Abstract
Background
Percutaneous cholecystostomy (PC) can be used to treat patients with acute calculous cholecystitis (ACC) who are considered to be unfit for surgery. However, this procedure has been insufficiently investigated. This paper presents the results of a 10-year experience with this treatment modality.
Methods
A retrospective observational study of all consecutive patients treated with PC for ACC in the period from 1 May 2002 to 30 April 2012 was conducted. All data were collected from patients' medical records.
Results
A total of 278 patients were treated with PC for ACC. Of these, 13 (4.7%) died within 30 days, 28 (10.1%) underwent early laparoscopic cholecystectomy and three (1.1%) patients were lost from follow-up. Of the remaining 234 patients, 55 (23.5%) were readmitted for the recurrence of cholecystitis. In 128 (54.7%) patients, PC was the definitive treatment (median follow-up time: 5 years), whereas 51 (21.8%) patients were treated with elective laparoscopic cholecystectomy. The frequency of recurrence of cholecystitis in patients with contrast passage to the duodenum on cholangiography was lower than that in patients without contrast passage (21.1% versus 36.7%; P = 0.037).
Conclusions
The present study, which is the largest ever conducted in this treatment area, supports the hypothesis that PC is an effective treatment modality for critically ill patients with ACC unfit for surgery and results in a low rate of 30-day mortality.
Introduction
Acute cholecystitis is a common condition which occurs in up to 20% of all patients with symptomatic gallstone disease and is best treated with early cholecystectomy.1 Generally, laparoscopic cholecystectomy is the treatment of choice; it is considered to be acceptable and safe, and is associated with low rates of morbidity and mortality.2,3 However, conversion from laparoscopic to open cholecystectomy substantially increases both morbidity and mortality.4–6 The risk for conversion increases with the duration of symptoms.7 In patients operated for acute calculous cholecystitis (ACC), conversion is required in up to 25% of subjects.8
Thus, in patients with a prolonged duration of symptoms and in critically ill patients who are considered to be unsuitable for surgery, percutaneous cholecystostomy (PC), in which the gallbladder is drained without the need for general anaesthesia,9 can be used as an alternative to laparoscopic cholecystectomy.10,11 Percutaneous cholecystostomy can be considered either as a bridging procedure to be followed by delayed laparoscopic cholecystectomy, or as a definitive treatment option for patients considered unfit for surgery.12–14
To date, only minor studies have evaluated the efficacy and safety of PC in the treatment of ACC. This paper presents the results of a 10-year experience of the use of PC as a treatment modality for ACC at a Danish university hospital.
Materials and methods
A retrospective study of outcomes in 278 consecutive patients treated with PC for ACC at Aarhus University Hospital over the 10-year period from 1 May 2002 to 30 April 2012 was conducted. The study was approved by the Danish Data Protection Agency (ref. j.nr. 2007-58-0010).
Study population and data on patients and procedures
Using the hospital's patient administration data, 345 consecutive patients treated with PC during the study period were identified using the following procedure codes from The Danish Classification of Surgical Procedures and Therapy: KJKA16 (percutaneous gallbladder drainage); UXRD46 (cholangiography through a catheter), and UXRD40 (cholangiography). From these 345 patients, all patients treated for indications other than ACC (acalculous cholecystitis and disease of unknown pathogenesis) were excluded, leaving a final study population of 278 patients.
All patients' medical records were retrospectively reviewed to obtain data on patient demographics, symptom duration, treatment modality and outcome, cholecystectomy and other operations performed during the index admission, recurrence of cholecystitis and 30-day mortality. All readmissions, both locally and nationally, were recorded. Readmission data were accessed through the Danish National Patient Registry, which includes information on all hospital admissions from 1977 and all outpatient clinic and emergency department visits from 1995 in Denmark.15
Diagnosis and treatment algorithm
The diagnosis of ACC was based on the presence of abdominal pain in the upper right quadrant, a positive Murphy's sign, fever, raised levels of C-reactive protein or leukocytes, and possibly affected liver function tests. The clinical evaluation was supplemented with ultrasonography or, rarely, computed tomography (CT) imaging. The presence of gallstones and thickening of the gallbladder wall (≥5 mm), probe tenderness and pericholecystic fluid were regarded as radiological signs of ACC.16 Indications for PC included a high burden of comorbidity and prolonged symptom duration (i.e. >5 days). Complicated symptomatology (e.g. suspected cholangitis) prompted endoscopic retrograde cholangiopancreaticography (ERCP) prior to PC.
