Abstract
Possible pathophysiological, clinical and epidemiological interactions between human immunodeficiency virus (HIV) and tropical pathogens, especially malaria parasites, constitute a concern in tropical areas. Two decades of research have shown that HIV-related immunosuppression is correlated with increased malaria infection, burden, and treatment failure, and with complicated malaria, irrespective of immune status. The recent role out of antiretroviral therapies and new antimalarials, such as artemisinin combination therapies, raise additional concerns regarding possible synergistic and antagonistic effects on efficacy and toxicity. Co-trimoxazole, which is used to prevent opportunistic infections, has been shown to have strong antimalarial prophylactic properties, despite its long-term use and increasing antifolate resistance. The administration of efavirenz, a non-nucleoside reverse transcriptase inhibitor, with amodiaquine–artesunate has been associated with increased toxicity. Recent in vivo observations have confirmed that protease inhibitors have strong antimalarial properties. Ritonavir-boosted lopinavir and artemether–lumefantrine have a synergistic effect in terms of improved malaria treatment outcomes, with no apparent increase in the risk of toxicity. Overall, for the prevention and treatment of malaria in HIV-infected populations, the current standard of care is similar to that in non-HIV-infected populations. The available data show that the wider use of insecticide-treated bed-nets, co-trimoxazole prophylaxis and antiretroviral therapy might substantially reduce the morbidity of malaria in HIV-infected patients. These observations show that those accessing care for HIV infection are now, paradoxically, well protected from malaria. These findings therefore highlight the need for confirmatory diagnosis of malaria in HIV-infected individuals receiving these interventions, and the provision of different artemisinin-based combination therapies to treat malaria only when the diagnosis is confirmed.
Keywords: Clinical epidemiology, diagnosis, HIV, immunology, interactions, malaria, public health, review, treatment
Introduction
Since it became obvious that resource-poor regions of the world, and in particular south-eastern Africa, carry the main burden of the human immunodeficiency virus (HIV) pandemic, there have been concerns about possible pathophysiological, clinical and epidemiological interactions between HIV and tropical pathogens. Especially Plasmodium falciparum malaria, one of the main tropical killers was envisaged as concomitantly malaria treatment and control were undermined by the emergence of resistance to commonly used antimalarial drugs such as chloroquine and sulphadoxine–pyrimethamine. The geographical distribution of HIV and malaria suggests that, for many sub-Saharan African countries, even a small link between the two diseases would be of extreme importance in terms of public health impact and control policies. Considering that the two diseases share similar immunological factors, such a link may be plausible, and needs to be assessed carefully. Furthermore, as malaria is not the only disease that could interact with HIV-1, information from malaria–HIV studies may be relevant for other parasitic, bacterial and viral co-infections. We present a short review of the literature, and attempt to reiterate the reasons for the above-mentioned concerns, to assemble the available evidence, and to address outstanding or possible future questions and concerns.
Pathophysiology
The impact of HIV infection on malaria
Most clinical problems in HIV-1-infected individuals are related to the specific loss of pathogen-specific CD4 cell immunity of the Th1 type, and, in developing/tropical countries, tuberculosis is probably the most common consequence of Th1 depletion [1,2]. Other protozoan parasites are often contributors to mortality in individuals with AIDS: Babesia,Toxoplasma,Giardia,Cryptosporidium,Isospora, and Leishmania [3,4]. As acquired immunity to blood-stage malaria was thought to be primarily antibody-mediated, one might predict that it would be largely unaffected, particularly as cytokine patterns in HIV-infected individuals are said to be associated with a shift to Th2-type responses [5]. B-cell polyclonal expansion and total immunoglobulin concentrations, including antimalarial antibodies, in HIV-1-infected patients can be higher than or the same as those in uninfected controls, [3,6]. Today, we know that HIV-1 CD4 T-cells, the prime targets for destruction by HIV-1, have a critical role in both Th1-type and Th2-type responses to malaria [4]. Enhanced T-cell activation in co-infected patients can worsen the immune response to both diseases [7]. Phagocytosis, proliferative and Th1 cytokine responses are reduced in pregnant women with HIV infection, and pregnancy may contribute to impaired control of malaria in HIV-infected individuals [8]. However, variant surface antigen antibody levels, which seem important for the control of parasite density and treatment outcome, seem to be marginally or not affected by HIV-1 in non-pregnant adults [9]. In pregnancy, although antimalarial antibody responses are mostly unaltered, there seem to be impaired responses to some antigens, including variant surface antigens expressed on infected erythrocytes binding chondroitin sulfate A, a key receptor for placental sequestration. This impairment is greatest in women with more advanced HIV disease, and occurs across all gravidities and in women with and without current malaria infection [10].
