Abstract
Objectives
The aim of this analysis was to examine prognostic features and outcomes in patients undergoing resection for intrahepatic cholangiocarcinoma (ICC).
Methods
A retrospective chart review was performed in all patients who underwent R0 or R1 resection for primary ICC between 1995 and 2011. Clinical data were abstracted and statistical analyses were conducted in the standard fashion.
Results
A total of 82 patients underwent curative hepatectomy for primary ICC; 51 patients in this cohort developed recurrence. The median follow-up of survivors was 27 months (range: 1–116 months). Recurrences were intrahepatic (65%), associated with multiple tumours (54%) and occurred during the first 2 years after hepatectomy (86%). The main factor associated with recurrence after resection was the presence of satellite lesions. Overall 5-year disease-free survival after primary resection was 16%. Factors associated with poor survival were transfusion and perineural invasion. Treatment of recurrence was undertaken in 89% of patients and repeat surgical resection was performed in 15 patients. The 3-year survival rate after recurrence was 25%. Prolonged survival after recurrence was associated with a solitary tumour recurrence.
Conclusions
Despite curative resection of ICC, recurrence can be expected to occur in 79% of patients at 5 years. Predictors of survival and recurrence after resection vary in the literature. In patients with recurrence, selection of the optimal treatment remains challenging.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy after hepatocellular carcinoma and accounts for 5–10% of all cholangiocarcinomas.1,2 In recent years, the increasing incidence reported in Western countries is at least in part related to growing recognition of this entity; in the past ICCs were often thought to be metastatic tumours from an unknown primary site.1–5 Hepatic resection is considered the first-line treatment in patients with ICC who have no evidence of distant metastases.6 In this setting, resection provides 5-year survival of 22–40%.6,7 However, 70% of patients present with advanced disease at diagnosis and have a median survival time of 9 months.6 Recurrence after resection remains high, with rates reported to lie between 44% and 70% at 5 years.8 Treatment of recurrent ICC remains challenging, with options including repeat resection, ablation, embolization, radiation and systemic chemotherapy.
Because of the rarity and low resectability rate of ICC, prognostic factors subsequent to resection have not been clearly established. In addition, options for the management of recurrence are not well described. The primary aim of this study was to define outcomes and identify prognostic features in patients undergoing curative treatment for ICC. In addition, the study aimed to determine risk factors and outcomes in patients with recurrent disease.
Materials and methods
Institutional review board approval was obtained and prospectively collected data from the Mount Sinai Medical Center were retrospectively reviewed. All consecutive patients with pathologically proven ICC who underwent hepatic resection with curative intent between January 1995 and January 2011 were included. The surgery was performed at a tertiary centre by two experienced hepatobiliary surgeons. The endpoint of the data collection was April 2014. Patients with hilar or distal cholangiocarcinoma, gallbladder cancer and hepatocellular carcinoma were excluded, as were patients with ICC who underwent liver transplantation. Patients who underwent R2 resection were also excluded. The primary endpoint analysed was overall survival from the date of primary resection. The secondary endpoint was time to progression from date of recurrence.
Preoperative variables examined included age, gender, underlying liver disease, extent of initial resection, interval from resection to recurrence, and Child–Pugh class. Pathological data on both the initial and recurrent tumours were examined, including data on size, number of tumours, presence of satellite nodules, grade of differentiation, presence and degree of vascular invasion, perineural invasion, and lymph node metastases. Satellite nodules were defined as tumour nodules of ≤2 cm in diameter located ≤2 cm from the primary tumour.
All patients underwent preoperative contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the chest and abdomen. Biopsy was not typically performed prior to surgery. Consideration for resection required the patient to be non-cirrhotic or to be cirrhotic with Child–Pugh class A status with no evidence of portal hypertension (platelet count >100 000/μL and absence of varices and splenomegaly on endoscopy/imaging).
Laparoscopy was performed routinely before laparotomy. Resection was undertaken only in the absence of peritoneal spread and contralateral liver metastasis. Limited lymph node involvement in the porta hepatis did not preclude resection, but nodal metastases extending to the coeliac or para-aortic region were considered to represent metastatic disease and a reason not to proceed. Intraoperative ultrasound was employed prior to and during resection. Systematic portal lymphadenectomy was performed routinely. Patients with lymph node involvement or positive surgical margins typically received adjuvant chemotherapy with or without external beam radiation. Routine common bile duct resection was not performed. Portal vein resection and reconstruction were performed in patients with macroscopic invasion.