Following PC, an antegrade cholangiography through the catheter was performed on postoperative day 3 (PoD 3). If no passage of contrast to the duodenum was shown, the procedure was repeated on PoD 5. If contrast passed to the duodenum and the patient's clinical response was satisfactory, the catheter was removed after 10 days of treatment. The presence of common bile duct stones prompted an ERCP.
Technique of PC
Percutaneous cholecystostomy was performed under ultrasound guidance by a dedicated interventional radiologist. The placement of the catheter (i.e. transperitoneal or transhepatic approach) varied according to the personal preference of the radiologist and the availability of the gallbladder under the given circumstances. The catheter was placed using an aseptic technique under local anaesthesia. In the context of transhepatic placement, the gallbladder was always punctured using Seldinger's technique and a 7-Fr pigtail catheter was placed in the gallbladder lumen. In the context of transperitoneal placement, a one-step method using a 7-Fr pigtail catheter (Skater Single Step Drainage Z-Locking; PBN Medicals Denmark A/S, Stenløse, Denmark) was used. The catheter was fixed to the skin using a patch and flushed up to three times daily.
Statistical analyses
Numerical data were described using the median and range as a measure of variation and compared using a median test. Categorical data were compared using Fisher's exact test. When appropriate, results are presented with 95% confidence intervals (CIs). Any two-sided P-value of <0.05 was considered to indicate statistical significance. All statistical analyses were carried out using stata Version 13.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 278 consecutive patients were treated with PC for ACC at the study institution during the study period (Table 1). There was no difference in demographic characteristics between patients treated with the transperitoneal method and those treated with the transhepatic approach (Table 2).
Table 1.
Data on patient characteristics and treatment procedures in 278 patients treated with percutaneous cholecystostomy (PC) for acute calculous cholecystitis
| Characteristic | Value |
|---|---|
| Age, years, median (range) | 72.5 (21–99) |
| Gender, n (%) | |
| Females | 157 (56.5%) |
| Males | 121 (43.5%) |
| Duration of symptoms, days, median (range)a | 4 (1–70) |
| Duration of PC treatment, days, median (range)b | 12 (0–193) |
| PC technique, n (%) | |
| Transperitoneal | 203 (73.0%) |
| Transhepatic | 62 (22.3%) |
| Unknown | 13 (4.7%) |
Missing values for 23 patients.
Missing values for five patients.
Table 2.
Data on patient characteristics and treatment procedures in 265 of 278 patients treated with percutaneous cholecystostomy for acute calculous cholecystitis according to treatment approach (transperitoneal or transhepatic)a
| Characteristic | Transperitoneal (n = 203) | Transhepatic (n = 62) | P-value |
|---|---|---|---|
| Age, years, median (range) | 73 (21–99) | 71 (31–94) | 0.135 |
| Gender, male, n (%) | 85 (41.9%) | 27 (43.5%) | 0.883 |
| Duration of symptoms, days, median (range)b | 4 (1–31) | 5 (1–70) | 0.184 |
| Duration of PC treatment, days, median (range)c | 12 (0–193) | 11.5 (1–109) | 0.918 |
| Cholangiography performed, n (%) | 180 (88.7%) | 53 (85.5%) | 0.508 |
| Contrast passage to the duodenum, n (%) | 126 (62.1%) | 43 (69.4%) | 0.365 |
Patients for whom information on approach is missing are omitted (n = 13).
Missing values for 22 patients.
Missing values for five patients.
Complications
All complications related to the procedures were recorded (Table 3). Catheter displacement was a frequent complication. Not all catheters were replaced, mainly because the patient in question demonstrated a good clinical response. No instance of bleeding required surgical intervention. If cholascos was suspected, an ultrasonography was performed. If cholascos was verified, drains were placed percutaneously guided by ultrasonography. There were no differences in complications between patients treated with the transperitoneal and transhepatic approaches, respectively.
Table 3.