The impact of malaria infection on HIV
The HIV-1 life cycle is intimately related to the level of activation of the immune cells supporting viral replication, which is enhanced by increased viral cellular entry, and reverse and proviral transcription [11]. Malaria infection is associated with strong CD4 cell activation and upregulation of proinflammatory cytokines; it provides the ideal microenvironment for the spread of the virus among the CD4 cells and for rapid HIV-1 replication [12]. In a malaria challenge trial, enhanced HIV production was related to the development of antimalaria immunity, and may have been mediated by proinflammatory cytokines [13]. HIV-1 viral load first increases in malaria-infected patients, and then decreases 4 weeks after antimalaria treatment [14]. Malaria parasitaemia also has an immediate impact on CD4 cells. Indeed, malaria in children is associated with reversible lymphopenia and an absolute lower CD4 cell count [15,16], and this has also been observed in HIV-1-infected adults [17]. Similar observations have been made in malaria-infected pregnant women. For example, the expression of CC-chemokine receptor 5, a co-receptor for HIV cell entry, is increased in the placentas of malaria-infected women, possibly contributing to intrauterine HIV transmission [18]. Malaria-infected pregnant women have an increased viral load, although lower than that observed in non-pregnant adults [19–21]. Of special concern is the risk of mother-to-child transmission (MTCT). Maternal HIV-1 viral load is currently considered to be the single most important determinant of MTCT. Studies addressing this issue produced conflicting results, and were all conducted prior to the introduction of antiretrovirals (ARVs) in Africa [18–22]. The discrepant results are probably attributable to the differences in diagnosing placental malaria, but might also reflect the complexity of maternal immune responses to malaria, which, on the one hand, may stimulate HIV viral replication in the placenta, thereby increasing the local viral load, and on the other hand may potentially control the severity of malarial infection and HIV replication [10]. The net result may be either an enhanced or a reduced risk of MTCT, depending on the specific situation. Furthermore, immunocompromised mothers with malaria–HIV co-infection have altered chemokine and cytokine profiles, less protective immune responses, and consequently higher parasite densities and viral load, leading to an increased risk of MTCT [23]. Today, schemes for the prevention of MTCT have reduced the risk to almost zero, so, in an interventional research setting, carefully designed studies, probably with huge sample sizes, will be needed to assess a modified risk of MTCT.
Epidemiology
Impact of malaria on HIV transmission
There is a strong correlation between viral load and the risk of heterosexual HIV-1 transmission, the predominant mode of transmission in Africa, and there is compelling evidence suggesting an epidemiological synergy between sexually transmitted infections and HIV [24,25]. Enhanced immune activation caused by malaria may contribute to increased HIV-1 transmission and disease progression. Transient but repeated elevated HIV viral loads resulting from recurrent co-infections, such as malaria, could influence and increase HIV prevalence [26]. However, we lack consistent data for some parameters used to measure the impact of malaria on HIV transmission. Only two reports have mentioned that HIV-1 viral load first increases in malaria-infected patients and then decreases 4 weeks after antimalarial treatment [14,27] (Fig.1).
Figure 1.
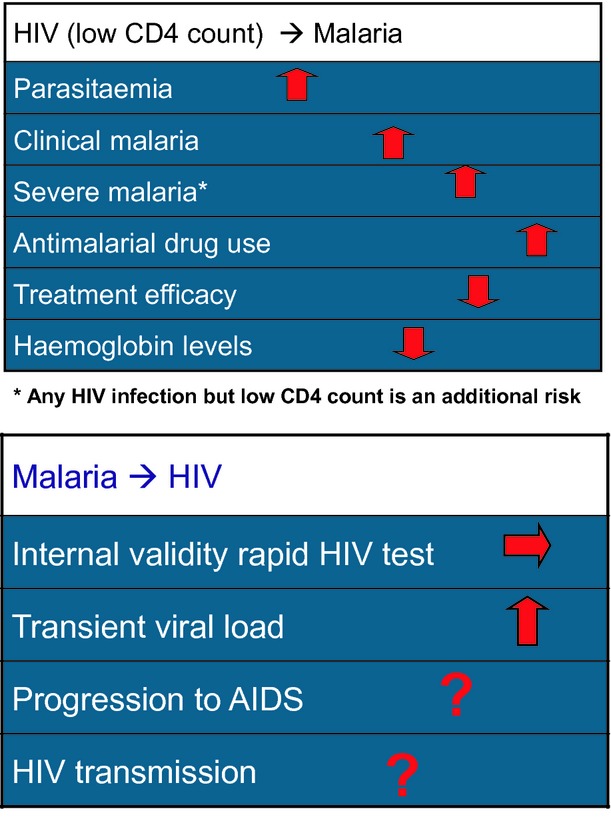
Clinical epidemiological impact of human immunodeficiency virus (HIV)–malaria interactions.