After hepatectomy, patients were followed with serial tumour marker [carbohydrate antigen (CA) 19-9] levels and contrast-enhanced imaging studies of the chest and abdomen. Surveillance was carried out every 3 months for the first year, every 4 months for the second year, and biannually thereafter. Diagnosis of recurrence was established by radiographic means, rising CA 19-9, or by tissue biopsy in select circumstances in patients with atypical imaging characteristics.
Repeat resection was considered in patients with a single site of recurrence, either intra- or extrahepatic. Patients with limited intrahepatic recurrence who were not eligible for resection were treated with radiofrequency ablation if feasible (one to three tumours of <4 cm in size). Patients with multinodular isolated hepatic recurrence exceeding these limits were treated with transarterial chemoembolization (TACE) or radioembolization. Systemic chemotherapy employing a variety of regimens was offered to patients with unresectable systemic disease, as well as to patients with intrahepatic recurrence concomitant with locoregional treatments. A multimodal approach to treatment was standard practice for patients presenting with recurrent ICC.
Categorical variables were expressed as percentages and compared using the chi-squared test. Continuous variables were expressed as the median (range) and tested with the Mann–Whitney U-test. Survival was calculated in months from the time of primary resection until the last clinical or telephone encounter, or death. Recurrence and time to recurrence were included to evaluate their impact on survival. Patient survival and recurrence rates were calculated using the Kaplan–Meier method and compared using the log-rank test. Perioperative deaths (at <30 days) were excluded from the outcome analysis. Variables found to be significantly associated with the endpoint on univariate analysis (P < 0.2) were entered into a Cox proportional hazards model to identify independent predictors. A P-value of <0.05 was considered significant on multivariate analysis. Analysis was carried out using IBM spss Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
During the study period, 86 patients underwent hepatic resection for ICC. Four of these patients had an R2 resection and were excluded. In the remaining 82 patients, an R0 resection was achieved in 67 (82%) and an R1 resection was performed in 15 (18%). Extrahepatic bile duct resection was performed in 14 (17%) patients in whom the intrahepatic tumour mass extended to the biliary confluence. Portal vein resection and reconstruction were performed in four (5%) patients with macroscopic invasion.
By the end of the study period, 49 (60%) patients had died and 51 (62%) had developed recurrence. One (1%) perioperative death occurred; this patient was excluded from the survival analysis. The median follow-up of survivors was 27 months (range: 1–116 months). The initial clinical, pathologic and operative data are summarized in Table 1.
Table 1.
Characteristics of patients with intrahepatic cholangiocarcinoma (ICC) at presentation (n = 82)
| Clinical, operative and pathological variables | At presentation | P-value | |
|---|---|---|---|
| Group 1 No recurrence n = 31 (38%) | Group 2 Recurrence n = 51 (62%) | ||
| Age, years, median (range) | 63 (44–77) | 60 (31–86) | 0.419 |
| Male gender, n (%) | 20 (64%) | 20 (39%) | 0.026 |
| Ethnicity, n (%) | |||
| White | 16 (52%) | 39 (77%) | 0.037 |
| Asian | 9 (31%) | 5 (10%) | |
| Other | 5 (17%) | 5 (10%) | |
| Symptoms at presentation, n (%) | 13 (42%) | 26 (51%) | 0.426 |
| Cirrhosis, n (%) | 4 (13%) | 3 (6%) | 0.261 |
| Extent of resection, n (%) | |||
| Right lobectomy | 12 (36%) | 13 (26%) | |
| Right trisectionectomy | 2 (6%) | 2 (6%) | |
| Left lobectomy | 7 (21%) | 16 (33%) | |
