasion | 0.350 | |||
| Yes | 21 (52.5) | 37 (43.5) | 58 (46.4) | |
| No | 19 (47.5) | 48 (56.5) | 67 (53.6) | |
| Not available | 3 | 1 | 4 | |
| Liver fibrosis stage (Laennec classification) | 0.930 | |||
| 0 | 1 (2.4) | 1 (1.2) | 2 (1.6) | |
| 1 | 7 (17.1) | 11 (12.9) | 18 (14.3) | |
| 2 | 8 (19.5) | 17 (20.0) | 25 (19.8) | |
| 3 | 7 (17.1) | 23 (27.1) | 30 (23.8) | |
| 4 | 18 (43.9) | 33 (38.8) | 51 (40.5) | |
| Not available | 2 | 1 | 3 | |
| Tumour number | 0.989 | |||
| 1 | 41 (95.3) | 81 (95.3) | 122 (95.3) | |
| >1 | 2 (4.7) | 4 (4.7) | 7 (4.7) | |
| Not available | 0 | 1 | 1 | |
| Greatest tumour diameter (cm; median, range) | 5.4 (2–16) | 4.4 (2–14) | 4.8 (2–16) | 0.189 |
| Margin distance (cm; median, range) | 1.3 (0.10-6.00) | 1.0 (0.04–8) | 1.0 (0.04–8.00) | 0.250 |
LLR, laparoscopic liver resection; OLR, open liver resection.
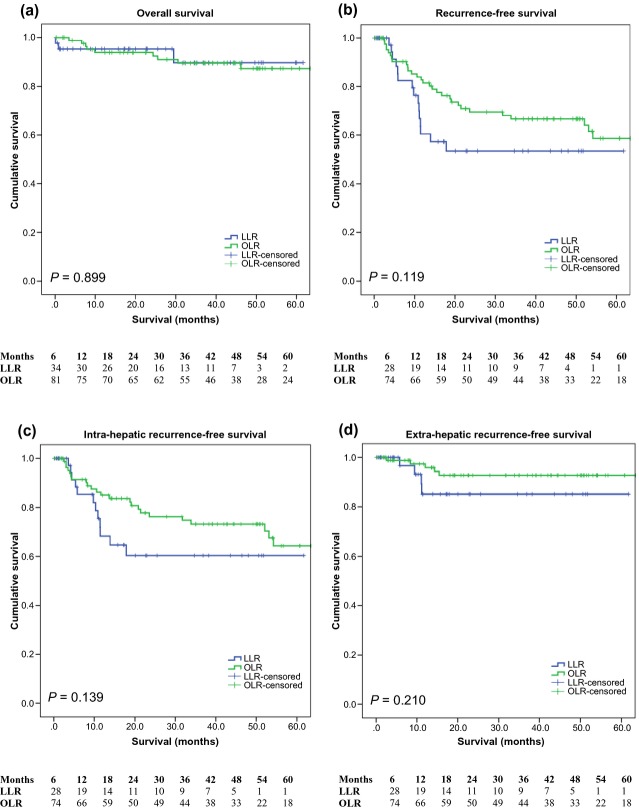
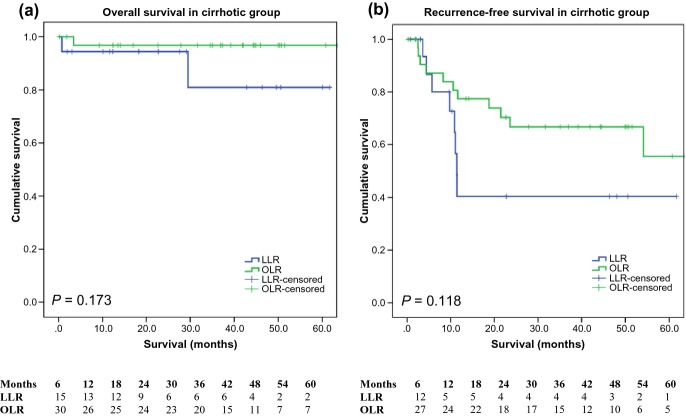
The median follow-up was 44.4 months for the OLR group and 22.7 months for the LLR group. The overall survival at 1, 3 and 5 years for LLR was 95.3%, 89.7% and 89.7%, respectively, and 93.9%, 89.5% and 87.3%, respectively, for OLR. Overall survival was similar (P = 0.899) and there was no statistical difference in the recurrence-free survival between the groups (P = 0.119) (Fig. 1). The 1-, 3- and 5-year recurrence-free survival was 60.5%, 53.5% and 53.5%, and 81.5%, 66.7% and 58.6% for the LLR and OLR groups, respectively. A subgroup analysis of the cirrhotic patients is illustrated in Fig. 2. Again, there was no statistical difference between the LLR and OLR groups in terms of overall survival (P = 0.173) and recurrence-free survival (P = 0.118). It is important to note that the numbers at risk in the LLR group at 5 years was low and should be interpreted with caution.
