Abstract
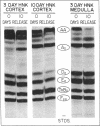
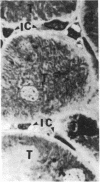
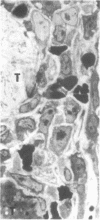
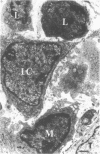
Unilateral ureter obstruction in rabbits produced profound changes in endogenous and exogenous renal arachidonic acid metabolism. Isolated perfused hydronephrotic kidneys (removed after 3 or 10 d of ureter obstruction) responded to bradykinin stimulation with a markedly enhanced release of prostaglandin E2 and thromboxane A2. Reversal (3 or 10 d) of the ureter obstruction resulted in a reduction in the vasoactive peptide-induced release of prostaglandin E2 and thromboxane A2 from the perfused hydronephrotic kidney. However, postobstruction reversal of prostaglandin production by the agonist-stimulated perfused kidney was not reflected in the cortical microsomal cyclooxygenase activity, which is greatly enhanced during ureter obstruction and does not decrease after removal of the obstruction. Histological analysis of the renal cortex in rabbits with ureteral obstruction revealed a proliferation of fibroblast-like cells and the presence of mononuclear cells; removal of the obstruction did not result in a disappearance of cortical fibroblasts but did result in a decrease of monocytes. The critical involvement of mononuclear cells in the exaggerated arachidonate metabolism that occurs during hydronephrosis was exhibited by the demonstration that: (a) only the perfused hydronephrotic rabbit kidney responded to administration of endotoxin with a sustained release of prostaglandin E2 and thromboxane A2; (b) the contralateral rabbit kidney, which is devoid of mononuclear cells, did not respond to endotoxin; and (c) the hydronephrotic cat kidney, which exhibits a fibroblast proliferation with a low number of mononuclear cells, did not respond to endotoxin. Thus, proliferation of fibroblast-like cells and the presence of mononuclear cells appear to be involved in the exaggerated prostaglandin and thromboxane production underlying hydronephrosis. The increase in microsomal cyclooxygenase activity is apparently most closely correlated with the increased fibroblastic activation and cellularity, whereas mononuclear cells (possibly via monokines) seem to be critical for the markedly enhanced prostaglandin and thromboxane release induced by endotoxin and bradykinin.
Full text
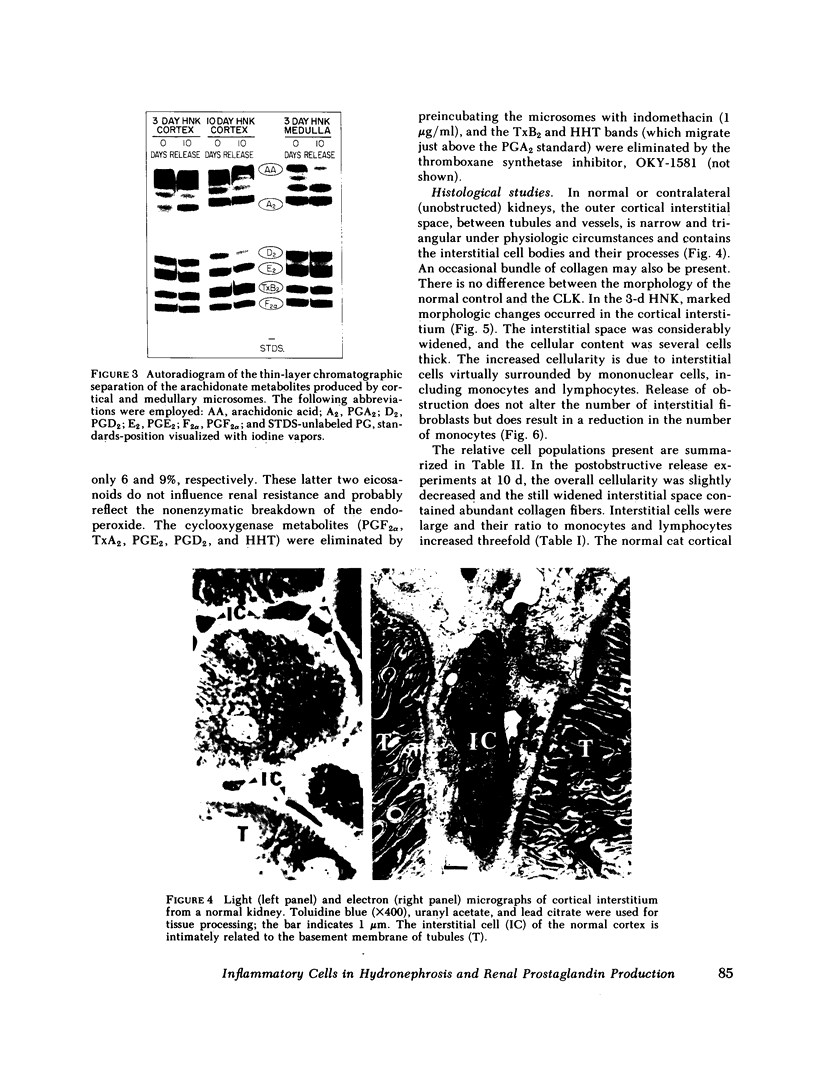
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benabe J. E., Klahr S., Hoffman M. K., Morrison A. R. Production of thromboxane A2 by the kidney in glycerol-induced acute renal failure in the rabbit. Prostaglandins. 1980 Mar;19(3):333–347. doi: 10.1016/0090-6980(80)90069-6. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Wise W. C., Halushka P. V. Thromboxane A2 and prostacyclin production by lipopolysaccharide-stimulated peritoneal macrophages. J Reticuloendothel Soc. 1981 Nov;30(5):445–450. [PubMed] [Google Scholar]