Impact of HIV on malaria transmission
At the population level, an impact of HIV on malaria transmission is suggested by the fact that families living in the same household as an HIV-infected individual have higher rates of malaria among HIV-negative children than do families without an HIV-infected individual in the household [28].
Mathematical modelling offers an alternative approach, and can be applied to different malaria and HIV settings, provided that the relevant parameters are included. Earlier models focusing on the direct effects in HIV-infected people concluded that the population-level effect was limited because of different geographical distributions and contrasting age patterns of the two diseases [29], and a second, more complex, model [26] estimated that the largest epidemiological impact occurs when the prevalence of one disease, HIV or malaria, is very high while the other is low and near its endemic threshold. However, when the prevalence of both diseases is either very high or very low, the epidemiological impact of the interaction will be minimal. The latter has been confirmed by a review evaluating multiple cross-sectional surveys in western sub-Saharan Africa [30]. However, several points should be considered. First, a ‘minimal’ impact in a setting with a high double burden will still be substantial in absolute terms. Second, with no or inefficacious treatment, the duration of heightened viral load and increased prevalence of parasitaemia resulting from HIV immunosuppression will be extended. Third, when both diseases reach an epidemic equilibrium, even if the direct epidemiological impact is minimal, interaction will occur frequently; viraemia will increase in HIV-infected patients as a result of malaria infection, and parasite load will also be higher in immunosuppressed co-infected patients. Therefore, co-infection is likely to produce a higher circulating biomass and a higher biodiversity of both species [31]. Finally, a wide variety of biological and behavioural factors are associated with the risk of HIV-1 sexual transmission [32], and the malaria morbidity effect on sexual behaviour is also not clearly documented and is not a sensitive parameter in HIV transmission models. No model has included indirect effects attributable to concurrent malaria and HIV-1 infection, such as anaemia or pregnancy, a vulnerable period for malaria morbidity and mortality, and HIV transmission has been not been considered. Therefore, the actual impact of the interaction, in the absence of any control intervention, might be more important than expected.
HIV-1 and malaria morbidity
A substantial overlap in the clinical and laboratory characteristics of malaria and HIV-related syndromes generates potential difficulties in AIDS staging and in the diagnosis and management of patients at risk of co-infection [33]. Several clinical or laboratory syndromes that are shared by malaria and AIDS-related conditions and that might cause diagnostic confusion have been identified [33]. For two decades, evidence for the impact of HIV-1 on malaria has accumulated. Increased risks of a higher parasite prevalence, malarial fever and clinical malaria have been reported in HIV-infected adults, and the risk was inversely correlated with the CD4 cell count [34,35] (Fig.1; Table1). However, the relationship between malaria and HIV-1 immunosuppression is more complex than expected. The incidence of recurrent malaria (>28 days after the first episode) was not associated with a low CD4 cell count [36], although a low CD4 cell count was associated with increased parasite density in patients with clinical malaria but not in people with asymptomatic parasite carriage [37]. Furthermore, for any parasite density, the probability of fever was inversely related to CD4 cell count. In children, HIV infection might be associated with severe malaria, although the magnitude of the effect may be relatively small [38]. In adults living in areas with low and unstable transmission, two-fold and seven-fold increases in mortality were observed [39,40], and a multifold increase was observed in an area of stable malaria transmission [41]. In both adult studies, HIV was an independent risk factor irrespective of CD4 cell count, although patients with a low CD4 cell count had an increased risk in the meso-endemic area. We should bear in mind that HIV-1 immunosuppression is associated with various opportunistic infections, and any fever in these patients with a positive malaria slide could be associated with an underlying disease [30], and that malaria parasitaemia, especially if symptomatic, transiently affects the CD4 cell count [17]. Because of the epidemiological overlap, particularly in eastern and southern Africa, co-infection with HIV-1 and malaria is a common phenomenon. Anaemia is frequently observed in both individuals with malaria and in those with HIV infection, although the physiopathological mechanisms differ. It is the most frequent cytopenia seen in HIV-infected individuals [42], partly because of bone marrow suppression by medications [43] and other factors, including haemolysis, gastrointestinal bleeding, nutritional deficiencies [44], and a diminished erythropoietin response [45]. Malaria is also often associated with anaemia, and, during a malaria attack, HIV-1-infected individuals, both infants and adults, have lower haemoglobin levels than HIV-1-uninfected patients [46]. Moreover, HIV-1-infected infants have an increased risk for severe anaemia [47]. Furthermore, HIV-1-infected malaria patients show a steeper haemoglobin decline in the first weeks after successful malaria treatment, and show slower haematological recovery (Fig.1). Possibly, this slow recovery could be explained by the impairments of erythropoiesis and iron mobilization [48].