Figure 1.
Overall survival, recurrence-free survival, intra-hepatic recurrence-free survival and extra-hepatic recurrence-free survival according to laparoscopic or open liver resection for hepatocellular carcinoma (P = 0.899, 0.119, 0.139 and 0.210, respectively). LLR, laparoscopic liver resection; OLR, open liver resection.
Figure 2.
Overall survival and recurrence-free survival in cirrhotic patients (P = 0.173 and 0.118, respectively)
The intra-hepatic recurrence-free survival at 1, 3 and 5 years was 68.2%, 60.3%, 60.3% for LLR and 85.0%, 73.1% and 64.3% for OLR (P = 0.139). Also, the extra-hepatic recurrence-free survival was 85.1%, 85.1% and 85.1% for LLR and 97.4%, 92.8% and 92.8% for OLR patients (P = 0.210).
Discussion
Surgical resection of HCC offers an excellent prospect of both overall- and disease-free survival in non- and mildly-cirrhotic patients. The outcomes reported in this series are commensurate with the highest standards reported to date.16–18 In spite of the increasing ubiquity of laparoscopy, its adoption in liver resection has been slower than in other subspecialties. In the setting of chronic liver disease, many centres are reluctant to employ a laparoscopic resection owing to what were, initially, well-founded technical concerns. However, there is now a growing body of literature to support the safety and adequacy of LLR in the setting of benign and metastatic disease.19–23 This study sought to examine the utility of LLR for HCC compared with OLR in a single, large volume, North American institution.
In two, well-matched groups of patients, LLR is associated with oncological outcomes similar to those of OLR at intermediate follow-up. The short-term benefits of laparoscopic surgery in terms of post-operative pain and return to normal activity are well recognized, but what is not clear in the context of LLR for malignant disease, is medium- to long-term oncological outcome. This study demonstrates that LLR is associated with oncological outcomes similar to OLR and in keeping with the best reported data.24
Overall survival appears equivalent after LLR and OLR; however, there appears to be a non-significant trend suggesting a potentially higher intra-hepatic recurrence rate after LLR in this series. While patients were matched on known pre-operative tumour and patient factors (age, tumour size and tumour number) these outcomes are notable given the potentially higher rates of poorly-differentiated, large-size lesions and microvascular invasion within the LLR cohort, factors typically associated with reductions in both disease-free and overall survival.25 While margin status is similar between LLR and OLR, it is acknowledged that there may be a predilection towards non-anatomic resections in LLR. Previous open resection series have demonstrated that non-anatomic resections is associated with higher intra-hepatic recurrence rates compared with anatomic resections.26 Finally, HCV was a more common predisposing condition in the LLR group and some series have suggested a higher recurrence rate among HCV patients compared with other liver disease aetiologies.12 Therefore, while the LLR and OLR groups were matched on selected pre-operative factors, some imbalances in the surgical approach, patient factors and histological findings may have led to a higher intra-hepatic recurrence rate in this series. Given the relatively small sample size and design of this study, these trends should be interpreted with caution and are worthy of further investigation. In particular, the discrepancy in length of follow-up between groups must be considered. While outcomes at a median follow-up of 22.7 months in the LLR group are comparable to the OLR group, it must be recognized that survival may drop-off further over time. This will be carefully assessed in further reports.
Typically of studies comparing laparoscopic and open surgery, reductions in LOS, estimated blood loss, readmission rate and ER attendance in the LLR group compared with OLR were demonstrated. Furthermore, LLR was associated with a significantly lower rate of severe peri-operative complications compared with OLR. It is reasonable to suggest that this reduction in complications may be attributed to the minimally-invasive approach. It is also worth noting that this cohort of patients was accrued during the early experience of LLR at the institution. During the study period, the 6 patients converted to OLR from LLR occurred in the first 3 years and there have been no further conversions since then. This suggests that as the learning curve is overcome, further improvements may be expected.