- Currie M. G., Davis B. B., Needleman P. Localization of exaggerated prostaglandin synthesis associated with renal damage. Prostaglandins. 1981 Dec;22(6):933–944. doi: 10.1016/0090-6980(81)90022-8. [DOI] [PubMed] [Google Scholar]
- D'Souza S. M., Englis D. J., Clark A., Russell R. G. Stimulation of production of prostaglandin E in gingival cells exposed to products of human blood mononuclear cells. Biochem J. 1981 Aug 15;198(2):391–396. doi: 10.1042/bj1980391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Passwell J. H., Schneeberger E. E., Krane S. M. Interactions among rheumatoid synovial cells and monocyte-macrophages: production of collagenase-stimulating factor by human monocytes exposed to concanavalin A or immunoglobulin Fc fragments. J Immunol. 1980 Apr;124(4):1712–1720. [PubMed] [Google Scholar]
- Dayer J. M., Robinson D. R., Krane S. M. Prostaglandin production by rheumatoid synovial cells: stimulation by a factor from human mononuclear Cells. J Exp Med. 1977 May 1;145(5):1399–1404. doi: 10.1084/jem.145.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenfels A., Vane J. R. Prostaglandins, oxygen tension and smooth muscle tone. Br J Pharmacol. 1972 Jul;45(3):451–462. doi: 10.1111/j.1476-5381.1972.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N., Foegh M., Ramwell P. W. Leukotrienes C4 and D4 induce prostaglandin and thromboxane release from rat peritoneal macrophages. Br J Pharmacol. 1981 Mar;72(3):389–391. doi: 10.1111/j.1476-5381.1981.tb10988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradet Y., Simard J., Grose J. H., Lebel M. Enhanced urinary prostaglandin E2 in postobstructive diuresis in humans. Prostaglandins Med. 1980 Jul;5(1):29–30. doi: 10.1016/0161-4630(80)90087-7. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Kuhn C., 3rd, Needleman P. Relationship of prostaglandin secretion by rabbit alveolar macrophages to phagocytosis and lysosomal enzyme release. Biochem J. 1979 Nov 15;184(2):345–354. doi: 10.1042/bj1840345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Sadowski S., Galavage M., Goldenberg M., Subers E., Bonney R. J., Kuehl F. A., Jr Evidence for two sources of arachidonic acid for oxidative metabolism by mouse peritoneal macrophages. J Biol Chem. 1982 Feb 25;257(4):1591–1594. [PubMed] [Google Scholar]
- Kawasaki A., Needleman P. Contribution of thromboxane to renal resistance changes in the isolated perfused hydronephrotic rabbit kidney. Circ Res. 1982 Apr;50(4):486–490. doi: 10.1161/01.res.50.4.486. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Anggård E. Increased juxtamedullary blood flow on stimulation of intrarenal prostaglandin biosynthesis. Eur J Pharmacol. 1974 Mar;25(3):326–334. doi: 10.1016/0014-2999(74)90263-5. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Lysz T. W., Needleman P. Evidence for two distinct forms of fatty acid cyclooxygenase in brain. J Neurochem. 1982 Apr;38(4):1111–1117. doi: 10.1111/j.1471-4159.1982.tb05355.x. [DOI] [PubMed] [Google Scholar]