Table 1.
Review of human immunodeficiency virus-1 infection and non-severe clinical malaria incidence
| First author, year | Risk of symptomatic malaria | Remarks |
|---|---|---|
| Witworth, 2000 [34] | OR: 6.0, 3.4 and 1.2 for CD4 cell counts of <200/μL, 200–499/μL, and ≥500/μL, respectively | Any symptomatic parasitaemia |
| French, 2001 [35] | Absolute Risk: 140, 93 and 57 per 1000 person-years for CD4 cell counts of <200/μL and 200–499/μL, and ≥500 parasites/μL, respectively | Any symptomatic parasitaemia |
| French, 2001 [35] | Absolute Risk: 90, 53 and 22 per 1000 person-years for CD4 cell counts of <200/μL, 200–499/μL and ≥500/μL, respectively | Symptomatic parasitaemia with >2800 parasites/μL |
| Laufer, 2006 [37] | Relative risks of 4.4 and 3 for CD4 cell counts of <200/μL and 200–499/μL, respectively, as compared with a CD4 cell count of ≥500/μL | |
| Patnaik, 2005 [36] | OR: 5.4, 4.9 and 3.2 per 1000 person-years for CD4 cell counts of <200/μL and 200–499/μL, and ≥400 parasites/μL, respectively | CD4 measures at baseline |
Observations in pregnant mothers, sometimes including their newborn children, have demonstrated an increased risk of malaria in those with HIV infection. The risk increment is more pronounced in multigravida than in primigravida, indicating that HIV-1 hinders the development of immunity. A two-fold increase in malaria incidence was also observed [10]. The relative risk (RR) for malaria parasitaemia in HIV-1-infected women has been estimated during pregnancy (RR 1.58), at delivery (RR 1.65), and for placenta malaria (RR 1.66). HIV-1 infection was also associated with lower birthweight [49], higher infant mortality, and a four-fold greater risk of malaria attacks in the newborn [50]. The increased risk of placental malaria in HIV-infected mothers was also associated with higher postnatal mortality [51]. As in non-pregnancy, the risk of anaemia is beyond the degree that would be expected from infections with malaria or HIV alone, suggesting a synergistic interaction between HIV and malaria, placing dually infected women at very high risk of developing severe anaemia [49,52].
Malaria and HIV-1 Disease Progression
Malaria infection leads to strong CD4 cell activation [12]. The selective infection of the antigen-specific memory CD4 cells by HIV leads to the loss of these cells [11]. Therefore, HIV patients could lose the de novo activated malaria-specific CD4 cells during each malaria attack, because these are more susceptible to HIV, and they could progress more rapidly to full-blown AIDS [53–55]. At present, there is no empirical evidence for this, but modelling from empirical data suggests that an additional 40 CD4 cells/μL per year may be lost because of repeated malaria episodes [54].
Case Management
Artemisinine combinations (ACTs) are now widely recommended as first-line drugs for the treatment of uncomplicated malaria in several African countries. The most commonly used ACTs in these settings include artemether–lumefantrine and amodiaquine–artesunate, with dihydroartemisinin–piperaquine also being utilized. Data on the safety and efficacy of antimalarials in HIV-infected populations are still limited (Table2). Only a few recent studies used ACTs and were able to determime CD4 cell counts. It has been reported that HIV-1-infected patients with malaria and with lower CD4 cell counts have a higher risk of experiencing a recrudescent infection and a new infection (Fig.1; Table2). Taking into account the reciprocal effect of malaria on CD4 cell count [17] and the results of a prospective cohort study with availability of baseline CD4 cell count values [36], the cut-off point for an increased risk of treatment failure, higher parasite prevalence and malarial fever probably has to be set at ≥400 cells/mL.
Table 2.