| Left trisectionectomy | 0 | 8 (16%) | |
| Segmentectomy | 8 (24%) | 6 (12%) | |
| Other | 4 (12%) | 5 (10%) | |
| Pringle manoeuvre, n (%) | 7 (87%) | 41 (80%) | 0.434 |
| Transfusion, n (%) | 10 (32%) | 15 (29%) | 0.786 |
| Solitary tumour nodule, n (%) | 29 (93%) | 44 (86%) | 0.307 |
| Tumour size, cm, median (range) | 7 (3–21) | 8 (3–17) | 0.405 |
| Histological differentiation, n (%) | 0.857 | ||
| Good | 1 (4%) | 2 (4%) | |
| Moderate | 12 (46%) | 25 (52%) | |
| Poor | 13 (50%) | 21 (44%) | |
| Vascular invasion, n (%) | 0.247 | ||
| None | 18 (58%) | 20 (39%) | |
| Microinvasion | 12 (39%) | 28 (55%) | |
| Macroinvasion | 1 (3%) | 3 (6%) | |
| Perineural invasion, n (%) | 7 (23%) | 15 (29%) | 0.498 |
| Lymph node involvement, n (%) | 6 (19%) | 15 (29%) | 0.312 |
| Satellite nodules, n (%) | 3 (10%) | 17 (33%) | 0.019 |
| Surgical margin, mm | |||
| Median (range) | 7 (0–35) | 6 (0–30) | 0.502 |
| Positive (R1), n (%) | 1 (3%) | 14 (28%) | 0.007 |
| Adjuvant chemotherapy ± radiotherapy | 1 (3%) | 22 (43%) | <0.001 |
| Follow-up, months, median (range) | 23 (1–116) | 29 (2–100) | 0.238 |
The median time from primary resection to recurrence was 18 months [95% confidence interval (CI) 12–25 months] and the median follow-up after recurrence was 16 months (range: 1–91 months). Data on overall recurrence is shown in Fig. 1. Recurrences were noted to be intrahepatic (n = 32, 63%), multiple lesions (n = 27, 53%) and to occur most often during the first 2 years after hepatectomy (n = 42, 82%). Sites of extrahepatic recurrence (n = 17) included lymph nodes (n = 7), lung (n = 3), bone (n = 3), peritoneum (n = 2), brain (n = 1) and ovary (n = 1). Factors associated with recurrence after primary resection are summarized in Table 2.
Figure 1.

Rates of overall recurrence in patients with intrahepatic cholangiocarcinoma (ICC) after primary hepatectomy (n = 81)
Table 2.
Predictors of recurrence after primary resection (n = 81) in univariate and multivariate analyses
| Variable | n (number of events) | Time to recurrence, months, median (95% CI) | P-value |
|---|---|---|---|
| Age | |||
| ≤60 years | 33 (23) | 15 (7–23) | 0.267 |
| >60 years | 48 (28) | 22 (12–32) | |
| Gender | |||
| Male | 39 (20) | 27 (8–46) | 0.165 |
| Female | 42 (31) | 17 (14–21) | |
| Underlying liver disease | |||
| None | 56 (37) | 18 (15–21) | 0.270 |
| Present | 25 (14) | 27 (0–57) | |
| Number of tumours | |||
| Single | 9 (7) | 16 (14–19) | 0.111 |
| Multiple | 72 (44) | 19 (13–25) | |
| Positive margin | |||
| Yes | 19 (14) | 23 (14–32) | 0.573 |
| No | 60 (36) | 18 (11–24) | |
| Adjuvant treatment | 0.003 | ||
| Yes | 23 (22) | 15 (5–25) | |
| No | 58 (29) | 24 (7–40) | |
| Vascular invasion | |||
| Yes | 44 (31) | 16 (9–24) | 0.011 |
| No | 37 (20) | 34 (5–64) | |
| Satellites | |||
| Yes | 20 (17) | 9 (5–13) | <0.001a |
| No | 60 (34) | 27 (10–43) | |
| Perineural invasion | |||
| Yes | 21 (15) | 9 (3–16) | 0.020 |
| No | 60 (36) | 22 (15–28) | |
| Lymph node involvement | |||
| Yes | 21 (15) | 11 (3–19) | 0.018 |
| No | 60 (36) | 23 (7–39) | |
| Symptoms | |||
| Yes | 39 (26) | 18 (13–22) | 0.515 |
| No | 42 (25) | 22 (15–29) | |
| Differentiation | |||
| Good | 3 (2) | 9 (–) | 0.529 |
| Moderate | 37 (25) | 22 (15–29) | |
| Poor | 33 (21) | 18 (1–34) | |
| Size | |||
| ≤5 cm | 24 (14) | 34 (5–63) | 0.149 |
| >5 cm | 56 (37) | 18 (11–25) | |
| Cirrhosis | |||
| Yes | 7 (3) | 23 (–) | 0.284 |
| No | 72 (47) | 18 (12–24) | |
| Transfusion requirement | |||
| No | 56 (36) | 22 (11–32) | 0.665 |
| Yes | 25 (15) | 18 (14–21) | |
| Extent of vascular invasion | |||
| No | 37 (20) | 34 (5–64) | 0.031 |
| Microvascular | 40 (28) | 16 (9–23) | |
| Macrovascular | 4 (3) | 8 (3–14) |
Significant on multivariate analysis: presence of satellites (P = 0.012, odds ratio 2.532, 95% CI 1–5).