The obvious limitations of the study are the single-centre, retrospective nature of the analysis and the inherent difficulties with matching studies. While the cohort size might be considered small in certain contexts, this cohort actually represents one of the largest reported to date from a North American centre. Although HCC remains relatively rarer in North America than elsewhere, this institution has a sizeable HCC practice. It was demonstrated that excellent outcomes can be achieved with LLR for HCC, comparable with those achieved with OLR. In addition, LLR offers significant benefits in terms of peri-operative outcomes compared with OLR and suggest that in appropriate centres, LLR should be considered as a first-line modality.
Conflicts of interest
None declared.
References
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Mehrabi A, Mollberg NM, Muller SA, Koch M, Buchler MW, et al. Hepatocellular carcinoma: current management and perspective for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- Davila JA, Morgan RO, Shaib Y, McGlyn KA, El-serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52:iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073–2082. doi: 10.1007/s00268-011-1161-0. [DOI] [PubMed] [Google Scholar]
- Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc. 2008;22:2208–2213. doi: 10.1007/s00464-008-0023-9. [DOI] [PubMed] [Google Scholar]
- Zhou YM, Shao WY, Zhao YF, Xu BL. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2011;56:1937–1943. doi: 10.1007/s10620-011-1572-7. [DOI] [PubMed] [Google Scholar]
- Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- Jun CH, Sim DW, Kim SH, Hong HJ, Chung MW, Myung E, et al. Predictive factors for recurrence and survival in hepatocellular carcinoma in South Korea. Anticancer Res. 2013;33:4129–4134. [PubMed] [Google Scholar]
- Hoffmann K, Muller-Butow V, Franz C, Hinz U, Longerich T, Buchler MW, et al. Factors predictive of survival after stapler hepatectomy of hepatocellular carcinoma: a multivariate, single-center analysis. Anticancer Res. 2014;23:767–776. [PubMed] [Google Scholar]
- Lee JJ, Kim PT, Fischer S, Fung S, Gallinger S, McGilvray I, et al. Impact of viral hepatitis on outcomes after liver resection for hepatocellular carcinoma: results from a north american center. Ann Surg Oncol. 2014;21:2708–2716. doi: 10.1245/s10434-014-3609-6. [DOI] [PubMed] [Google Scholar]
- Bhojani FD, Fox A, Pitzul K, Gallinger S, Wei A, Moulton CA, et al. Clinical and economic comparison of laparoscopic to open liver resections using a 2-to-1 matched pair analysis: an institutional experience. J Am Coll Surg. 2012;214:184–195. doi: 10.1016/j.jamcollsurg.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien P. Classification of surgical complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Cho MY, Baik SK, Park HJ, Jeon HK, Im CK, et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol. 2001;55:1004–1009. doi: 10.1016/j.jhep.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Kim HH, Park EK, Seoung JS, Hur YH, Yang SK, Jung CK, et al. Liver resection for hepatocellular carcinoma: case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc. 2011;80:412–419. doi: 10.4174/jkss.2011.80.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg. 2011;35:2268–2274. doi: 10.1007/s00268-011-1212-6. [DOI] [PubMed] [Google Scholar]
- Truant S, Boras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc. 2011;25:3668–3677. doi: 10.1007/s00464-011-1775-1. [DOI] [PubMed] [Google Scholar]
- Long TCD, Bac NH, Thuan ND, Dat LT, Viet DQ, Hoang LC. Laparoscopic liver resection: 5-year experience at a single center. Surg Endosc. 2014;28:796–802. doi: 10.1007/s00464-013-3259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli G, Fantini C, Belli A, Limongelli P. Laparoscopic liver resection for hepatocellular carcinoma in cirrhosis: long-term outcomes. Dig Surg. 2011;28:134–140. doi: 10.1159/000323824. [DOI] [PubMed] [Google Scholar]
- Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–1176. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- Parks KR, Kuo YH, Davis JM, O'Brien B, Hagopian EJ. Laparoscopic versus open liver resection: a meta-analysis of long-term outcome. HPB. 2014;16:109–118. doi: 10.1111/hpb.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6657–6668. doi: 10.3748/wjg.v18.i45.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195–202. doi: 10.1002/jso.10178. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for Hepatocellular carcinoma. Ann Surg. 2005;242:252–259. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]