Human immunodeficiency virus-1 (HIV)-1 infection and antimalarial treatment failure
| First author, year | Age (years) | Treatment | Follow-up (days) | HIV-negative | HIV-positive | PCR | CD4 | Result |
|---|---|---|---|---|---|---|---|---|
| Muller, 1990 [70] | <5 | CQ–SP | 3 | 40 | 35 | − | − | NS |
| Muller, 1990 [70] | 26 | CQ–SP | 3 | 58 | 142 | − | − | NS |
| Colebunders, 1990 [71] | 7 | QN | 7 | 83 | 59 | − | − | NS |
| Greenberg, 1991 [72] | <1 | SP | 7 | 166 | 32 | − | − | NS |
| Kamya, 2001 [73] | <5 | CQ | 14/28 | 186 | 6 | − | − | NS |
| Kamya, 2001 [73] | 10 | CQ | 14/28 | 124 | 23 | − | − | NS |
| Birku, 2002 [74] | 32 | AS | 3 | − | − | Decreased parasite density and fever clearance | ||
| Kamya, 2006 [75] | 28.5 | CQ–SP AQ–SP AQ–AS |
28 | 113 | 50 | + | − | Increased total treatment failure Increase in new infections |
| Van Geertruyden, 2006 [76] | 27.6 | SP–AL | 28 | 530 | 266 | + | + | Increased total treatment failure in low-CD4 HIV-positive vs. HIV-negative individuals: NS |
PCR: genotyping for recrudescence or new infection.
AL, artemether–lumefantrine; AQ, amodiaquine; AS, artesunate or artemisinine; CQ, chloroquine; NS, not significant; QN, quinine; SP, sulphadoxine–pyrimethamine.
With the introduction of ARTs and co-trimoxazole prophylaxis, the situation is changing drastically. First, co-trimoxazole has been rediscovered as an antimalarial [2]. The protective efficacy of co-trimoxazole prophylaxis against clinical malaria ranges between 46% and 97% in HIV-1-infected individuals [56,57], and between 39.5% and 99.5% in HIV-negative infants and children [58,59]. The lowest figures were observed where malaria transmission and sulphadoxine–pyrimethamine resistance were extremely high.
Drug–drug Interactions
Interactions between ARTs and antimalarials also deserve attention. Interactions may affect antimalarial activity and exacerbate toxicity. Furthermore, if individuals are exposed to repeated malaria infections requiring treatment, the effect of drug interactions combined with the increased transient viral replication and viral load during malaria infection [27] may be similar to the effects of suboptimal adherence to ARTs. The currently used ARTs fall into three classes: nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors. Possible treatment interactions have been discussed previously [60]. Both malaria and HIV case management involve combination therapies, and many drugs follow the same metabolic pathways. Therefore, detailed clinical studies that also assess pharmacokinetic and pharmacodynamic interactions are needed. Two recent in vivo observations showed that co-administration of artemether–lumefantrine with efavirenz or nevirapine resulted in a reduction in artemether and dihydroartemisinin exposure, but the findings on the impact on lumefantrine were conflicting [61,62]. One of the studies indicated a reduction in nevirapine exposure [62]. Fortunately, safety and tolerability may not be affected [61]. Pharmacokinetic and pharmacodynamic trials evaluating the impact of these drug interactions are urgently needed, as treatment efficacy and the selection of resistant strains may be affected. Another in vivo study showed a dramatic benefit of the interaction between ritonavir-boosted lopinavir and artemether–lumefantrine in terms of improved malaria treatment outcomes, with no apparent increase in the risk of toxicity [63]. Clearly, in these settings, the boosted lopinavir-based ART regimen had significant advantages over the non-nucleoside reverse transcriptase inhibitor-based ART regimen, as it was associated with a marked reduction in malaria risk. This regimen could therefore be strategically utilized in high-transmission settings in high-risk populations, such as children <5 years of age, for the dual benefits of protection against malaria and as a superior regimen for HIV treatment [64]. For ARVs combined with amodiaquine–artesunate, in particular efavirenz, a higher risk of neutropenia has been documented, and cases of hepatitis have been reported [65,66]. This provides good guidance for clinicians in their evaluations. There is limited literature on the interaction between quinine and ARVs, but the available literature does not suggest any obvious contraindications for its use [67]. Overall, the spectrum of potential adverse events with concurrent administration of the other antimalarial drugs and ARVs is not well characterized. Adjustment of antimalarial dosing may be necessary to avoid toxicity, but adequate data to guide dosing adjustments are under investigation and not yet available. These will need standardized evaluations to ensure that any such events are consistently documented. This will become increasingly important with the development of newer antimalarial drugs and ARVs. The strategies for controlling malaria during pregnancy rely on case management and a package of preventive measures, including insecticide-treated bed-nets (ITNs) and intermittent preventive treatment with sulphadoxine–pyrimethamine, a folate inhibitor [68]. HIV-1 infection decreases the efficacy of sulphadoxine–pyrimethamine intermittent preventive treatment during pregnancy [69]. Co-trimoxazole prophylaxis is currently recommended by the WHO to prevent opportunistic infections in pregnant women living with HIV/AIDS. In HIV-infected pregnant women on daily co-trimoxazole, sulphadoxine–pyrimethamine intermittent preventive treatment is not indicated, as it may be associated with overlapping toxicities. Fortunately, co-trimoxazole prophylaxis has been shown to decrease the prevalence of placental malaria in HIV-infected women as much as sulphadoxine–pyrimethamine intermittent preventive treatment in HIV-uninfected women [2].