95% CI, 95% confidence interval.
Treatment of recurrence was undertaken in 41 (89%) patients and included surgical resection (n = 15), locoregional therapy (n = 13), systemic therapy alone (n = 13) and supportive care (n = 5). Surgical resection (n = 15) included repeat liver resection for localized intrahepatic recurrence (n = 10) or metastasectomy (ovary, n = 1; brain, n = 1; lung, n = 2; vertebra, n = 1).
The overall survival of patients with recurrent disease is shown in Fig. 2. Median disease-free survival was 16 months (95% CI 11–21 months), with 1-, 3- and 5-year disease-free survival rates of 60%, 24% and 16%, respectively. Predictors of survival after primary hepatic resection are shown in Table 3. The median survival after recurrence was 19 months (95% CI 15–23 months) and 1- and 3-year survival rates were 74% and 25%, respectively. Age, gender, underlying liver disease, site of recurrence, and time to recurrence (<12 months) were not significant predictors of survival after recurrence. Prolonged survival after recurrence was associated with the presence of a solitary tumour recurrence (P = 0.032).
Figure 2.
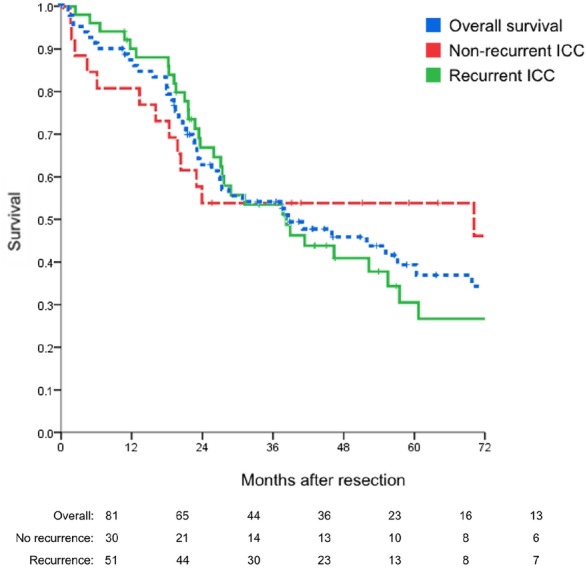
Rates of overall survival in patients with intrahepatic cholangiocarcinoma (ICC) after primary hepatectomy, with recurrent and non-recurrent disease (P = 0.275) (n = 81)
Table 3.