Procedure-related complications according to treatment approach in 265 of 278 patients treated with percutaneous cholecystostomy for acute calculous cholecystitisa
| Complication | Transperitoneal (n = 203) | Transhepatic (n = 62) | P-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Bile leak | 10 (4.9%) | 1 (1.6%) | 0.467 |
| Bleeding | 2 (1.0%) | 0 | 1.000 |
| Catheter displaced, replaced | 41 (20.2%) | 10 (16.1%) | 0.582 |
| Catheter displaced, discontinued | 19 (9.4%) | 8 (12.9%) | 0.472 |
| Fistula to skin | 4 (2.0%) | 0 | 0.576 |
| Abscess formation/infection | 3 (1.5%) | 0 | 1.000 |
Patients for whom information on approach is missing are omitted (n = 13).
Of the 278 patients treated with PC, 28 (10.1%) underwent laparoscopic cholecystectomy during the index admission. Drains in these patients were usually removed at surgery. The rate of 30-day mortality was 4.7% (13 patients). Three (1.1%) patients were transferred to another hospital and were thus lost from follow-up. The remaining 234 patients were discharged and considered to have been treated with PC for ACC (Fig. 1).
Figure 1.
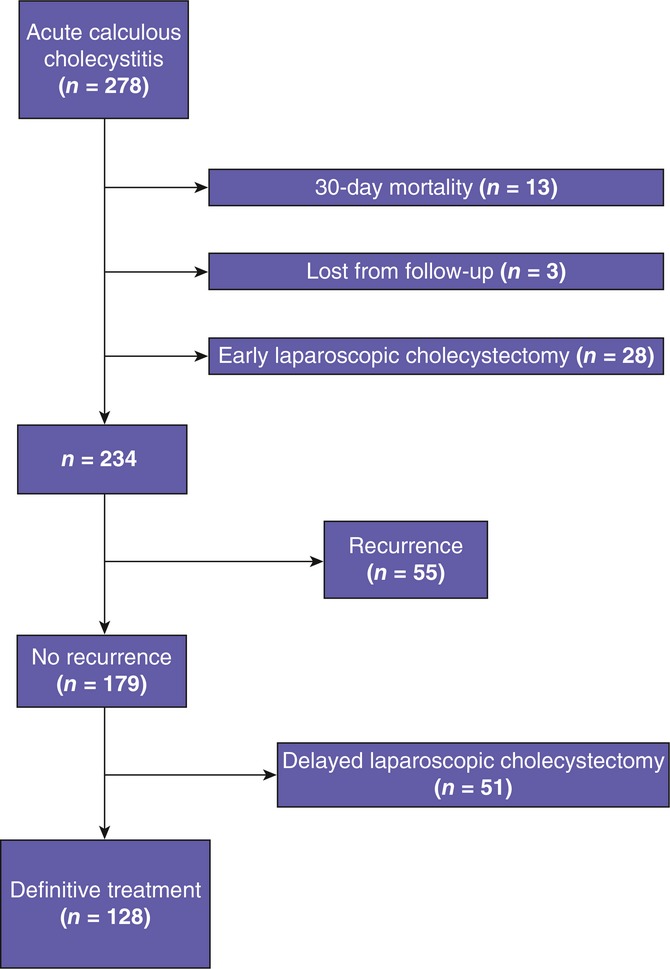
Flowchart of 278 patients treated with percutaneous cholecystostomy for acute calculous cholecystitis
Disease recurrence and cholangiography
Of these 234 patients, 55 (23.5%) were readmitted for the recurrence of cholecystitis and treated accordingly (Table 4). Patients with and without recurrence were similar in terms of median age, symptom duration and duration of PC treatment (data not shown). Among patients with recurrence of cholecystitis, a significantly lower proportion was treated with a transhepatic approach compared with patients without recurrence of cholecystitis (13.0% versus 26.5%; P = 0.043) when missing values for mode of approach were omitted. The median time from the end of the initial PC treatment to readmission was 54.5 days (range: 0–1518 days).
Table 4.