Conclusion
For the prevention and treatment of malaria in HIV-infected populations, the current standard of care should include the wide availability of ITNs, the use of co-trimoxazole prophylaxis, the wide availability of different ACTs and intermittent preventive treatment with sulphadoxine–pyrimethamine or co-trimoxazole in pregnant women. For the treatment of HIV infection, the increasing availability and rapid scale-up of ARV therapy has ensured wider coverage of the population that requires this treatment. The available data show that the wider implementation of multiple interventions such as ITNs, co-trimoxazole prophylaxis and ARV therapy may substantially reduce the morbidity of malaria in HIV-infected patients [3,56]. HIV infection may therefore no longer be considered to be a risk factor for malaria among those accessing care for HIV infection, as such individuals are now, paradoxically, protected from malaria by co-trimoxazole prophylaxis and ITNs. These findings therefore highlight the need for confirmatory diagnosis of malaria in HIV-infected individuals receiving these interventions, and the provision of malaria therapy only when the diagnosis is confirmed. The fact that protease inhibitors have proven antimalarial properties may have huge public health implications regarding the potential benefit of this kind of interaction for co-infected individuals, particularly those living in areas of high malaria transmission intensity.
Transparency Declaration
The authors declare that they have no conflicts of interest.
References
- 1.Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 2.Manyando C, Njunju EM, D'Alessandro U, Van Geertruyden JP. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS ONE. 2013;8:e56916. doi: 10.1371/journal.pone.0056916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mermin J, Ekwaru JP, Liechty CA, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 4.Artavanis-Tsakonas K, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol. 2003;133:145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici M, Shearer GM. The Th1–Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 6.Nnedu ON, O'Leary MP, Mutua D, et al. Humoral immune responses to Plasmodium falciparum among HIV-1-infected Kenyan adults. Proteomics Clin Appl. 2011;5:613–623. doi: 10.1002/prca.201100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavale H, Santos-Oliveira JR, Da-Cruz AM, Enosse S. Enhanced T cell activation in Plasmodium falciparum malaria-infected human immunodeficiency virus-1 patients from Mozambique. Mem Inst Oswaldo Cruz. 2012;107:985–992. doi: 10.1590/s0074-02762012000800004. [DOI] [PubMed] [Google Scholar]
- 8.Ludlow LE, Zhou J, Tippett E, et al. HIV-1 inhibits phagocytosis and inflammatory cytokine responses of human monocyte-derived macrophages to P. falciparum infected erythrocytes. PLoS ONE. 2012;7:e32102. doi: 10.1371/journal.pone.0032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Geertruyden JP, Van EE, Yosaatmadja F, et al. The relationship of Plasmodium falciparum humoral immunity with HIV-1 immunosuppression and treatment efficacy in Zambia. Malar J. 2009;8:258–266. doi: 10.1186/1475-2875-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:41–54. [PubMed] [Google Scholar]
- 11.Lawn SD. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Worku S, Bjorkman A, Troye-Blomberg M, Jemaneh L, Farnert A, Christensson B. Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gammadelta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol. 1997;108:34–41. doi: 10.1046/j.1365-2249.1997.d01-981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlov M, Vaida F, Finney OC, et al. P. falciparum enhances HIV replication in an experimental malaria challenge system. PLoS ONE. 2012;7:e39000. doi: 10.1371/journal.pone.0039000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman IF, Jere CS, Taylor TE, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood BM, Oduloju AJ, Stratton D. Lymphocyte changes in acute malaria. Trans R Soc Trop Med Hyg. 1977;71:408–410. doi: 10.1016/0035-9203(77)90039-6. [DOI] [PubMed] [Google Scholar]
- 16.Hviid L, Kurtzhals JA, Goka BQ, Oliver-Commey JO, Nkrumah FK, Theander TG. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Geertruyden JP, Mulenga M, Kasongo W, et al. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr. 2006;43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 18.Tkachuk AN, Moormann AM, Poore JA, et al. Malaria enhances expression of CC chemokine receptor 5 on placental macrophages. J Infect Dis. 2001;183:967–972. doi: 10.1086/319248. [DOI] [PubMed] [Google Scholar]
- 19.Inion I, Mwanyumba F, Gaillard P, et al. Placental malaria and perinatal transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;188:1675–1678. doi: 10.1086/379737. [DOI] [PubMed] [Google Scholar]