Predictors of survival after primary resection (n = 81) in univariate and multivariate analyses
| Variable | n (number of events) | Survival, months, median (95% CI) | P-value |
|---|---|---|---|
| Age | |||
| ≤60 years | 33 (17) | 52 (32–73) | 0.330 |
| >60 years | 48 (31) | 27 (5–50) | |
| Gender | 0.586 | ||
| Male | 39 (25) | 27 (2–52) | |
| Female | 42 (23) | 46 (23–70) | |
| Underlying liver disease | 0.131 | ||
| None | 56 (37) | 31 (19–44) | |
| Present | 25 (11) | 97 (26–168) | |
| Number of tumours | |||
| Single | 73 (42) | 41 (15–67) | 0.153 |
| Multiple | 9 (7) | 27 (3–51) | |
| Recurrence | |||
| Yes | 51 (35) | 38 (23–54) | 0.193 |
| No | 30 (13) | >median | |
| Positive margin | |||
| Yes | 19 (11) | 38 (6–70) | 0.850 |
| No | 60 (35) | 41 (14–68) | |
| Adjuvant treatment | |||
| Yes | 23 (15) | 31 (16–36) | 0.241 |
| No | 58 (33) | 46 (15–78) | |
| Vascular invasion | |||
| Yes | 42 (26) | 24 (17–30) | 0.020 |
| No | 38 (22) | 57 (19–96) | |
| Satellites | |||
| Yes | 20 (12) | 24 (18–30) | 0.028 |
| No | 60 (35) | 52 (30–74) | |
| Perineural invasion | |||
| Yes | 21 (15) | 20 (12–27) | 0.001a |
| No | 60 (33) | 55 (35–76) | |
| Lymph node involvement | |||
| Yes | 21 (11) | 26 (14–37) | 0.250 |
| No | 60 (37) | 46 (26–66) | |
| Symptoms | |||
| Yes | 39 (23) | 38 (14–62) | 0.769 |
| No | 42 (25) | 41 (12–71) | |
| Differentiation | |||
| Good | 3 (1) | 22 (–) | 0.824 |
| Moderate | 37 (21) | 41 (29–54) | |
| Poor | 33 (19) | 55 (32–79) | |
| Size | |||
| ≤5 cm | 24 (14) | 57 (9–106) | 0.869 |
| >5 cm | 56 (33) | 39 (20–58) | |
| Cirrhosis | |||
| Yes | 7 (4) | 52 (0–111) | 0.851 |
| No | 72 (43) | 39 (20–58) | |
| Transfusion requirement | |||
| No | 56 (29) | 55 (33–78) | 0.014a |
| Yes | 25 (19) | 23 (10–36) | |
| Extent of vascular invasion | |||
| No | 37 (21) | 57 (20–95) | 0.027 |
| Microvascular | 40 (23) | 27 (8–46) | |
| Macrovascular | 4 (4) | 7 (0–26) |
Significant on multivariate analysis.
Perineural invasion (P = 0.015, odds ratio 2.403, 95% CI 1−5).
Transfusion requirement (P = 0.021, odds ratio 2.134, 95% CI 1–4).
95% CI, 95% confidence interval.
Discussion
The role of hepatectomy in the treatment of ICC is well established.3,6,9 However, recurrence is common despite resection of ICC, as is apparent by the 79% rate of recurrence at 5 years identified in the current series. This study analysed the outcomes of 82 consecutive patients with ICC treated with curative resection at a single Western centre over a 16-year period.
Recurrence in this series occurred most often (86%) during the first 2 years after hepatectomy, with a median time to recurrence of 18 months. The liver remnant was the most common site of recurrence (65%). In this series, predictors of recurrence were satellite nodules. Endo et al. reported that multiple tumours, regional nodal involvement and large tumour size represented independent predictors of poor recurrence-free survival following resection.6
This study demonstrates a 5-year disease-free survival rate of 16% with a median follow-up of 27 months. Predictors of poor survival in the present series were transfusion requirements and perineural invasion. Independent predictors of survival in other published studies were age <40 years, lymphatic invasion, and preoperative CA 19-9 levels of >37 ng/dL.3,10–12 Interestingly, the current series did not demonstrate an association between positive margins and poor survival. In other series, margins of <1 cm were associated with decreased survival rates.9 Efforts to achieve wide surgical margins whenever possible are therefore advocated.