- Martz E., Steinberg M. S. The role of cell-cell contact in "contact" inhibition of cell division: a review and new evidence. J Cell Physiol. 1972 Apr;79(2):189–210. doi: 10.1002/jcp.1040790205. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Thromboxane A2 biosynthesis in the ureter obstructed isolated perfused kidney of the rabbit. J Pharmacol Exp Ther. 1978 Apr;205(1):1–8. [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Unmasking of thromboxane A2 synthesis by ureteral obstruction in the rabbit kidney. Nature. 1977 May 19;267(5608):259–260. doi: 10.1038/267259a0. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Bulger R. E., Cutler R. E., Jervis H. R., Benditt E. P. Unilateral obstructive nephropathy in the rabbit. I. Early morphologic, physiologic, and histochemical changes. Lab Invest. 1973 Apr;28(4):456–467. [PubMed] [Google Scholar]
- Nagle R. B., Johnson M. E., Jervis H. R. Proliferation of renal interstitial cells following injury induced by ureteral obstruction. Lab Invest. 1976 Jul;35(1):18–22. [PubMed] [Google Scholar]
- Needleman P., Kauffman A. H., Douglas J. R., Jr, Johnson E. M., Jr, Marshall G. R. Specific stimulation and inhibition of renal prostaglandin release by angiotensin analogs. Am J Physiol. 1973 Jun;224(6):1415–1419. doi: 10.1152/ajplegacy.1973.224.6.1415. [DOI] [PubMed] [Google Scholar]
- Needleman P., Wyche A., Bronson S. D., Holmberg S., Morrison A. R. Specific regulation of peptide-induced renal prostaglandin synthesis. J Biol Chem. 1979 Oct 10;254(19):9772–9779. [PubMed] [Google Scholar]
- Nishikawa K., Morrison A., Needleman P. Exaggerated prostaglandin biosynthesis and its influence on renal resistance in the isolated hydronephrotic rabbit kidney. J Clin Invest. 1977 Jun;59(6):1143–1150. doi: 10.1172/JCI108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold D. F., Watters K., Holmberg S., Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [PubMed] [Google Scholar]
- Schwartzman M., Raz A. Selective induction of de novo prostaglandin biosynthesis in rabbit kidney cortex. Biochim Biophys Acta. 1981 Jun 23;664(3):469–474. doi: 10.1016/0005-2760(81)90125-9. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972 Aug 15;11(17):3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- Yarger W. E., Schocken D. D., Harris R. H. Obstructive nephropathy in the rat: possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J Clin Invest. 1980 Feb;65(2):400–412. doi: 10.1172/JCI109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser R., Myers S., Needleman P. Exaggerated prostaglandin and thromboxane synthesis in the rabbit with renal vein constriction. Circ Res. 1980 Aug;47(2):231–237. doi: 10.1161/01.res.47.2.231. [DOI] [PubMed] [Google Scholar]