- 20.Mwapasa V, Rogerson SJ, Molyneux ME, et al. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS. 2004;18:1051–1059. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 21.Ayisi JG, van Eijk AM, Newman RD, et al. Maternal malaria and perinatal HIV transmission, western Kenya. Emerg Infect Dis. 2004;10:643–652. doi: 10.3201/eid1004.030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, et al. The effects of placental malaria on mother-to-child HIV transmission in Rakai, Uganda. AIDS. 2003;17:2539–2541. doi: 10.1097/00002030-200311210-00020. [DOI] [PubMed] [Google Scholar]
- 23.Ned RM, Moore JM, Chaisavaneeyakorn S, Udhayakumar V. Modulation of immune responses during HIV–malaria co-infection in pregnancy. Trends Parasitol. 2005;21:284–291. doi: 10.1016/j.pt.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 26.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 27.Kublin JG, Patnaik P, Jere CS, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–240. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 28.Mermin J, Lule J, Ekwaru JP, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. AIDS. 2005;19:1035–1042. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]
- 29.Korenromp EL, Williams BG, de Vlas SJ, et al. Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa. Emerg Infect Dis. 2005;11:1410–1419. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuadros DF, Branscum AJ, Garcia-Ramos G. No evidence of association between HIV-1 and malaria in populations with low HIV-1 prevalence. PLoS ONE. 2011;6:e23458. doi: 10.1371/journal.pone.0023458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Geertruyden JP, Menten J, Colebunders R, Korenromp E, D'Alessandro U. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar J. 2008;7:134–146. doi: 10.1186/1475-2875-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 33.Brentlinger PE, Behrens CB, Micek MA. Challenges in the concurrent management of malaria and HIV in pregnancy in sub-Saharan Africa. Lancet Infect Dis. 2006;6:100–111. doi: 10.1016/S1473-3099(06)70383-8. [DOI] [PubMed] [Google Scholar]
- 34.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–1056. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 35.French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS. 2001;15:899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 36.Patnaik P, Jere CS, Miller WC, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- 37.Laufer MK, van Oosterhout JJ, Thesing PC, et al. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 38.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. Childhood malaria in a region of unstable transmission and high human immunodeficiency virus prevalence. Pediatr Infect Dis J. 2003;22:1057–1063. doi: 10.1097/01.inf.0000101188.95433.60. [DOI] [PubMed] [Google Scholar]
- 39.Cohen C, Karstaedt A, Frean J, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis. 2005;41:1631–1637. doi: 10.1086/498023. [DOI] [PubMed] [Google Scholar]
- 40.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–554. doi: 10.1097/00002030-200402200-00023. [DOI] [PubMed] [Google Scholar]
- 41.Chalwe V, Van Geertruyden JP, Mukwamataba D, et al. Increased risk for severe malaria in HIV-1-infected adults, Zambia. Emerg Infect Dis. 2009;15:749–756. doi: 10.3201/eid1505.081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sloand E. Hematologic complications of HIV infection. AIDS Rev. 2005;7:187–196. [PubMed] [Google Scholar]
- 43.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- 44.Markle HV. Cobalamin. Crit Rev Clin Lab Sci. 1996;33:247–356. doi: 10.3109/10408369609081009. [DOI] [PubMed] [Google Scholar]
- 45.Spivak JL, Barnes DC, Fuchs E, Quinn TC. Serum immunoreactive erythropoietin in HIV-infected patients. JAMA. 1989;261:3104–3107. [PubMed] [Google Scholar]
- 46.van Eijk AM, Ayisi JG, ter Kuile FO, et al. Malaria and human immunodeficiency virus infection as risk factors for anemia in infants in Kisumu, western Kenya. Am J Trop Med Hyg. 2002;67:44–53. doi: 10.4269/ajtmh.2002.67.44. [DOI] [PubMed] [Google Scholar]
- 47.Otieno RO, Ouma C, Ong'echa JM, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–280. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 48.Van Geertruyden JP, Mulenga M, Chalwe V, et al. Impact of HIV-1 infection on the hematological recovery after clinical malaria. J Acquir Immune Defic Syndr. 2009;50:200–205. doi: 10.1097/QAI.0b013e3181900159. [DOI] [PubMed] [Google Scholar]
- 49.Ayisi JG, van Eijk AM, ter Kuile FO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003;17:585–594. doi: 10.1097/00002030-200303070-00014. [DOI] [PubMed] [Google Scholar]
- 50.Ticconi C, Mapfumo M, Dorrucci M, et al. Effect of maternal HIV and malaria infection on pregnancy and perinatal outcome in Zimbabwe. J Acquir Immune Defic Syndr. 2003;34:289–294. doi: 10.1097/00126334-200311010-00005. [DOI] [PubMed] [Google Scholar]