Choice of treatment in recurrence of acute calculous cholecystitis in 55 patients initially treated with percutaneous cholecystostomy
| Treatment modality | n (%) |
|---|---|
| Percutaneous cholecystostomy | 26 (47.3%) |
| Percutaneous cholecystostomy + laparoscopic cholecystectomy | 12 (21.8%) |
| Laparoscopic cholecystectomy | 9 (16.4%) |
| Conservative | 7 (12.7%) |
| Unknown | 1 (1.8%) |
In total, 128 patients (54.7%) were given PC as their definitive treatment and were followed for a median duration of 5 years (range: 1.1–10.5 years) without recurrence. Elective laparoscopic cholecystectomy was subsequently performed in 51 (21.8%) patients. In total, 215 patients underwent cholangiography during the index admission (Fig. 2). The odds ratio for the recurrence of cholecystitis was 2.17 (95% CI 1.02–4.55; P = 0.037) for patients without contrast passage to the duodenum (36.7%) compared with patients with contrast passage to the duodenum (21.1%) on cholangiography.
Figure 2.
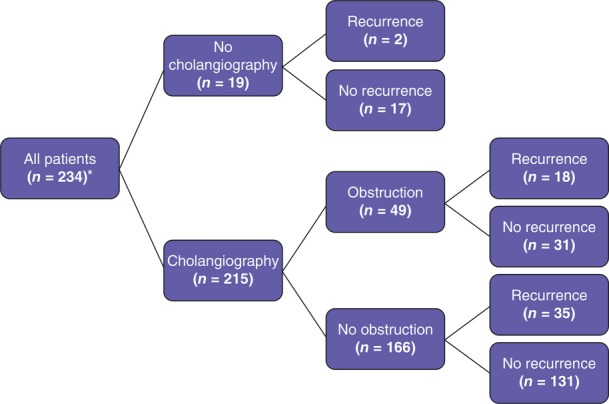
Results of cholangiography in 234 patients treated with percutaneous cholecystostomy (PC) for acute calculous cholecystitis. *Patients lost from follow-up (n = 3), patients who died within 30 days of PC (n = 13), and patients submitted to early laparoscopic cholecystectomy (n = 28) are excluded
Discussion
The incidence of gallstone disease, which is the primary risk factor for ACC, increases with age.17,18 Although the reference standard treatment of patients with ACC is laparoscopic cholecystectomy, some patients, especially those with a prolonged duration of symptoms and those who are critically ill, are considered to be unfit for surgery. For these patients, PC, either as a bridging procedure to subsequent elective cholecystectomy or as a definitive treatment modality, can be used in the management of ACC.10,11
The present study reports the results of a 10-year experience of the use of PC as a treatment modality in 278 ACC patients who were deemed unfit for laparoscopic cholecystectomy and thus describes the largest series to be reported to date. The initial PC served as a definitive treatment in 54.7% of patients and as a bridging procedure to subsequent elective cholecystectomy in 21.8% of patients. The recurrence rate was 23.5%, which corresponds to results by Sanjay et al.19
The present series demonstrated a 30-day mortality rate of 4.7%, which is lower than results presented by previous studies with smaller populations.19,20 This discrepancy may be partially explained by the fact that these earlier studies included patients with both calculous and acalculous cholecystitis. As the latter usually occurs in critically ill patients,21 the present population may have had a lower burden of comorbidity, on which no information was available, and thus a lower rate of mortality. However, Sanjay et al.19 found no differences in need for intensive care or frequency of readmission between patients with acalculous and those with calculous cholecystitis.
In this hospital department, the vast majority of patients with ACC are treated with either laparoscopic cholecystectomy or PC. Only a minor proportion of patients are treated with antibiotics as standalone therapy. The exact proportion of patients with ACC who were treated with PC is unknown, but is estimated to be approximately 12%. This accords with a study by Chang et al.,22 who found that 10.4% of their patients with ACC were treated with PC. A retrospective study by Cherng et al.23 reported on 185 patients with ACC treated with PC, but did not indicate the total number of patients diagnosed with ACC. Data from the study by Chang et al.22 suggest that patients in the present series were comparable with those in the earlier study in terms of comorbidities.
The optimal time at which the catheter should be removed after PC treatment for ACC remains controversial. Some authors advocate a minimum treatment duration of 6 weeks,19 although Hsieh et al.24 reported a higher risk for the early recurrence of cholecystitis if the placement of the catheter exceeded 2 weeks.24 In the present study, the median interval between treatment and catheter removal was 12 days, although there was a high degree of variation. Furthermore, it seems preferable to perform cholangiography through the PC catheter to ensure bile passage to the duodenum before catheter removal, as recurrence rates were lower in patients with contrast passage to the duodenum compared with those without contrast passage. Therefore, in patients who do not show passage to the duodenum on cholangiography, a lower threshold for cholecystectomy should be considered.