Recurrence rates are high despite attempted curative treatment; however, approaches to recurrent ICC remain controversial and challenging. Limited data exist on the treatment of recurrence after primary resection for ICC.8,9,13–16 Various treatment modalities have been proposed in recent years in patients with recurrent disease. Locoregional therapies such as radiofrequency ablation (RFA), TACE and transarterial radioembolization have been shown to be safe and effective in the treatment of ICC.17–20 Locoregional therapies were offered to 26% of patients in the present study and achieved a 3-year survival rate of 35% in patients with unresectable recurrent disease. Elsewhere, the treatment of recurrent cholangiocarcinoma after curative resection with RFA resulted in a median overall survival of 27.4 months in a series of 20 patients.18 Sulpice et al. discussed the role of yttrium-90 radioembolization and demonstrated a significant survival benefit in the treatment of recurrent ICC.16 Similarly, Saxena et al.19 reported disease control in 72% of treated patients, and Whitney et al.21 demonstrated tumour downstaging in a subgroup of patients treated with yttrium-90. Kiefer et al. reported local tumour control with the use of TACE in the treatment of unresectable ICC.22
There are even fewer data on the role of repeat resection in the management of isolated recurrent disease.9,13,16,23 Recent published series report the resection of recurrent ICC in 8.5–30% of patients.8,9,13–16 Repeat resection in the treatment of recurrent ICC has been demonstrated as an independent predictor of overall survival in several series. Among the patients with liver-only recurrence in the present series, 10 had preserved liver function and isolated disease, and thus were candidates for second hepatectomy. In addition, five patients with isolated extrahepatic disease were selected for repeat resection. Low statistical power and the heterogeneity of this group of patients limit the ability to further evaluate this subgroup.
Conclusions
In summary, despite curative resection of ICC, recurrence can be expected to occur in 79% of patients at 5 years. Predictors of survival and recurrence after resection vary in the literature. In patients with recurrence, selection of the optimal treatment remains challenging.
Conflicts of interest
None declared.
References
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl. 6):1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumours. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. discussion 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolani G, Vetrone G, Grazi GL, Aramaki O, Cescon M, Ravaioli M, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252:107–114. doi: 10.1097/SLA.0b013e3181e462e6. [DOI] [PubMed] [Google Scholar]
- Cherqui D, Tantawi B, Alon R, Piedbois P, Rahmouni A, Dhumeaux D, et al. Intrahepatic cholangiocarcinoma. Results of aggressive surgical management. Arch Surg. 1995;130:1073–1078. doi: 10.1001/archsurg.1995.01430100051011. [DOI] [PubMed] [Google Scholar]
- Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Hamba H, Tanaka H, et al. Histologic factors affecting prognosis following hepatectomy for intrahepatic cholangiocarcinoma. World J Surg. 2001;25:865–869. doi: 10.1007/s00268-001-0042-3. [DOI] [PubMed] [Google Scholar]
- Jan YY, Yeh CN, Yeh TS, Chen TC. Prognostic analysis of surgical treatment of peripheral cholangiocarcinoma: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:1779–1784. doi: 10.3748/wjg.v11.i12.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CN, Jan YY, Chen MF. Influence of age on surgical treatment of peripheral cholangiocarcinoma. Am J Surg. 2004;187:559–563. doi: 10.1016/j.amjsurg.2003.12.051. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sakaguchi T, Yokoi Y, Okamoto K, Kurachi K, Tsuchiya Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002;26:687–693. doi: 10.1007/s00268-001-0291-1. [DOI] [PubMed] [Google Scholar]
- Lang H, Sotiropoulos GC, Frühauf NR, Dömland M, Paul A, Kind EM, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single centre experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241:134–143. doi: 10.1097/01.sla.0000149426.08580.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Significance of repeated resection for recurrent intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2009;56:1–5. [PubMed] [Google Scholar]
- Sulpice L, Rayar M, Boucher E, Pracht M, Meunier B, Boudjema K. Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg. 2012;99:1711–1717. doi: 10.1002/bjs.8953. [DOI] [PubMed] [Google Scholar]
- Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2011;80:e221–e225. doi: 10.1016/j.ejrad.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–491. doi: 10.1245/s10434-009-0777-x. [DOI] [PubMed] [Google Scholar]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113:2119–2128. doi: 10.1002/cncr.23818. [DOI] [PubMed] [Google Scholar]
- Whitney R, Tatum C, Hahl M, Ellis S, Scoggins CR, McMasters K, et al. Safety of hepatic resection in metastatic disease to the liver after yttrium-90 therapy. J Surg Res. 2011;166:236–240. doi: 10.1016/j.jss.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-centre study. Cancer. 2011;117:1498–1505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos GC, Lang H, Broelsch CE. Surgical management of recurrent intrahepatic cholangiocellular carcinoma after liver resection. Surgery. 2005;137:669–670. doi: 10.1016/j.surg.2005.03.007. [DOI] [PubMed] [Google Scholar]