- 51.Bloland PB, Wirima JJ, Steketee RW, Chilima B, Hightower A, Breman JG. Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. AIDS. 1995;9:721–726. doi: 10.1097/00002030-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 52.van Eijk AM, Ayisi JG, ter Kuile FO, et al. Risk factors for malaria in pregnancy in an urban and peri-urban population in western Kenya. Trans R Soc Trop Med Hyg. 2002;96:586–592. doi: 10.1016/s0035-9203(02)90319-6. [DOI] [PubMed] [Google Scholar]
- 53.Whitworth JA, Hewitt KA. Effect of malaria on HIV-1 progression and transmission. Lancet. 2005;365:196–197. doi: 10.1016/S0140-6736(05)17752-6. [DOI] [PubMed] [Google Scholar]
- 54.Mermin J, Lule JR, Ekwaru JP. Association between malaria and CD4 cell count decline among persons with HIV. J Acquir Immune Defic Syndr. 2006;41:129–130. doi: 10.1097/01.qai.0000179427.11789.a7. [DOI] [PubMed] [Google Scholar]
- 55.Bentwich Z. Concurrent infections that rise the HIV viral load. J HIV Ther. 2003;8:72–75. [PubMed] [Google Scholar]
- 56.Kamya MR, Gasasira AF, Achan J, et al. Effects of trimethoprim–sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 57.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 58.Thera MA, Sehdev PS, Coulibaly D, et al. Impact of trimethoprim–sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–1829. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandison TG, Homsy J, Arinaitwe E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011:342. doi: 10.1136/bmj.d1617. d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skinner-Adams TS, McCarthy JS, Gardiner DL, Andrews KT. HIV and malaria co-infection: interactions and consequences of chemotherapy. Trends Parasitol. 2008;24:264–271. doi: 10.1016/j.pt.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Kredo T, Mauff K, Van der Walt JS, et al. Interaction between artemether–lumefantrine and nevirapine-based antiretroviral therapy in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5616–5623. doi: 10.1128/AAC.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byakika-Kibwika P, Lamorde M, Mayito J, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67:2213–2221. doi: 10.1093/jac/dks207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012;367:2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gasasira AF, Kamya MR, Achan J, et al. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46:985–991. doi: 10.1086/529192. [DOI] [PubMed] [Google Scholar]
- 66.German P, Greenhouse B, Coates C, et al. Hepatotoxicity due to a drug interaction between amodiaquine plus artesunate and efavirenz. Clin Infect Dis. 2007;44:889–891. doi: 10.1086/511882. [DOI] [PubMed] [Google Scholar]
- 67.Soyinka JO, Onyeji CO, Omoruyi SI, Owolabi AR, Sarma PV, Cook JM. Pharmacokinetic interactions between ritonavir and quinine in healthy volunteers following concurrent administration. Br J Clin Pharmacol. 2010;69:262–270. doi: 10.1111/j.1365-2125.2009.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine–pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–2616. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 69.Mathanga DP, Uthman OA, Chinkhumba J. Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women. Cochrane Database Syst Rev. 2011:CD006689. doi: 10.1002/14651858.CD006689.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller O, Moser R. The clinical and parasitological presentation of Plasmodium falciparum malaria in Uganda is unaffected by HIV-1 infection. Trans R Soc Trop Med Hyg. 1990;84:336–338. doi: 10.1016/0035-9203(90)90306-y. [DOI] [PubMed] [Google Scholar]
- 71.Colebunders R, Bahwe Y, Nekwei W, et al. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa. Zaire J Infect. 1990;21:167–173. doi: 10.1016/0163-4453(90)91701-e. [DOI] [PubMed] [Google Scholar]
- 72.Greenberg AE, Nsa W, Ryder RW, et al. Plasmodium falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325:105–109. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 73.Kamya MR, Kigonya CN, McFarland W. HIV infection may adversely affect clinical response to chloroquine therapy for uncomplicated malaria in children. AIDS. 2001;15:1187–1188. doi: 10.1097/00002030-200106150-00019. [DOI] [PubMed] [Google Scholar]
- 74.Birku Y, Mekonnen E, Bjorkman A, Wolday D. Delayed clearance of Plasmodium falciparum in patients with human immunodeficiency virus co-infection treated with artemisinin. Ethiop Med J. 2002;40(suppl 1):17–26. [PubMed] [Google Scholar]
- 75.Kamya MR, Gasasira AF, Yeka A, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 76.Van Geertruyden JP, Mulenga M, Mwananyanda L, et al. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–925. doi: 10.1086/507310. [DOI] [PubMed] [Google Scholar]