Both the transperitoneal and transhepatic routes for PC have been described in the literature, although the latter is more common.19,25,26 However, Sanjay et al.19 suggested that the transperitoneal route should be used when the gallbladder is grossly distended and adherent to the abdominal wall, or when unfavourable anatomy renders transhepatic access difficult. In the present series, the transperitoneal approach was used more frequently. Although there was no significant difference in the rate of complications between the two routes, the frequency of complications in this series was higher than those reported in studies in which the transhepatic approach was preferred.24,27,28
Furthermore, the transhepatic approach was found to be the more effective option in the treatment of ACC in this study. This finding may, of course, have occurred by chance, or it may indicate that the position in which the catheter is placed is more favourable with regard to gallbladder drainage in the transhepatic approach because the catheter can be stabilized by the liver parenchyma. Other than the rate of recurrence of cholecystitis, which was found to be higher among patients treated with the transperitoneal approach, no differences emerged in the characteristics of patients assigned to the transhepatic and the transperitoneal treatment modality, respectively. Thus, the transhepatic route seems superior to the transperitoneal. To the present authors' knowledge, this study is the first to demonstrate a difference in recurrence rate between these two approaches.
The limitations of this study include its retrospective design and thus a possible selection bias. In addition, the present data provide no information on the severity of comorbidities in the study patients, which limits possibilities for the comparison of these data with those from other studies. However, the major strengths of this study derive from the fact that the study population is large in comparison with patient series in previous work in this field, and from its longterm and almost complete follow-up, which was facilitated by access to online Danish medical registries.
In conclusion, the present study supports the suggestion that PC is a feasible treatment modality for patients with ACC who are deemed to be unfit for surgery. The rate of 30-day mortality in the present series is low in comparison with those in previous studies. In addition, the transhepatic route seems to be superior to the transperitoneal route in avoiding the recurrence of cholecystitis.
Conflicts of interest
None declared.
References
- Strasberg SM. Clinical practice. Acute calculous cholecystitis. N Engl J Med. 2008;358:2804–2811. doi: 10.1056/NEJMcp0800929. [DOI] [PubMed] [Google Scholar]
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of day-case laparoscopic cholecystectomy. Br J Surg. 2008;95:161–168. doi: 10.1002/bjs.6105. [DOI] [PubMed] [Google Scholar]
- Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:141–150. doi: 10.1002/bjs.6870. [DOI] [PubMed] [Google Scholar]
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997–2006. J Gastrointest Surg. 2009;13:2292–2301. doi: 10.1007/s11605-009-0988-2. [DOI] [PubMed] [Google Scholar]
- Kaafarani HM, Smith TS, Neumayer L, Berger DH, Depalma RG, Itani KM. Trends, outcomes, and predictors of open and conversion to open cholecystectomy in Veterans Health Administration hospitals. Am J Surg. 2010;200:32–40. doi: 10.1016/j.amjsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- To KB, Cherry-Bukowiec JR, Englesbe MJ, Terjimanian MN, Shijie C, Campbell DA, Jr, et al. Emergent versus elective cholecystectomy: conversion rates and outcomes. Surg Infect (Larchmt) 2013;14:512–519. doi: 10.1089/sur.2012.160. [DOI] [PubMed] [Google Scholar]
- Hadad SM, Vaidya JS, Baker L, Koh HC, Heron TP, Hussain K, et al. Delay from symptom onset increases the conversion rate in laparoscopic cholecystectomy for acute cholecystitis. World J Surg. 2007;31:1298–1301. doi: 10.1007/s00268-007-9050-2. ; discussion 1302–1303. [DOI] [PubMed] [Google Scholar]
- Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205–211. doi: 10.1016/j.amjsurg.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Akhan O, Akinci D, Ozmen MN. Percutaneous cholecystostomy. Eur J Radiol. 2002;43:229–236. doi: 10.1016/s0720-048x(02)00158-4. [DOI] [PubMed] [Google Scholar]
- Hultman CS, Herbst CA, McCall JM, Mauro MA. The efficacy of percutaneous cholecystostomy in critically ill patients. Am Surg. 1996;62:263–269. [PubMed] [Google Scholar]
- Vogelzang RL, Nemcek AA., Jr Percutaneous cholecystostomy: diagnostic and therapeutic efficacy. Radiology. 1988;168:29–34. doi: 10.1148/radiology.168.1.3289094. [DOI] [PubMed] [Google Scholar]
- Leveau P, Andersson E, Carlgren I, Willner J, Andersson R. Percutaneous cholecystostomy: a bridge to surgery or definite management of acute cholecystitis in high-risk patients? Scand J Gastroenterol. 2008;43:593–596. doi: 10.1080/00365520701851673. [DOI] [PubMed] [Google Scholar]
- Paran H, Zissin R, Rosenberg E, Griton I, Kots E, Gutman M. Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg. 2006;4:101–105. doi: 10.1016/j.ijsu.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Spira RM, Nissan A, Zamir O, Cohen T, Fields SI, Freund HR. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculus cholecystitis. Am J Surg. 2002;183:62–66. doi: 10.1016/s0002-9610(01)00849-2. [DOI] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:35–46. doi: 10.1007/s00534-012-0568-9. [DOI] [PubMed] [Google Scholar]
- Gouma DJ, Obertop H. Acute calculous cholecystitis. What is new in diagnosis and therapy? HPB Surg. 1992;6:69–78. doi: 10.1155/1992/46529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Sanjay P, Mittapalli D, Marioud A, White RD, Ram R, Alijani A. Clinical outcomes of a percutaneous cholecystostomy for acute cholecystitis: a multicentre analysis. HPB. 2013;15:511–516. doi: 10.1111/j.1477-2574.2012.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atar E, Bachar GN, Berlin S, Neiman C, Bleich-Belenky E, Litvin S, et al. Percutaneous cholecystostomy in critically ill patients with acute cholecystitis: complications and late outcome. Clin Radiol. 2014;69:e247–e252. doi: 10.1016/j.crad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Barie PS, Eachempati SR. Acute acalculous cholecystitis. Gastroenterol Clin North Am. 2010;39:343–357. doi: 10.1016/j.gtc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Chang YR, Ahn YJ, Jang JY, Kang MJ, Kwon W, Jung WH, et al. Percutaneous cholecystostomy for acute cholecystitis in patients with high comorbidity and re-evaluation of treatment efficacy. Surgery. 2014;155:615–622. doi: 10.1016/j.surg.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Cherng N, Witkowski ET, Sneider EB, Wiseman JT, Lewis J, Litwin DE, et al. Use of cholecystostomy tubes in the management of patients with primary diagnosis of acute cholecystitis. J Am Coll Surg. 2012;214:196–201. doi: 10.1016/j.jamcollsurg.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Chen CK, Su CW, Chan CC, Huo TI, Liu CJ, et al. Outcome after percutaneous cholecystostomy for acute cholecystitis: a single-centre experience. J Gastrointest Surg. 2012;16:1860–1868. doi: 10.1007/s11605-012-1965-8. [DOI] [PubMed] [Google Scholar]
- Chok KS, Chu FS, Cheung TT, Lam VW, Yuen WK, Ng KK, et al. Results of percutaneous transhepatic cholecystostomy for high surgical risk patients with acute cholecystitis. ANZ J Surg. 2010;80:280–283. doi: 10.1111/j.1445-2197.2009.05105.x. [DOI] [PubMed] [Google Scholar]
- Yun SS, Hwang DW, Kim SW, Park SH, Park SJ, Lee DS, et al. Better treatment strategies for patients with acute cholecystitis and American Society of Anesthesiologists classification 3 or greater. Yonsei Med J. 2010;51:540–545. doi: 10.3349/ymj.2010.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JC, Lee DW, Lai CW, Li AC, Chu DW, Chan AC. Percutaneous cholecystostomy for the treatment of acute cholecystitis in the critically ill and elderly. Hong Kong Med J. 2004;10:389–393. [PubMed] [Google Scholar]
- Melloul E, Denys A, Demartines N, Calmes JM, Schafer M. Percutaneous drainage versus emergency cholecystectomy for the treatment of acute cholecystitis in critically ill patients: does it matter? World J Surg. 2011;35:826–833. doi: 10.1007/s00268-011-0985-y. [DOI] [PubMed] [Google Scholar]


